The Influence of the Type of Dry-Cured Italian PDO Ham on Cathepsin B Activity Trend during Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thigh Origin
2.2. Plan of Distribution and Ripening of the Thighs
- -
- T0, slaughter
- -
- T1, out of salting (salting)
- -
- T2, introduction to the resting room (resting)
- -
- T3, after washing and drying (drying)
- -
- T4, mid-curing, after greasing (greasing)
- -
- T5, end of curing (curing)
- -
- out of salting
- -
- pre- and post-trimming
- -
- post-washing and drying
- -
- pre- and post-sugnatura
- -
- pre- and post-‘stuccatura’ (only for SD, a second greasing using ‘stucco’, a sugna mixture richer in flour)
- -
- end of curing.
2.3. Collection of the Sample for Analysis
- -
- Moisture content [24];
- -
- Sodium chloride content, using the Volhard method [24];
- -
- aw (water activity; AquaLab Dew Point Water Activity Meter 4TE, Decagon Devices, Inc., Pullman, WA, USA);
- -
- Proteolysis index: the ratio between the Non Protein Nitrogen (NPN) and Total Nitrogen (TN); i.e., NPN/TN × 100. The proteins were precipitated using 20% (w/v) trichloroacetic acid and NPN and the TN contents were determined according to the Kjeldahl method [24].
2.4. Extraction of Cathepsin B from Biceps Femoris
2.5. Quantification of Enzymatic Activity
2.6. Statistical Analyses
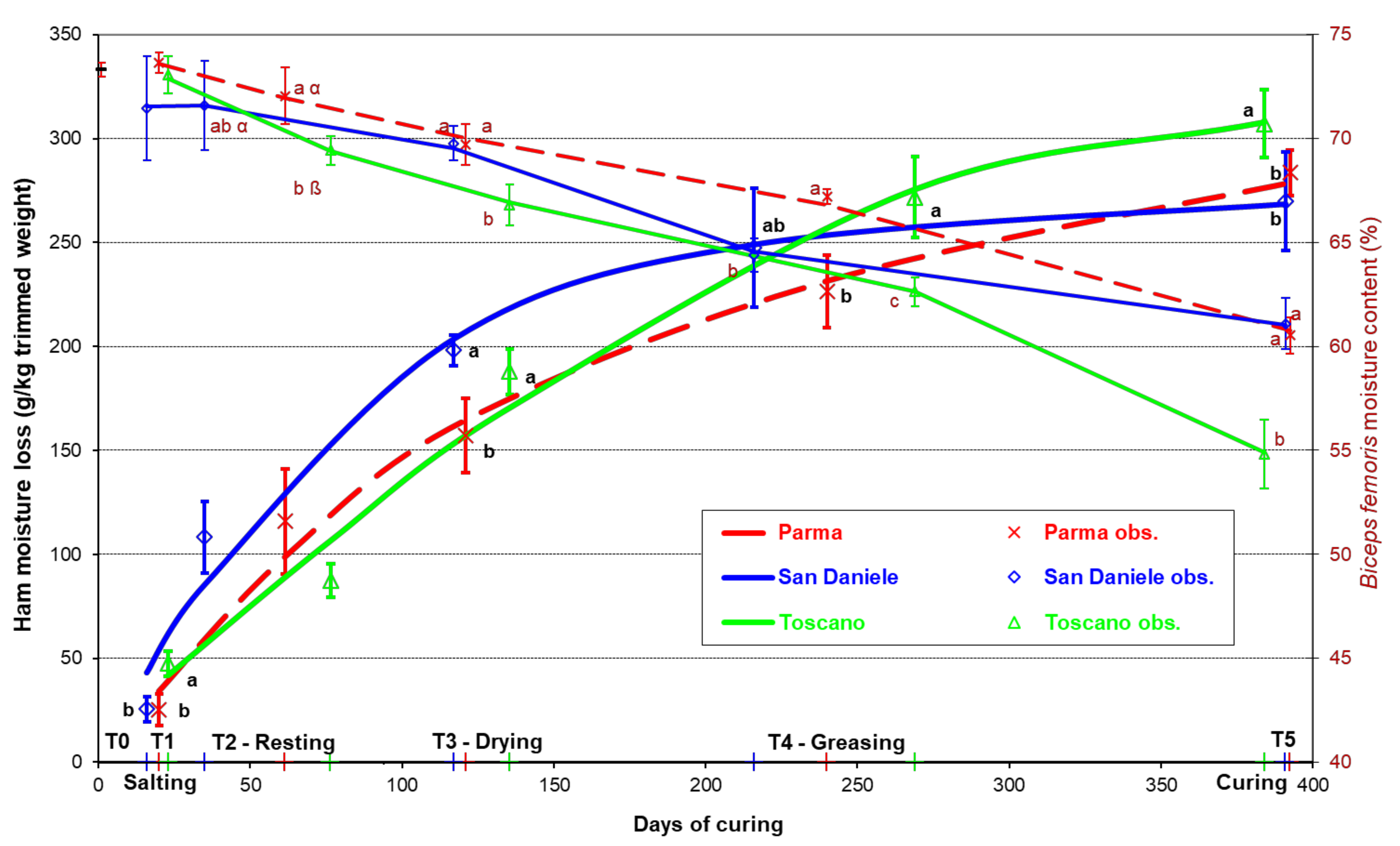
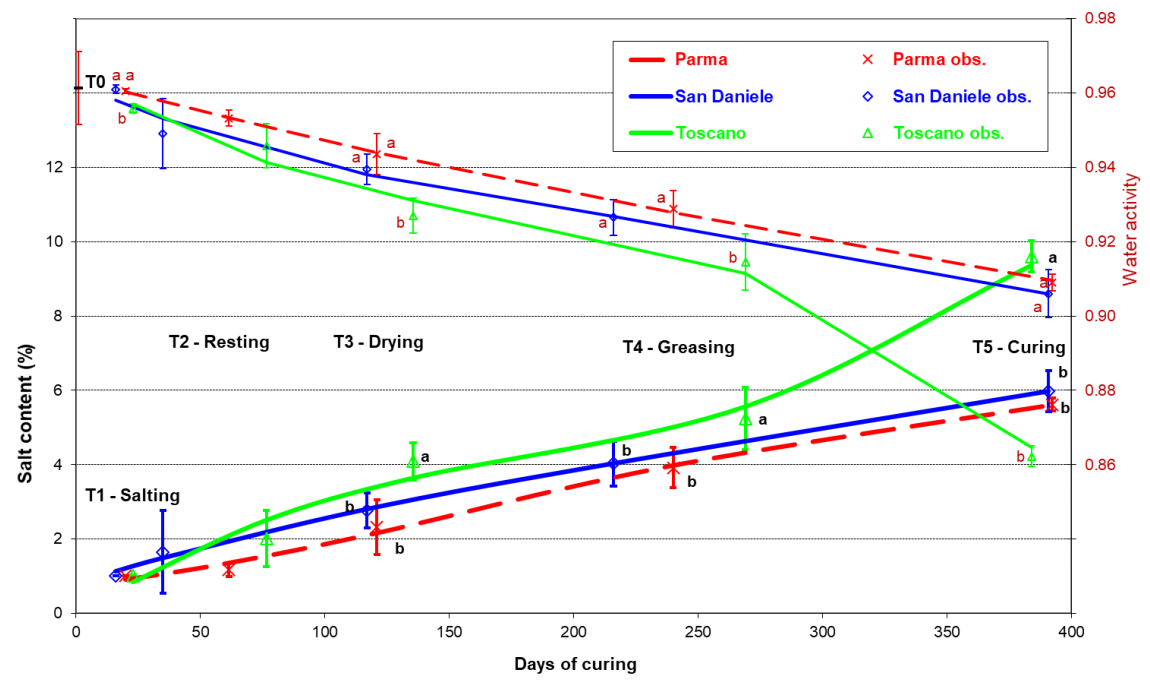
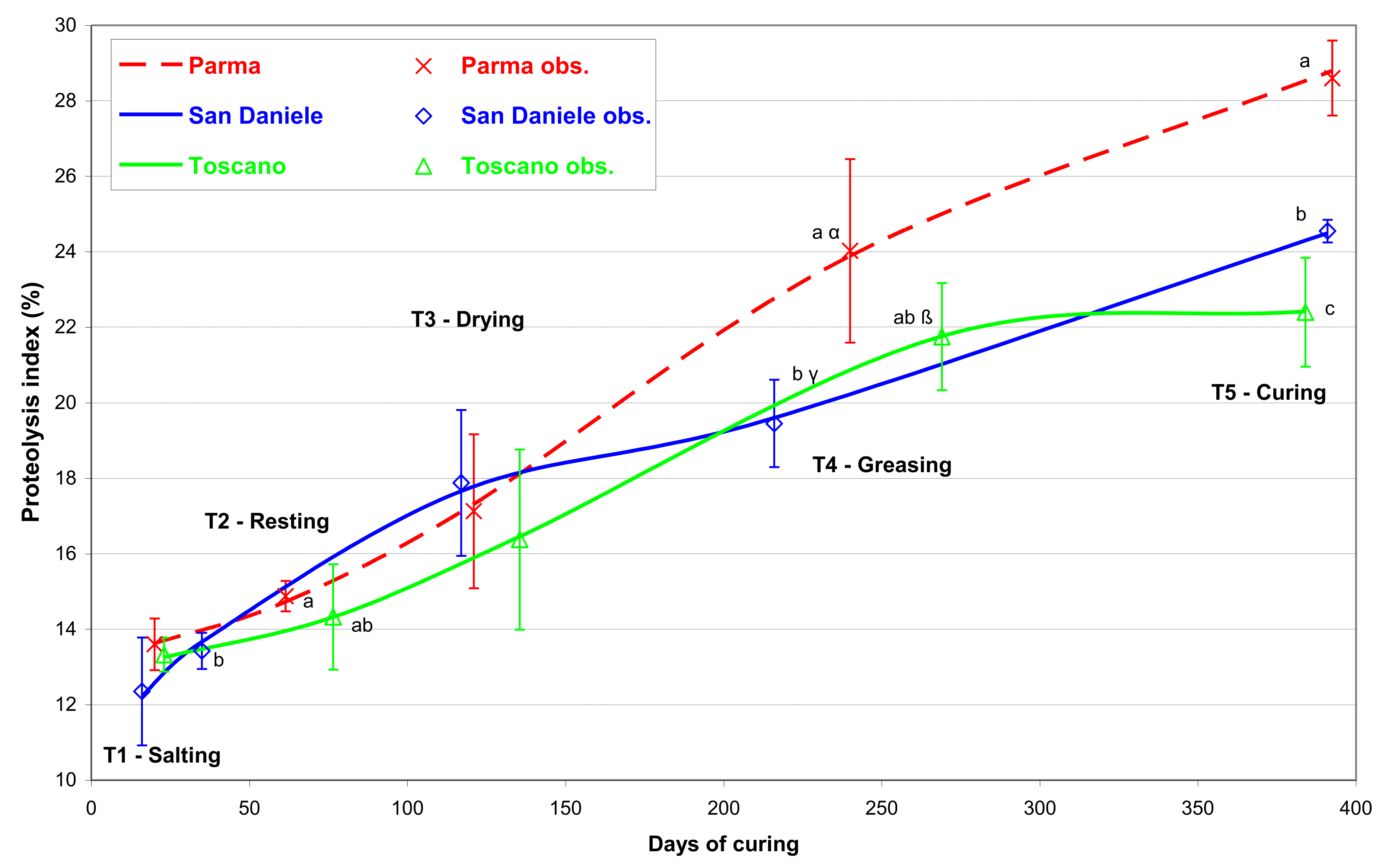
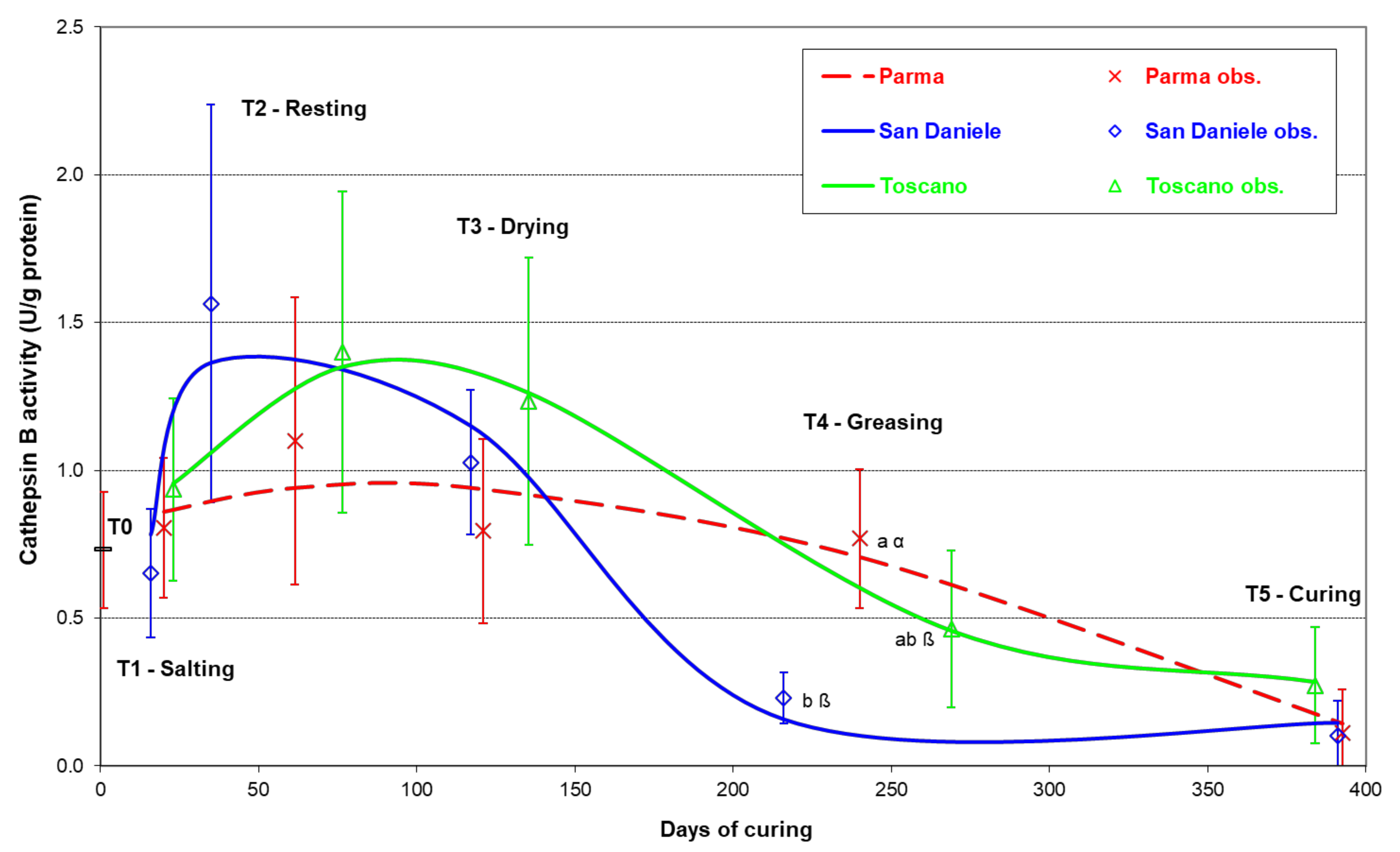
3. Results
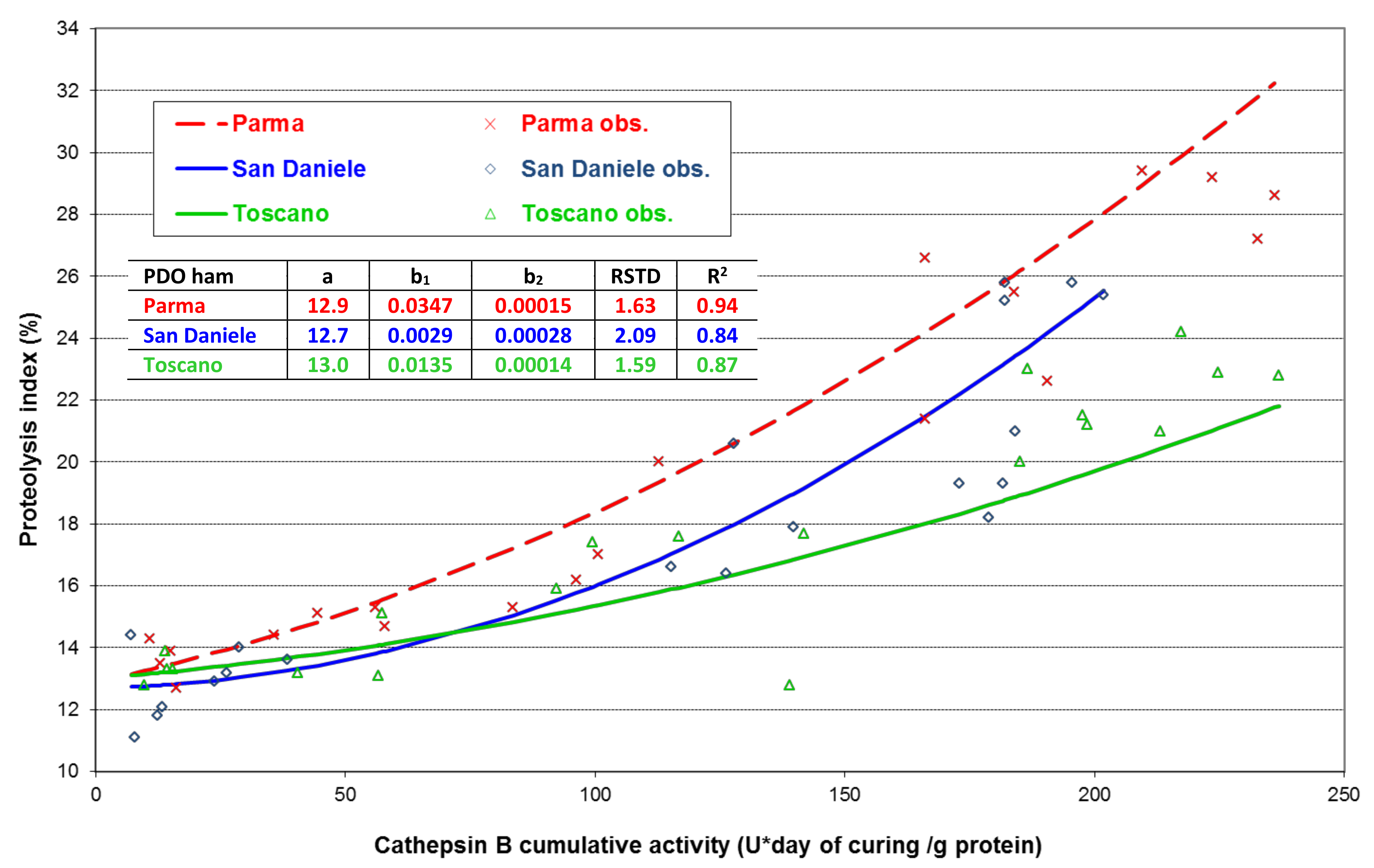
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- RIFT-Registro Italiano Filiera Tutelata. Available online: https://www.csqa.it/CSQA/Download/PROSCIUTTO-DI-PARMA-DOP (accessed on 26 August 2021).
- Toldrá, F. The role of muscle enzymes in dry-cured meat products with different drying conditions. Trends Food. Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Toldrá, F. Manufacturing of dry-cured ham. In Dry-Cured Meat Products; Food & Nutrition Press: Trumbull, CT, USA, 2002; pp. 27–62. [Google Scholar]
- Pugliese, C.; Sirtori, F.; Skrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Candek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, Y.; Wang, G.; Zhu, R.; Ge, C.; Liao, G. Changes in the physicochemical properties and volatile flavor compounds of dry-cured Chinese Laowo ham during processing. J. Food Process. Preserv. 2020, 44, e14593. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M.; Sanz, Y. Dry-cured ham flavour: Enzymatic generation ad process influence. Food Chem. 1997, 59, 523–530. [Google Scholar] [CrossRef]
- Goll, D.E.; Neti, G.; Mares, S.W.; Thompson, V.F. Myofibrillar protein turnover: The proteasome and the calpains. J. Anim. Sci. 2008, 86, E19–E35. [Google Scholar] [CrossRef]
- Söderstroöm, M.; Salminen, H.; Glumoff, V.; Kirschke, H.; Aro, H.; Vuorio, E. Cathepsin expression during skeletal development. Biochim. Biophys. Acta 1999, 1446, 35–46. [Google Scholar] [CrossRef]
- Turk, B.; Turk, D.; Turk, V. Lysosomal cysteine proteases: More than scavengers. Biochim. Biophys. Acta 2000, 1477, 98–111. [Google Scholar] [CrossRef]
- Bechet, D.; Tassa, A.; Taillandier, D.; Combaret, L.; Attaix, D. Lysosomal proteolysis in skeletal muscle. Int. J. Biochem. Cell Biol. 2005, 37, 2098–2114. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Ramoni, R.; Parolari, G.; Virgili, R.; Grolli, S.; Accornero, P.; Fermi, P.; Biffi, R.; Bignetti, E. Evalution of cathepsin B levels in fresh thighs selected for cured raw ham product. Meat Sci. 1997, 46, 401–414. [Google Scholar] [CrossRef]
- Riccio, M.; Di Giaimo, R.; Pianetti, S.; Palmieri, P.; Melli, M.; Santi, S. Nuclear Localization of Cystatin B, the Cathepsin Inhibitor Implicated in Myoclonus Epilepsy (EPM1). Exp. Cell Res. 2001, 262, 84–94. [Google Scholar] [CrossRef]
- Larrea, V.; Hernando, I.; Quiles, A.; Lluch, M.A.; Pérez-Munuera, I. Changes in proteins during Teruel dry-cured ham processing. Meat Sci. 2006, 74, 586–593. [Google Scholar] [CrossRef]
- Baron, P.C.; Jacobsen, S.; Purslow, P. Cleavage of desmin by cysteine proteases: Calpains and cathepsin B. Meat Sci. 2004, 68, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Schivazappa, C.; Degni, M.; Nanni Costa, L.; Russo, V.; Buttazzoni, L.; Virgili, R. Analysis of raw meat to predict proteolysis in PA ham. Meat Sci. 2002, 60, 77–83. [Google Scholar] [CrossRef]
- Škrlep, M.; Čandek-Potokar, M.; Mandelc, S.; Javornik, B.; Gou, P.; Chambon, C.; Santé-Lhoutellier, V. Proteomic profile of dry-cured ham relative to PRKAG3 or CAST genotype, level of salt and pastiness. Meat Sci. 2011, 88, 657–667. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Pan, D.D.; Cao, J.X.; Zhou, G.H. A comprehensive review on molecular mechanism of defective dry-cured ham with excessive pastiness, adhesiveness, and bitterness by proteomics insights. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3838–3857. [Google Scholar] [CrossRef]
- Zhao, G.M.; Zhou, G.H.; Wang, Y.L.; Xu, X.L.; Huan, Y.J.; Wu, J.Q. Time-related changes in cathepsin B and L activities during processing of Jinhua ham as a function of pH, salt and temperature. Meat Sci. 2005, 70, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Parolari, G.; Virgili, R.; Schivazappa, C. Relationship between cathepsin B activity and compositional parameters in dry-cured hams of normal and defective texture. Meat Sci. 1994, 38, 117–122. [Google Scholar] [CrossRef]
- García-Garrido, J.A.; Quiles-Zafra, R.; Tapiador, J.; Luque de Castro, M.D. Activity of cathepsin B, D, H and L in Spanish dry-cured ham of normal and defective texture. Meat Sci. 2000, 56, 1–6. [Google Scholar] [CrossRef]
- Laureati, M.; Buratti, S.; Giovanelli, G.; Corazzin, M.; Lo Fiego, D.P.; Pagliarini, E. Characterization and differentiation of Italian Parma, San Daniele and Toscano dry-cured hams: A multi-disciplinary approach. Meat Sci. 2014, 96, 288–294. [Google Scholar] [CrossRef]
- Pagliarini, E.; Laureati, M.; Dinnella, C.; Monteleone, E.; Proserpio, C.; Piasentier, E. Influence of pig genetic type on sensory properties and consumer acceptance of Parma, San Daniele and Toscano dry-cured hams. J. Sci. Food Agric. 2016, 96, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, A.; Bencivenni, M.; Piasentier, E.; Sforza, S.; Stecchini, M.L.; Lippe, G. Proteolytic resistance of actin but not of myosin heavy chain during processing of Italian PDO (protected designation of origin) dry-cured hams. Eur. Food Res. Technol. 2016, 242, 881–889. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, of AOAC International, 20th ed.; 39.1.02, 39.1.10, 39.1.15; AOAC International: Gaithersburg, MD, USA, 2016; Volume II. [Google Scholar]
- Sturaro, E.; Gallo, L.; Noventa, M.; Carnier, P. The genetic relationship between enzymatic activity of cathepsin B and firmness of dry-cured hams. Meat Sci. 2008, 79, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Flores, M. The use of muscle enzymes as predictors of pork meat quality. Food Chem. 2000, 69, 387–395. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Armero, E.; Barbosa, J.; Toldrá, F.; Baselga, M.; Pla, M. Effects of the terminal sire type and sex on pork muscle cathepsins (B, B+L and H), cysteine proteinase inhibitors and lipolytic enzyme. Meat Sci. 1999, 51, 185–189. [Google Scholar] [CrossRef]
- Renaville, B.; Piasentier, E.; Fan, B.; Vitale, M.; Prandi, A.; Rothschild, M.F. Candidate gene markers involved in San Daniele ham quality. Meat Sci. 2010, 85, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Corazzin, M.; Favotto, S.; Saccà, E.; Piasentier, E. Variability in the characteristics of fresh meat and thighs in relationship to genetic type of the heavy pig. Ital. J. Anim. Sci. 2009, 8 (Suppl. 2), 561–563. [Google Scholar] [CrossRef]
- Monin, G.; Marinava, P.; Talmant, A.; Martin, J.F.; Cornet, M.; Lanore, D.; Grasso, F. Chemical and Structural changes in Dry-cured ham, (Bayonne hams) during processing and effects of the dehairing technique. Meat Sci. 1997, 47, 29–47. [Google Scholar] [CrossRef]
- Innocente, N.; Manzocco, L.; Nicoli, M.C. Monitoring moisture distribution in San Daniele ham by magnetic resonance imaging. In Qualità e Sicurezza Nella Filiera del Prosciutto [Quality and Safety in Prosciutto Chain]; Piasentier, E., Ed.; SOZOOALP: Trento, Italy, 2009; pp. 132–145. [Google Scholar]
- Bechet, D.; Deval, C.; Robelin, J.; Ferrara, M.; Obled, A. Developmental Control of Cathepsin B Expression in Bovine Fetal Muscles. Arch. Biochem. Biophys. 1996, 334, 362–368. [Google Scholar] [CrossRef]
- Sárraga, C.; Gil, M.; García-Regueiro, J.A. Comparison of Calpain and Cathepsin (B, L e D) Activities during Dry-Cured Ham Processing from Heavy and Light Large White Pigs. J. Sci. Food Agric. 1993, 62, 71–75. [Google Scholar] [CrossRef]
- Sforza, S.; Piazzani, A.; Motti, M.; Porta, C.; Virgili, R.; Galaverna, G.; Dossena, A.; Marchelli, R. Oligopeptides and free amino acids in Parma hams of known cathepsin B activity. Food Chem. 2001, 75, 267–273. [Google Scholar] [CrossRef]
- Takahiko, A.; Masaki, Y.; Ryuji, U. A cathepsin B-like enzyme from mackerel white muscle is a precursor of cathepsin B. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 133, 307–316. [Google Scholar]
- Mort, J.; Buttle, D. Molecules in focus: Cathepsin B. Int. J. Biochem. Cell Biol. 1997, 29, 715–720. [Google Scholar] [CrossRef]
- Toldrá, F.; Rico, E.; Flores, J. Cathepsins B, D, H and L activity in the processing of dry-cured ham. J. Sci. Food Agric. 1993, 62, 157–161. [Google Scholar] [CrossRef]
- Rico, E.; Toldrà, F.; Flores, M. Effect of dry-curing process parameters on pork muscle cathepsin B, H and L. Eur. Food Res. Technol. 1991, 193, 541–544. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Martin, L.; Cordoba, J.J.; Antequera, T.; Timón, M.; Ventanas, J. Effects of salt and temperature on proteolysis during ripening of Iberian ham. Meat Sci. 1998, 49, 145–153. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Effect of pH24, NaCl content and proteolysis index on the relationship between water content and texture parameters in biceps femoris and semimembranosus muscles in dry-cured ham. Meat Sci. 2006, 72, 185–194. [Google Scholar] [CrossRef]
- Virgili, R.; Parolari, G.; Schivazappa, C.; Bordini, C.S.; Borri, M. Sensory and texture quality of dry-cured ham as affected by endogenous cathepsin B activity and muscle composition. J. Food Sci. 1995, 60, 1183–1186. [Google Scholar] [CrossRef]
| Item | Prosciutto PDO Type | RSTD | Significance | |||||
|---|---|---|---|---|---|---|---|---|
| Parma | San Daniele | Toscano | PDO | Ripening Phase (T) | PDOxT | |||
| Initial thigh weight | kg | 14.20 a,b | 14.43 a | 13.59 b | 0.993 | 0.03 | 0.15 | 0.78 |
| Moisture Loss of Ham | Moisture Content | aw | Salt Content | Proteolysis Index | |
|---|---|---|---|---|---|
| Cathepsin B activity | −0.485 | 0.586 | 0.522 | −0.541 | −0.580 |
| Moisture loss of ham | −0.867 | −0.870 | 0.868 | 0.851 | |
| Moisture content | 0.950 | −0.949 | −0.771 | ||
| aw | −0.990 | −0.741 | |||
| Salt content | 0.754 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piasentier, E.; Pizzutti, N.; Lippe, G. The Influence of the Type of Dry-Cured Italian PDO Ham on Cathepsin B Activity Trend during Processing. Foods 2021, 10, 3123. https://doi.org/10.3390/foods10123123
Piasentier E, Pizzutti N, Lippe G. The Influence of the Type of Dry-Cured Italian PDO Ham on Cathepsin B Activity Trend during Processing. Foods. 2021; 10(12):3123. https://doi.org/10.3390/foods10123123
Chicago/Turabian StylePiasentier, Edi, Nicoletta Pizzutti, and Giovanna Lippe. 2021. "The Influence of the Type of Dry-Cured Italian PDO Ham on Cathepsin B Activity Trend during Processing" Foods 10, no. 12: 3123. https://doi.org/10.3390/foods10123123
APA StylePiasentier, E., Pizzutti, N., & Lippe, G. (2021). The Influence of the Type of Dry-Cured Italian PDO Ham on Cathepsin B Activity Trend during Processing. Foods, 10(12), 3123. https://doi.org/10.3390/foods10123123






