Metabolomic Profile and Biological Properties of Sea Lavender (Limonium algarvense Erben) Plants Cultivated with Aquaculture Wastewaters: Implications for Its Use in Herbal Formulations and Food Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of the Extracts
2.4. Liquid Chromatography-Tandem High-Resolution Mass Spectrometry (LC-HRMS/MS) Analysis
2.5. In Vitro Antioxidant Activity
2.5.1. Radical Scavenging Activity (RSA) on DPPH• and ABTS+•
2.5.2. Ferric Reducing Antioxidant Power (FRAP)
2.5.3. Metal Chelating Activity on Iron (ICA) and Copper (CCA)
2.6. Ex Vivo Antioxidant Activity
2.6.1. Oxidative Haemolysis Inhibition Assay (OxHLIA)
2.6.2. Inhibition of Lipid Peroxidation by the Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.7. In Vitro Tyrosinase Inhibition
2.8. Cell Culture
2.8.1. In Vitro Anti-Inflammatory Activity
2.8.2. In Vitro Anti-Melanogenic Properties
2.8.3. In Vitro Cytotoxicity
2.9. Statistical Analyses
3. Results and Discussion
3.1. Metabolomic Profile
| Id | Rt (min) | Proposed Structure | [M-H]− [m/z (∆ ppm)] | MS/MS [(m/z) (∆ ppm) (Attribution) (%)] | Proposed Compound | Flowers | Peduncles | Leaves | ||||
| FWt | 300 mM | FWt | 300 mM | FWt | 300 mM | 600 mM | ||||||
| 1 | 2.9 | - | 272.9591 | 158.9782 | n.i. | - | - | - | - | ++ | +++ | ++++ |
| 2 | 3.1 | C12H22O11 | 341.1094 (−1.3) | 179.0558 (+1.8) [C6H11O6]− (100) 119.0333 (+14.2) [C4H7O]− (70) | Sucrose or isomers | - | - | - | - | ++ | +++ | ++++ |
| 3 | 3.3 | C6H7O6SO3 | 254.9820 (−1.4) | 175.0245 (+1.8) [C6H7O6]− (100) 115.0022 (+12) [C4H3O4]− (80) | Ascorbic acid sulphate | - | - | - | - | ++++ | +++ | +++ |
| 4 | 3.4 | C6H6O7 | 189.0032 (+4.3) | 189.0034 (4.5) [C6H5O7]− (100) 127.0046 (−7.2) [C5H3O4]− (50) | Oxalosuccinic acid | ++++ | +++ | ++++ | +++ | + | ++ | ++ |
| 5 | 3.6 | C11H17NO8 | 290.0885 (−1.3) | 170.0445 (8.5) [C7H8NO4]− (10) 128.0361 (−6.4) [C5H6NO3]− (100) | 2-Deoxy-2,3-dehydro N-acetylneuraminic acid | - | ++++ | - | - | - | - | - |
| 6 | 3.7 | C6H8O7 | 191.0187 (+5.4) | 111.0088 (−9.9) [C5H3O4]− (100) | Citric acid | ++ | ++ | - | - | +++ | +++ | +++ |
| 7 | 3.8 | C13H10O8 | 293.0339 (−2.2) | 169.0133 (−5.6) [C7H5O5]− (100) 137.0220 (−12.6) [C7H5O3]− (7−0) 125.0261 (−10.2) [C6H5O3]− (40) | Pyrogallol gallate | + | + | - | - | - | - | - |
| 8 | 3.9 | C13H16O8SO3 | 379.0348 (−2.1) | 299.0765 (+2.4) [C13H15O8]− (10) 241.0023 (+0.4) [C6H9O8S]− (100) | Salicylic acid glucosyl sulphate | - | - | - | - | - | ++ | + |
| 9 | 3.9 | C7H6O5 | 169.0135(−5.0) | 125.0241 (−6.4) [C6H5O3]− (100) | Gallic acid | ++ | ++ | - | - | - | - | - |
| 10 | 4.0 | C13H16O10SO3 | 411.0235 (+0.9) | 331.0671 (−5.4) [C13H15O10]− (10) 241.0023 (+0.5) [C6H9O8S]− (100) 169.0134 (−4.9) [C7H5O5]− (20) | Glucogallin sulphate | ++ | ++ | ++++ | +++ | +++ | ++++ | ++++ |
| 11 | 4.8 | C14H18O10SO3 | 425.0398 (−0.6) | 345.0828 (−0.3) [C14H17O10]− (7) 241.0023 (+0.5) [C6H9O8S]− (100) 183.0299(+0.1) [C8H7O5]− (50) | Glucosyl methyl gallate sulphate | - | - | - | - | - | + | ++ |
| 12 | 5.0 | C15H20O10(SO3)2 | 519.01914 (−0.6) | 439.0552 (−0.3) [C15H19O13S]− (30) 241.0025 (−0.4) [C6H9O8S]− (100) | Glucosyringic acid disulphate | ++ | + | - | - | ++++ | ++++ | +++ |
| 13 | 5.0 | C15H20O10SO3 | 439.0554 (−0.4) | 359.1010 (−8.1) [C15H19O10]− (10) 241.0023 (−0.5) [C6H9O8S]− (100) 197.0447 (−4.3) [C9H9O5] (10) | Glucosyringic acid sulphate | ++ | ++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 14 | 5.4 | C15H18O8(SO3)2 | 485.0062 (−0.6) | 405.0498 (+0.2) [C15H17O15S]− (90) 325.0928 (+0.2) [C15H17O8]− (10) | Glucosyl coumaric acid disulphate | - | - | - | - | ++ | ++ | +++ |
| 15 | 5.4 | C15H20O10SO3 | 439.0558 (−0.6) | 359.1010 (−8.1) [C15H19O10]− (20) 241.0026 (−0.8) [C6H9O8S]− (100) | Glucosyringic acid sulphate isomer | +++ | +++ | - | - | - | - | - |
| 16 | 5.8 | C13H16O16SO3 | 379.0348 (−2.1) | 299.0768 (+1.4) [C13H15O8]− (15) 241.0023 (−0.5) [C6H9O8S]− (100) | Salicylic acid glucoside | - | - | - | - | + | - | + |
| 17 | 5.9 | C18H24O13 | 447.1144 (−1.0) | 429.1041 (−0.6) [C18H21O12]− (60) 339.0722 (−0.1) [C15H15O9]− (30) 301.0568 (−1.1) [C12H13O9]− (20) | Aralidioside | ++ | ++ | - | - | - | - | - |
| 18 | 5.9 | C15H18O8SO3 | 405.0496 (+0.1) | 325.0926 (+0.8) [C15H17O8]− (10) 241.0025 (−0.4) [C6H9O8S]− (100) 163.0396 (+2.2) [C9H7O3]− (10) | Glucosyl coumaric acid sulphate | ++ | +++ | - | - | +++ | ++++ | ++++ |
| 19 | 6.2 | C15H18O9SO3 | 421.0451 (−1.1) | 341.0660 (−1.3) [C15H17O9]− (7) 241.0018 (+0.5) [C6H9O8S]− (100) 179.0344 (+3.4) [C9H7O4]− (40) | Glucosyl-caffeic acid sulphate | + | + | - | - | - | - | - |
| 20 | 6.4 | C20H20O14 | 483.0779 (+0.3) | 313.0671 (−0.1) [C13H15O10]− (20) 313.0564 (+0.3) [C13H13O9]− (60) 271.0461 (−0.5) [C11H11O8]− (100)169.0129 (+8.1) [C7H5O5]− (40) | Digalloyl glucose | + | ++ | ++ | ++ | - | - | - |
| 21 | 6.5 | C37H30O18 | 761.1356 (0.4) | 609.1249 (+0.1) [C30H25O14]− (20) 423.0721 (+0.0) [C22H15O9]− (100) 305.0667 (+0.0) [C15H13O7]− (70)169.0135 (−3.5) [C7H5O5]− (7) | Theasinensin B | - | +++ | - | - | - | - | - |
| 22 | 6.5 | C15H18O8SO3 | 405.0496 (−1.6) | 241.0025 (−0.4) [C6H9O8S]− (100) 163.0395 (+3.6) [C9H7O3]− (30) | Glucosyl coumaric acid sulphate isomer | ++ | - | ++ | +++ | - | - | - |
| 23 | 6.8 | C15H18O8(SO3)2 | 485.0061 (+0.7) | 405.0497 (+0.1) [C15H17O11S]− (40) 325.0928 (+0.2) [C15H17O8]− (5) 241.0024 (−0.1) [C6H9O8S]− (100) | Glucosyl coumaric acid disulphate isomer | +++ | ++++ | - | - | +++ | +++ | ++++ |
| 24 | 6.8 | C15H18O8SO3 | 405.0498 (−0.3) | 325.0930 (−0.5) (C15H17O8)− (10) 241.0023 (−1.8) [C6H9O8S]− (100) 163.0397 (2.5) [C9H7O3]− (10) | Glucosyl coumaric acid sulphate isomer | +++ | ++ | +++ | +++ | - | ++++ | ++++ |
| 25 | 7.0 | C37H28O18 | 759.1207 (−0.6) | 423.0724 (−0.6) [C22H15O9]− (100) 301.0354 (−0.2) [C15H9O7]− (80) | ProdelphinidinA2 3′-gallate | ++++ | +++ | - | - | - | - | - |
| 26 | 7.0 | C20H22O12 | 453.1040 (−0.3) | 313.0567 (+0.7) [C13H13O9]− (100) 169.0131 (+6.5) [C7H5O5]− (40) | Galloyl glucose derivative | - | - | +++ | +++ | - | - | - |
| 27 | 7.4 | C11H14O4SO3 | 289.0393 (−1.8) | 209.0826 (−3.1) [C11H13O4]− (60) 149.0599 (5.9) [C9H9O2]− (100) | Sinapyl alcohol sulphate | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 28 | 7.5 | C15H16O10SO3 | 435.0240 (−0.2) | 355.0671 (−0.1) [C15H15O10]− (20) 197.0444 (+6.0) [C9H9O5]− (100) | Caffeic acid-3-glucuronide sulphate | ++ | ++ | - | - | ++++ | +++ | ++++ |
| 29 | 7.7 | C17H30O10 | 393.1770 (−0.9) | 271.0610 (+0.8) [C15H11O5]− (20) 205.0709 (+4.3) [C8H13O6]− (100) 119.0337 (+7.8) [C4H7O4]− (80) | Cis-3-hexenyl-b-primeveroside | ++++ | ++++ | - | - | - | - | - |
| 30 | 7.8 | C28H24O17 | 631.0944 (−0.6) | 479.0830 (+0.2) [C21H19O13]− (90) 316.019 (−1.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-galloyl-hexoside | +++ | +++ | +++ | +++ | ++ | +++ | ++++ |
| 31 | 8.0 | C18H24O12 | 431.1192 (−0.2) | 285.0625 (−3.1) [C12H13O8]− (10) 225.0409 (−1.8) [C10H9O8]− (100) | Licoagroside B | + | +++ | - | - | - | - | - |
| 32 | 8.0 | C27H30O17 | 625.1418 (−1.2) | 316.0233 (−2.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-rutinoside | - | - | ++ | ++ | ++ | +++ | +++ |
| 33 | 8.2 | C21H20O13 | 479.0835 (+0.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) 271.0253 (−1.9) (C14H7O6)− (20) | Myricetin-3-O-glucoside | +++ | +++ | - | - | ++ | +++ | ++++ |
| 34 | 8.5 | C22H22O12 | 477.1036 (+0.4) | 433.1145 (−1.1) (C21H21O10)− (20) 313.0567 (−0.7) (C13H13O9)− (100) 169.0141 (+1.0) [C7H5O5]− (40) | Galloylhexoside derivative | ++ | ++ | ++ | +++ | - | - | - |
| 35 | 8.6 | C28H24O16 | 615.0985 (+0.1) | 463.0882 (+0.1) [C21H19O12]− (100) 301.0345 (+3.0) [Y0]− [C15H9O7]− (80) 300.0279 (−1.0) [Y0−H]−∙ [C15H8O7]−∙ (90) | Quercetin-3-O-galloyl-hexoside | - | - | - | + | + | + | + |
| 36 | 8.7 | C18H20O10 | 371.0981 (+0.6) | 249.0615 (+0.2) [C9H13O8]− (100) | Hydroxyferuloylglucose | - | - | - | - | - | +++ | +++ |
| 37 | 8.7 | C27H30O16 | 609.1456 (+0.8) | 463.0882 (−0.1) [C22H19O12]− (400) 300.0275 (−0.8) [Y0−H]−∙ [C15H8O7]−∙ (100) | Quercetin-3-O-rutinoside | - | - | - | - | ++ | ++ | +++ |
| 38 | 8.8 | C19H26O12 | 445.1350 (+0.2) | 285.0623 (−2.6) [C12H13O8]− (7) 225.0429 (−1.9) [C10H9O8]− (100) | Methyl licoagroside B | +++ | +++ | - | - | - | - | - |
| 39 | 8.8 | C14H14O9 SO3 | 405.0145 (−1.2) | 325.0564 (0.3) [C14H13O9]− (20) 209.0454 (+0.5) [C10H9O5]− (50) 167.0344 (+3.2) [C8H7O4]− (100) | Galloylshikimic acid sulphate | - | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 40 | 9.0 | C21H20O12 | 463.0872 (+2.2) | 316.020 (−1.8) [Y0−H]−∙ [C15H8O8]−∙ (100) 217.0250 (−0.8) [C14H7O6]− (20) | Myricetin-3-O-rhamnoside | - | ++++ | ++ | ++ | ++ | +++ | ++++ |
| 41 | 9.0 | C15H16O10SO3 | 435.0251 (−2.9) | 355.0677 (−1.7) [C15H15O10]− (10) 197.0455 (+5.5) [C9H9O5]− (100) | Caffeic acid glucuronic sulphate | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| 42 | 9.3 | C21H20O13 | 479.0835 (+0.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-glucoside | + | + | - | - | - | - | - |
| 43 | 9.3 | C20H24O8SO3 | 471.0967 (−0.5) | 275.0228 +(0.9) [C10H11O4SO3]− (100) 195.0651 (+6.1) [C10H11O4]− (50) | Acetosyringone sulphate | - | - | - | - | ++ | ++ | ++ |
| 44 | 9.3 | C28H24O15 | 599.1036 (+0.7) | 316.022 (−0.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-pentoside-gallate | - | - | - | - | - | - | + |
| 45 | 9.3 | C30H28O17 | 659.1244 (+1.5) | 316.019 (−1.7) [Y0−H]−∙ [C15H8O8]−∙ (100) 479.0811 (+4.2) [C21H19O13]− (20) | Myricetin-3-O-(3-caffeic acid-glucoside) | - | - | - | - | + | ++ | +++ |
| 46 | 9.4 | C23H22O14 | 521.0931 (−1.1) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin 3-O-(6-acetylgalactoside) | - | - | + | ++ | +++ | ||
| 47 | 9.4 | C29H26O16 | 629.1143 (+0.9) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | 2’-C-methyl-myricetin-3-O-rhamnoside-gallate | - | - | - | - | - | + | ++ |
| 48 | 9.4 | C22H26O8SO3 | 599.1042 (+0.8) | 447.0932 (+0.2) [C21H19O11]− (30) 313.0563 (+0.5) [C13H13O9]− (100) 169.0141 (+1.0) [C7H5O5]− (40) | Di-galloyl-hexose malic acid | - | - | + | + | - | - | - |
| 49 | 9.4 | C28H24O15 | 599.1045 (−0.4) | 447.0943 (−0.8) [C21H19O11]− (50) 285.0404 (0.1) [Y0−H]−∙ [C15H9O6]− (100) | Kaempferol-galloyl-hexoside | - | ++ | - | - | - | - | - |
| 50 | 9.5 | C9H10O5 | 197.0445 (+5.5) | 124.0166 (−0.4) [C6H4O3]− (100) | Syringic acid | +++ | - | - | - | - | - | - |
| 51 | 9.5 | C28H24O15 | 599.1037 (+0.9) | 447.0931 (+0.3) [C21H19O11]− (30) 316.022 (−0.8) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-pentoside-gallate isomer | - | - | - | - | ++ | ++ | ++ |
| 52 | 9.6 | C29H26O16 | 629.1143 (+0.9) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | 2′-C-methyl-myricetin-3-O-rhamnoside-gallate isomer | - | - | - | - | + | ++ | - |
| 53 | 9.8 | C13H14N2O3 | 203.0818 (+4.0) | 142.0648 (+9.2) [C10H8N]− (40) | Tryptophan | - | - | ++++ | ++ | - | - | - |
| 54 | 9.8 | C13H14N2O3 | 245.0932 (+0.8) | 203.0818 (+4.0) [C11H11N2O2]− (100) 142.0648 (+9.2) [C10H8N]− (40) | N-acetyl-tryptophan | ++++ | + | - | - | ++++ | +++ | +++ |
| 55 | 9.8 | C14H14N2O5 | 289.0830 (−0.1) | 203.0818 (+4.0) [C11H11N2O2]− (100) | N-manoyl-tryptophan | +++ | +++ | +++ | - | +++ | ++ | ++ |
| 56 | 9.8 | C24H22O5 | 549.0891 (−1.0) | 316.025 (−2.2) [Y0−H]−∙ [C15H8O8]−∙ (100) 217.0250 (−0.8) [C14H7O6]− (20) | Myricetin-3-O-acetyl--malonyl-deoxyhexose | - | - | - | + | - | - | - |
| 57 | 10.0 | C27H36O12 | 551.2134 (+0.0) | 357.1346 (−0.7) [C20H12O6]− (100) | Pinoresinol derivative | - | - | + | - | - | - | - |
| 58 | 10.1 | C21H22O10 | 433.1136 (+1.0) | 271.0610 (−0.1) [Y0]− [C15H11O5]− (100) | Naringenin-7-O-glucoside | ++ | ++ | - | - | - | - | - |
| 59 | 10.1 | C21H18O11 | 445.0775 (+0.3) | 269.0456 (−0.1) [Y0]− [C15H9O5]− (100) | Apigenin-7-O-glucuronide | ++++ | +++ | - | - | - | - | - |
| 60 | 10.2 | C23H22O13 | 505.0995 (−1.2) | 316.0230 (−0.9)[Y0−H]−∙ [C15H8O8]−, (100) 217.0253 (−1.9) [C14H7O6]− (15) | Myricetin-3-O-acetyl-deoxyhexose | - | - | - | - | +++ | +++ | ++++ |
| 61 | 10.5 | C28H26O14 | 585.1247 (+0.4) | 439.0880 (+0.5) [C19H19O13]− (100) 271.0608 (+1.2) [Y0]− [C15H11O5]− (10) | Prunin-6′’-O-gallate | +++ | +++ | - | - | |||
| 62 | 10.5 | C18H18O7SO3 | 425.0545 (+0.3) | 345.0980 (+0.0) [C18H17O7]− (40) 315.0872 (+0.5) [C17H15O6]− (60) 300.0638 (+0.6) [C16H12O6]−∙ (100) | 3′,4′,5′-Trimethoxyflavanone sulphate | - | - | - | +++ | +++ | +++ | |
| 63 | 10.6 | C22H26O8SO3 | 497.1125 (−0.3) | 417.1556 (−0.3) [C22H25O8]− (100) 181.0493 (+7.4) [C9H9O4]− (90) | Syringaresinol sulphate | ++ | +++ | ++ | ++ | +++ | +++ | ++++ |
| 64 | 10.7 | C17H24O7SO3 | 467.1019 (+1.1) | 387.1448 (+0.4) [C17H23O7]− (100) 372.1212 (+0.5) [C16H20O7]−∙ (90) 357.0978 (+0.4) [C19H17O7]− (40) 181.0494 (+7.0) [C9H9O4]− (50) 151.0309 (+6.8) [C8H7O3]− (40) | Medioresinol sulphate | - | - | - | - | ++ | +++ | +++ |
| 65 | 10.7 | C23H22O12 | 489.1042 (−0.8) | 300.0280 (−1.6) [Y0−H]−∙ [C15H8O7]−∙ (100) 271.0153 (−1.9) [C14H7O6]− (30) | Quercetin-3-O-acetyl-rhamnoside | + | ++ | - | - | - | - | - |
| 66 | 11.0 | C29H26O14 | 597.1245 (−0.5) | 413.0887 (−2.2) [C21H17O9]− (10) 301.03607 (−1.9) [C15H9O7]− (20) 269.0456 (−0.3) [Y0]− [C15H9O5]−∙ (100) | Apigenin derivative | + | ++ | - | - | - | - | - |
| 67 | 11.2 | C15H12O6 | 287.0563 (+0.6) | 151.0031 (+3.9) [1,3A−] [C7H3O4]− (20) 135.0435 (−0.8) [1,3B−] [C8H7O2]− (100) | 2-Hydroxynaringenin | ++ | ++ | - | - | - | - | - |
| 68 | 11.3 | C18H18O7SO3 | 425.0553 (+0.9) | - | 3′,4′,5′-Trimethoxyflavanone sulphate isomer | - | - | - | - | ++ | ++ | ++ |
| 69 | 11.5 | C22H26O8SO3 | 497.1135 (−2.3) | 417.1164 (−2.1) [C22H25O8]− (50) 402.1326 (−1.5) [C21H22O8]− (90) 387.1093 (−2.0) [C20H19O8]− (60) 181.0500 (+3.4) [C9H9O4]− (100) | Syringaresinol sulphate isomer | - | - | - | - | + | ++ | ++ |
| 70 | 11.5 | C20H22O6SO3 | 437.0904 (+1.0) | 357.1339 (+1.4) [C20H12O6]− (100) 342.1104 (+1.3) [C19H18O6]− (85) 151.0391 (+6.3) [C8H7O3] (70) | Pinoresinol sulphate | ++++ | +++ | +++ | +++ | ++++ | ++++ | ++++ |
| 71 | 12.1 | C20H22O6SO3 | 437.0916 (+1.8) | 357.1339 (+1.4) [C20H12O6]− (100) | Pinoresinol sulphate isomer | ++ | + | - | - | +++ | +++ | +++ |
| 72 | 12.2 | C15H20O6 | 285.0403 (+0.6) | 199.0392 (+4.2) [C12H7O3]− (50) 175.0390 (+6.1) [C10H7O3]− (60) 151.0029 (+4.9) [1,3A−] [C7H3O4]− (40) 133.0279 (−8.3) [1,3B−] [C8H5O2]− (100) | Luteolin | ++ | ++ | - | - | - | - | - |
| 73 | 12.2 | C25H24O14 | 547.1102 (−1.6) | 316.023 (−0.9) [Y0−H]−∙ [C15H8O8]−∙ (100) | Myricetin-3-O-diacetylrhamnoside | + | + | - | + | +++ | ++ | ++++ |
| 74 | 12.3 | C15H12O6 | 287.0565 (−1.2) | 269.0458 (−1.0) [C15H9O5]− (30) 259.0614 (+0.6) [C14H11O5]− (30) 177.0546 (+6.6) [C10H9O3]− (100) 151.0031(+3.8) [1,3A−] [C7H3O4]− (40) | Dihydrokaempferol | ++ | +++ | - | - | - | - | - |
| 75 | 13.3 | C18H22O5 | 327.2175 (−0.6) | - | Trihydroxy-10,15-octadecadienoic acid | +++ | +++ | +++ | +++ | ++ | ++ | ++ |
| 76 | 13.5 | C15H10O5 | 269.0457 (−0.6) | 227.0347 (−1.1) [C13H7O4]− (60) 151.0030 (+2.5) [1,3A−] [C7H3O4]− (70) 117.0324 (+9.1) [1,3B−] [C8H7O]− (100) | Apigenin | +++ | + | - | - | - | - | - |
| 77 | 13.7 | C15H12O5 | 271.0615 (−1.3) | 187.0393 (+4.3) [C11H7O3]− (40) 151.0030 (+3.4) [1,3A−] [C7H3O4]− (50) 119.0490 (+9.9) [1,3B−] [C8H7O]− (100) | Naringenin | +++ | ++++ | - | - | - | - | - |
| 78 | 14.1 | C18H34O5 | 329.2332 (−0.5) | - | Trihydroxy-10-octadecenoic acid | +++ | ++++ | ++ | ++ | ++ | ++ | ++ |
| 79 | 14.3 | C13H24O3SO3 | 307.1220(−0.2) | - | Oxo-tridecanoic acid sulphate | + | - | ++++ | ++ | ++ | +++ | +++ |
| 80 | 14.9 | C18H12O4 | 287.2227 (+0.4) | - | 10, 16-Dihydroxyhexadecanoic acid | + | - | - | - | +++ | ++ | ++ |
3.2. In Vitro Antioxidant Activity
3.3. Ex Vivo Antioxidant Activity
3.4. Tyrosinase Inhibition
3.5. In Vitro Anti-Inflammatory Properties
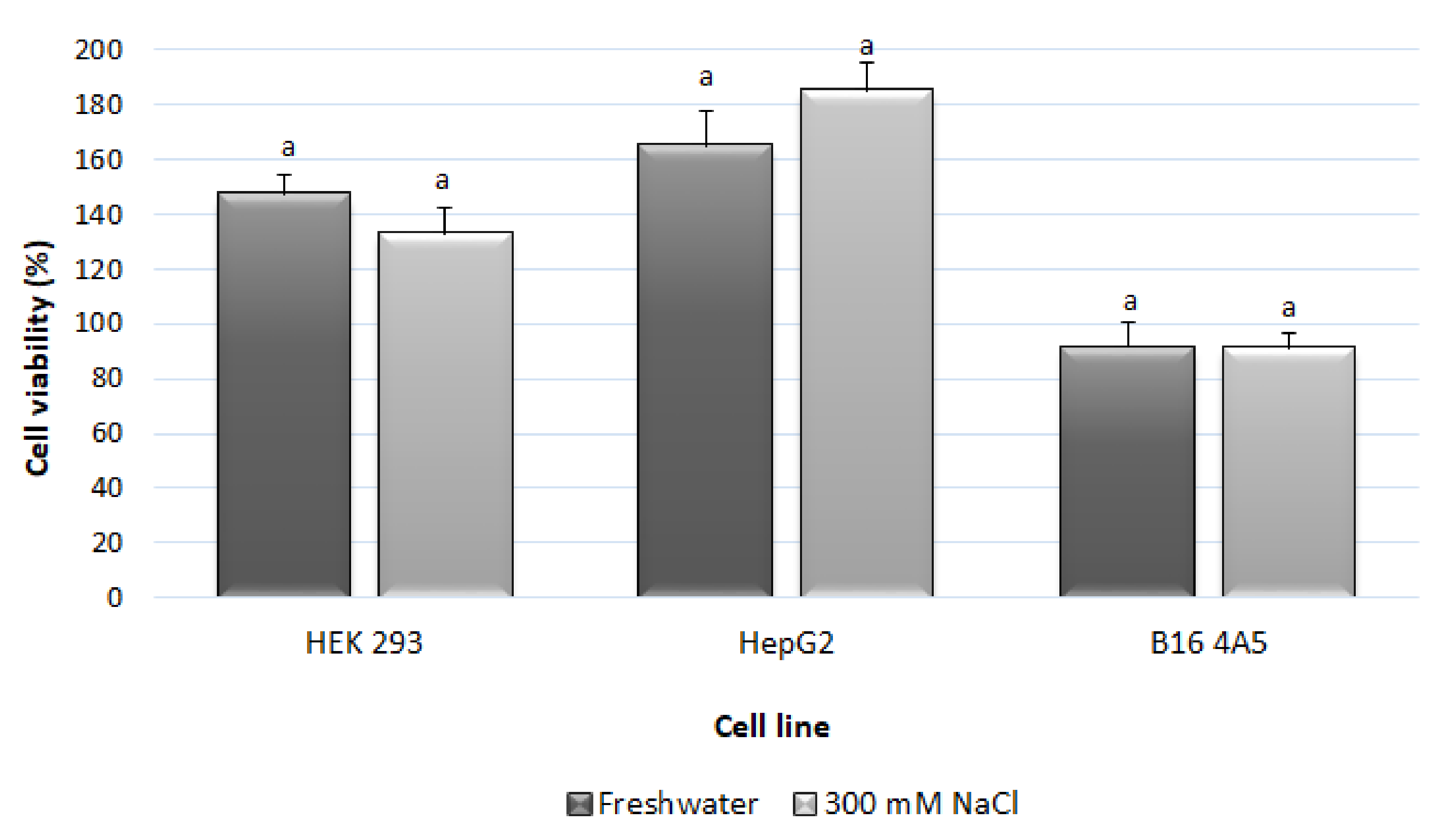
3.6. Toxicological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charen, E.; Harbord, N. Toxicity of Herbs, Vitamins, and Supplements. Adv. Chronic Kidney Dis. 2020, 27, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Herbal Supplements Market Size, Share & Trends Analysis Report by Product (Turmeric, Echinacea), by Formulation (Capsules, Powder and Granules), by Consumer (Pregnant Women, Adults), and Segment Forecasts, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/herbal-supplements-market (accessed on 30 October 2021).
- Persistance Market Research. Botanical Supplements Market. Global Market Study on Botanical Supplements: Drugs Application Segment to Hold Maximum Value Share During 2017–2025. Available online: https://www.persistencemarketresearch.com/market-research/botanical-supplements-market.asp (accessed on 30 October 2021).
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. IJPSR 2013, 4, 2496. [Google Scholar]
- Arnold, E.; Lofthouse, N.; Hurt, E. Artificial Food Colors and Attention-Deficit/Hyperactivity Symptoms: Conclusions to dye for. Neurotherapeutics 2012, 9, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tükoğlu, S. Evaluation of genotoxic effects of five flavour enhancers (glutamates) on the root meristem cells of Allium cepa. Toxicol. Ind. Health 2015, 31, 792. [Google Scholar] [CrossRef] [PubMed]
- Minioti, K.S.; Sakellariou, C.F.; Thomaidis, N.T. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase high-performance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta 2007, 583, 103. [Google Scholar] [CrossRef]
- Kanarek, R.B. Artificial food dyes and attention deficit hyperactivity disorder. Nutr. Rev. 2011, 69, 385–391. [Google Scholar] [CrossRef]
- Bearth, A.; Cousin, M.-E.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Pref. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Huang, H.-W. Current status and future trends of high-pressure processing in food industry. Food Control. 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Scaglioni, P.S.; Badiale-Furlong, E. Can Microalgae Act as Source of Preservatives in Food Chain? J. Food. Sci. Eng. 2017, 7, 283–296. [Google Scholar]
- Che, C.-T.; Zhang, H. Plant Natural Products for Human Health. Int. J. Mol. Sci. 2019, 20, 830. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef] [Green Version]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Neng, N.R.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 27536. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Ladeiro, B. Saline agriculture in the 21st Century: Using salt contaminated resources to cope food requirements. J. Bot. 2012, 2012, 310705. [Google Scholar] [CrossRef]
- Holguin Peña, R.J.; Medina-Hernández, D.; Ghasemi, M.; Rueda Puente, E.O. Salt tolerant plants as a valuable resource for sustainable food production in arid and saline coastal zones. Acta Biol. Colomb. 2021, 26, 116–126. [Google Scholar] [CrossRef]
- Custódio, M.; Villasante, S.; Cremades, J.; Calado, R.; Lillebø, A.I. Unravelling the potential of halophytes for marine integrated multi-trophic aquaculture (IMTA)—A perspective on performance, opportunities and challenges. Aquac. Environ. Interact. 2017, 9, 445–460. [Google Scholar] [CrossRef] [Green Version]
- Jerónimo, D.; Lillebø, A.I.; Maciel, E.; Domingues, M.R.M.; Cremades, J.; Calado, R. Unravelling the fatty acid profiles of different polychaete species cultured under integrated multi-trophic aquaculture (IMTA). Sci. Rep. 2021, 11, 10812. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Soszynski, A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crop. Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Oliveira, M.; Neves, V.; Ovelheiro, A.; Pereira, C.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Coupling Sea lavender (Limonium algarvense Erben) and green tea (Camellia sinensis (L.) Kuntze) to produce an innovative herbal beverage with enhanced enzymatic inhibitory properties. S. Afr. J. Bot. 2019, 120, 87–94. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Monteiro, I.; Castañeda-Loaiza, V.; Placines, C.; Oliveira, M.C.; Caperta, A.D.; Pousão-Ferreira, P.; Pereira, C.; Custódio, L. Growth performance, in vitro antioxidant properties and chemical composition of the halophyte Limonium algarvense Erben are strongly influenced by the salinity irrigation. Ind. Crop. Prod. 2020, 143, 111930. [Google Scholar] [CrossRef]
- Silva de Sá, I.; Peron, A.P.; Leimann, F.V.; Bressan, G.N.; Krum, B.N.; Fachinetto, R.; Pinela, J.; Calhelha, R.C.; Barreiro, M.F.; Ferreira, I.C.F.R.; et al. In vitro and in vivo evaluation of enzymatic and antioxidant activity, cytotoxicity and genotoxicity of curcumin-loaded solid dispersions. Food Chem. Toxicol. 2019, 125, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, R.C.G.; de Souza, A.H.P.; Calhelha, R.C.; Barros, L.; Glamoclija, J.; Sokovic, M.; Peralta, R.M.; Bracht, A.; Ferreira, I.C.F.R. Bioactive formulations prepared from fruiting bodies and submerged culture mycelia of the Brazilian edible mushroom Pleurotus ostreatoroseus Singer. Food Funct. 2015, 6, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.J.; Pereira, C.; Oliveira, M.; Neng, N.R.; Nogueira, J.M.F.; Zengin, G.; Mahomoodally, M.F.; Custódio, L. Sea rose (Armeria pungens (Link) Hoffmanns. & Link) as a potential source of innovative industrial products for anti-ageing applications. Ind. Crop. Prod. 2018, 121, 250–257. [Google Scholar]
- Rodrigues, M.J.; Matkowski, A.; Slusarczyk, S.; Magné, C.; Poleze, T.; Pereira, C.; Custódio, L. Sea knotgrass (Polygonum maritimum L.) as a potential source of innovative industrial products for skincare applications. Ind. Crop. Prod. 2019, 128, 391–398. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petigny, L.; Ozel, M.Z.; Périno, S.; Wajsman, J.; Chemat, F. Water as green solvent for extraction of natural products. In Green Extraction of Natural Products; Chemat, F., Strube, J., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; pp. 237–264. [Google Scholar]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; Souza, M.D.F.V.D. Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barron, D.; Varin, L.; Ibrahim, R.K.; Harborne, J.B.; Williams, C.A. Sulphated Flavonoids—An update. Phytochem 1988, 27, 2375–2395. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef] [Green Version]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Avaz, S. Afyonkarahisar’da Doğal Olarak Yetişen Limonium Mill. Türlerinin Antimikrobiyal Aktiviteleri. Master’s Thesis, Afyon Kocatepe University, Afyonkarahisar, Turkey, 2010. [Google Scholar]
- Ruiz-Riaguas, A.; Zengin, G.; Sinan, K.I.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Phenolic profile, antioxidant activity, and enzyme inhibitory properties of Limonium delicatulum (Girard) Kuntze and Limonium quesadense Erben. J. Chem. 2020, 2020, 1016208. [Google Scholar] [CrossRef] [Green Version]
- Geng, D.; Chi, X.; Dong, Q.; Hu, F. Antioxidants screening in Limonium aureum by optimized on-line HPLC–DPPH assay. Ind. Crop. Prod. 2015, 67, 492–497. [Google Scholar] [CrossRef]
- Gadetskaya, A.V.; Tarawneh, A.H.; Zhusupova, G.E.; Gemejiyeva, N.G.; Cantrell, C.L.; Cutler, S.J.; Ross, S.A. Sulfated phenolic compounds from Limonium caspium: Isolation, structural elucidation, and biological evaluation. Fitoterapia 2015, 104, 80–85. [Google Scholar] [CrossRef] [Green Version]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on Salt Tolerance of Two Endemic Limonium Species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Miguel-Chávez, R.S. Phenolic antioxidant capacity: A review of the state of the art. In Phenolic Compounds—Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.R., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Slama, I.; M’Rabet, R.; Ksouri, R.; Talbi, O.; Debez, A.; Abdelly, C. Water deficit stress applied only or combined with salinity affects physiological parameters and antioxidant capacity in Sesuvium portulacastrum. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 213, 69–76. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Haz-Map: Occupational Exposure to Hazardous Agents; National Library of Medicine: Bethesda, US, 2010. Available online: http://hazmap.nlm.nih.gov (accessed on 11 December 2021).
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In International Agency for Research on Cancer; WHO: Paris, France, 1986.
- Study on Enhancing the Endocrine Disrupter Priority List with a Focus on Low Production Volume Chemicals, Revised Report to DG Environment; DHI Water and Environment: Hersholm, Denmark, 2007; Available online: http://ec.europa.eu/environment/endocrine/documents/final_report_2007.pdf (accessed on 11 December 2021).
- UNEP and OECD, 2,6-di-tert-butyl-p-cresol (BHT) Screening Information Data Set: Initial Assessment Report. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=6d30349e-ef9f-496c-a2af-6d497d4f1cca (accessed on 11 December 2021).
- Permal, R.; Chang, W.L.; Chen, T.; Seale, B.; Hamid, N.; Kam, R. Optimising the spray drying of avocado wastewater and use of the powder as a food preservative for preventing lipid peroxidation. Foods 2020, 9, 1187. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Jiménez, A.M.; Ruggieri, S.; Frega, N.; Strabbioli, R.; Murcia, M.A. Antioxidant properties of mediterranean spices compared with common food additives. J. Food Prot. 2001, 64, 1412–1419. [Google Scholar] [CrossRef]
- Takebayashi, J.; Chen, J.; Tai, A. A Method for evaluation of antioxidant activity based on inhibition of free radical-induced erythrocyte hemolysis. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: London, UK, 2009; pp. 287–296. [Google Scholar]
- Takebayashi, J.; Iwahashi, N.; Ishimi, Y.; Tai, A. Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA). Food Chem. 2012, 134, 606–610. [Google Scholar] [CrossRef]
- Hakozaki, T.; Swanson, C.L.; Bissett, D.L. Hyperpigmentaion in aging skin. In Textbook of Aging Skin; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 495–501. [Google Scholar]
- Cestari, T.F.; Dantas, L.P.; Boza, J.C. Acquired hyperpigmentations. An. Bras. Dermatol. 2014, 89, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Nouveau, S.; Agrawal, D.; Kohli, M.; Bernerd, F.; Misra, N.; Nayak, C.S. Skin hyperpigmentation in Indian population: Insights and best practice. Indian J. Dermatol. 2016, 61, 487–495. [Google Scholar]
- Dorga, S.; Sarangal, R. Pigmentary disorders: An insight. Pigment Int. 2014, 1, 5–7. [Google Scholar]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [Green Version]
- Kusumawati, I.G.A.W.; Putra, I.M.W.A.; Yogeswara, I.B.A. In vitro ACE inhibitory activity and bioactive-compounds of aqueous extract of Citrus amblycarpa. Trad. Med. J. 2021, 26, 118–122. [Google Scholar]
- Bakhouche, I.; Toufik, A.; Tahar, B.; Lynda, G.; Aysen, S.; Yuva, B. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and antiinflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. S. Afr. J. Bot. 2021, 139, 42–49. [Google Scholar] [CrossRef]
- Baysal, I.; Ekizoglu, M.; Ertas, A.; Temiz, B.; Agalar, H.G.; Yabanoglu-Ciftci, S.; Temel, H.; Ucar, G.; Turkmenoglu, F.P. Identification of Phenolic Compounds by LC-MS/MS and Evaluation of Bioactive Properties of Two Edible Halophytes: Limonium effusum and L. sinuatum. Molecules 2021, 26, 4040. [Google Scholar] [CrossRef]
- Obaida, R.J.; Mughal, E.U.; Naeemb, N.; Sadiqc, A.; Alsantalid, R.I.; Jassase, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaston, T.E.; Mendrick, D.L.; Paine, M.F.; Roe, A.L.; Yeung, C.K. Natural” is not synonymous with “Safe”: Toxicity of natural products alone and in combination with pharmaceutical agents. Regul. Toxicol. Pharmacol. 2020, 113, 104642. [Google Scholar] [CrossRef] [PubMed]
| Irrigation Salinity/Treatment | Plant Organ | DPPH | ABTS | CCA | FRAP |
|---|---|---|---|---|---|
| Freshwater | Flower | 604 ± 4 d | 397 ± 2 b | 642 ± 21 b | 129 ± 6 a |
| Peduncles | 532 ± 17 c | 800 ± 13 e | 922 ± 31 d | 251 ± 22 c | |
| Leaves | 549 ± 7 c | 793 ± 11 e | 953 ± 27 d | 339 ± 14 d | |
| 300 mM NaCl | Flower | 692 ± 11 f | 617 ± 14 c | 720 ± 8 c | 191 ± 17 b |
| Peduncles | 383 ± 7 b | 745 ± 9 d | - | 228 ± 10 c | |
| Leaves | - | - | - | 351 ± 11 d | |
| 600 mM NaCl | Leaves | - | - | - | 251 ± 7 c |
| Positive control * | 111 ± 9 a | 142 ± 11 a | 171 ± 9 a | na |
| Biological Activity | Method | Freshwater | 300 mM | Positive Control * |
|---|---|---|---|---|
| Antioxidant | TBARS | 127 ± 45 c | 81 ± 28 b | 9.1 ± 0.3 a |
| OxHLIA (Δt = 60 min) | 136 ± 4 b | 140 ± 4 b | 21 ± 1 a | |
| Anti-inflammatory | NO reduction | - | - | 16 ± 1 |
| Anti-melanogenic/anti-browning | Tyrosinase inhibition | - | 873 ± 59 b | 137 ± 6 a |
| Anti-melanogenic | Inhibition of melanin synthesis by B16 4A5 cells | - | - | 16 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.J.; Castañeda-Loaiza, V.; Monteiro, I.; Pinela, J.; Barros, L.; Abreu, R.M.V.; Oliveira, M.C.; Reis, C.; Soares, F.; Pousão-Ferreira, P.; et al. Metabolomic Profile and Biological Properties of Sea Lavender (Limonium algarvense Erben) Plants Cultivated with Aquaculture Wastewaters: Implications for Its Use in Herbal Formulations and Food Additives. Foods 2021, 10, 3104. https://doi.org/10.3390/foods10123104
Rodrigues MJ, Castañeda-Loaiza V, Monteiro I, Pinela J, Barros L, Abreu RMV, Oliveira MC, Reis C, Soares F, Pousão-Ferreira P, et al. Metabolomic Profile and Biological Properties of Sea Lavender (Limonium algarvense Erben) Plants Cultivated with Aquaculture Wastewaters: Implications for Its Use in Herbal Formulations and Food Additives. Foods. 2021; 10(12):3104. https://doi.org/10.3390/foods10123104
Chicago/Turabian StyleRodrigues, Maria João, Viana Castañeda-Loaiza, Ivo Monteiro, José Pinela, Lillian Barros, Rui M. V. Abreu, Maria Conceição Oliveira, Catarina Reis, Florbela Soares, Pedro Pousão-Ferreira, and et al. 2021. "Metabolomic Profile and Biological Properties of Sea Lavender (Limonium algarvense Erben) Plants Cultivated with Aquaculture Wastewaters: Implications for Its Use in Herbal Formulations and Food Additives" Foods 10, no. 12: 3104. https://doi.org/10.3390/foods10123104
APA StyleRodrigues, M. J., Castañeda-Loaiza, V., Monteiro, I., Pinela, J., Barros, L., Abreu, R. M. V., Oliveira, M. C., Reis, C., Soares, F., Pousão-Ferreira, P., Pereira, C. G., & Custódio, L. (2021). Metabolomic Profile and Biological Properties of Sea Lavender (Limonium algarvense Erben) Plants Cultivated with Aquaculture Wastewaters: Implications for Its Use in Herbal Formulations and Food Additives. Foods, 10(12), 3104. https://doi.org/10.3390/foods10123104












