Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development
Abstract
1. Introduction
2. Sources of Bioactive Compounds
3. Extraction Methods for Bioactive Compounds
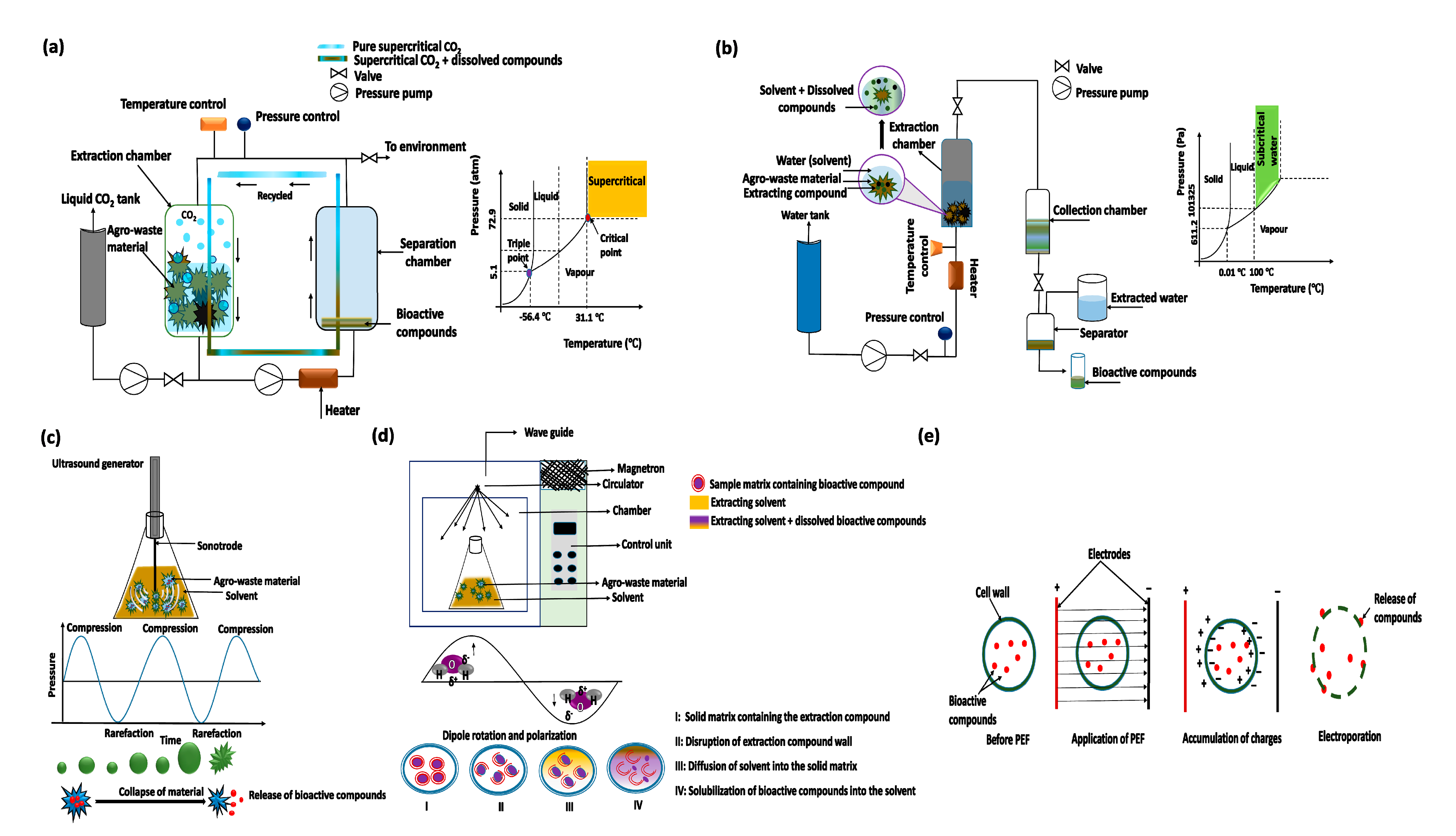
3.1. Supercritical Fluid Extraction
3.2. Subcritical Water Extraction
3.3. Ultrasound-Assisted Extraction
3.4. Microwave-Assisted Extraction
3.5. Pulsed Electric Field Extraction
4. Bulk Encapsulation of Bioactive Compounds
4.1. Ultrasound for Bulk Encapsulation
4.2. Spray Drying for Bulk Encapsulation
4.3. Spray Chilling for Bulk Encapsulation of Temperature-Sensitive Bioactives
4.4. Fluidised Bed for Additional Coating
4.5. Freeze Drying Bulk Encapsulation
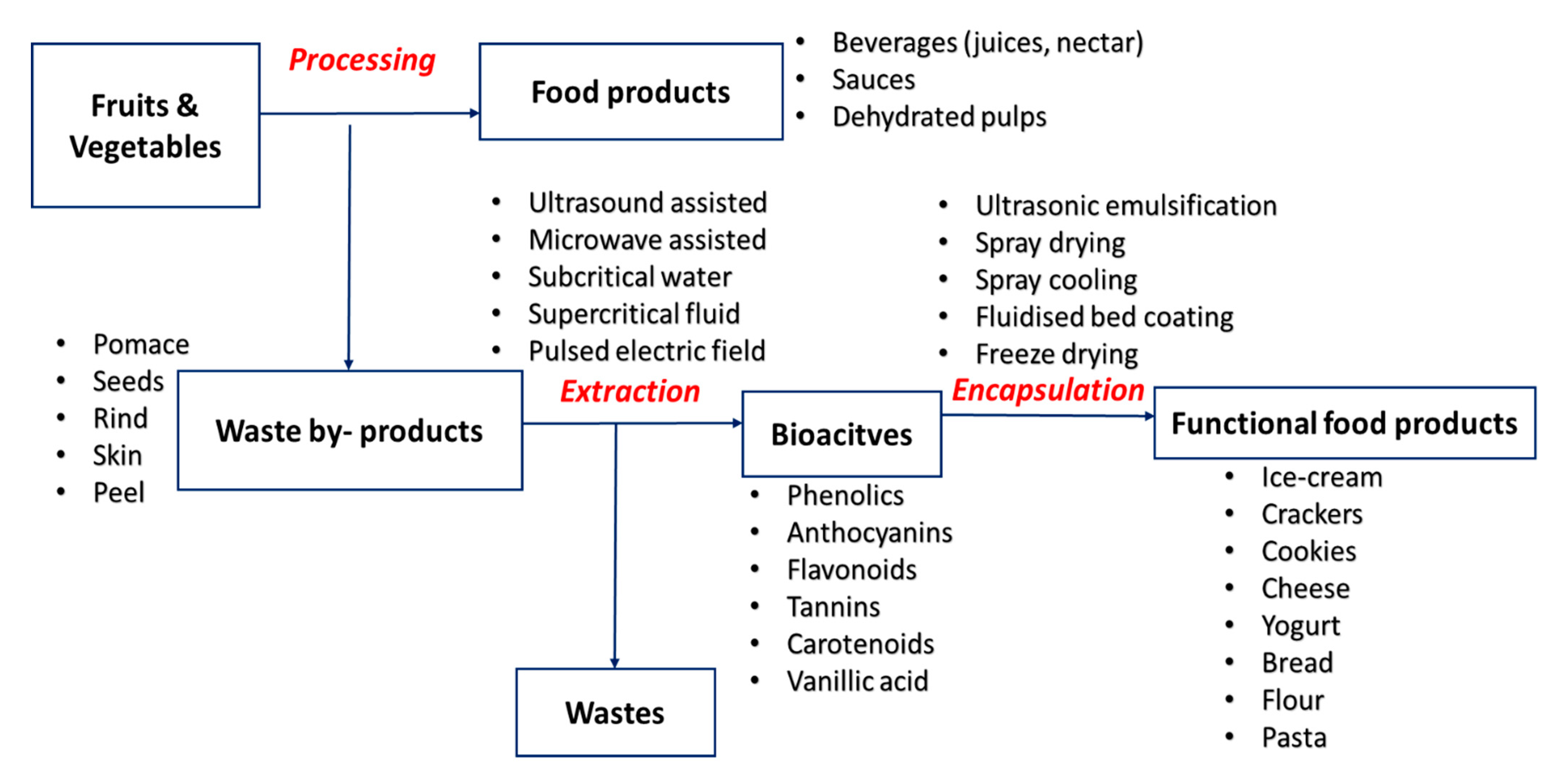
5. Development of Functional and Nutraceutical Food Products
6. Summary and Future Trends
7. Methodology of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choudhary, M.; Tripathi, S.; Kesharwani, R.K. Nutraceuticals Role in Stress, Aging, and Neuro-degenerative Disorders. In Nutraceutical and Functional Foods in Disease Prevention; IGI Global: Philadelphia, PSA, USA, 2019; pp. 288–306. [Google Scholar]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- Sawicka, B. Post-harvest Losses of Agricultural Produce. Hist. Sci. 2019, 1–16. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Process of fruit peel waste biorefinery: A case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ. Sci. Pollut. Res. 2019, 26, 34713–34722. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Dorta, E.; Sogi, D.S. Value added processing and utilization of pineapple by-products. In Hand-Book of Pineapple Technology, Production, Postharvest Science, Processing and Nutrition; John Wiley and Sons: Oxford, UK, 2017; pp. 196–220. [Google Scholar]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Norfezah, M.N.; Hardacre, A.; Brennan, C.S. Comparison of waste pumpkin material and its potential use in extruded snack foods. Food Sci. Technol. Int. 2011, 17, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Dry. Technol. 2017, 35, 139–162. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Tong, Q. Pec-tin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S.; Ahmad, S. Role of By-Products of Fruits and Vegetables in Functional Foods Functional Food Products and Sustainable Healt; Springer: Berlin/Heidelberg, Germany, 2020; pp. 199–218. [Google Scholar]
- Kruczek, M.; Gumul, D.; Kačániová, M.; Ivanišhová, E.; Mareček, J.; Gambuś, H. Industrial Apple Pomace By-Products as A Potential Source of Pro-Health Compounds In Functional Food. J. Microbiol. Biotechnol. Food Sci. 2019, 2019, 22–26. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S. Formulation and Processing of papaya by products. Int. J. Food Sci. Nutr. 2019, 4, 143–148. [Google Scholar]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Valdez-Morales, M.; Aparicio-Fernandez, X.; Medina-Godoy, S.; Guevara-Lara, F. Vegetable By-products. In Food Wastes By-Products Nutraceutical Health Potential; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 223–266. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef]
- Jan, N.U.; Ahmad, B.; Ali, S.; Adhikari, A.; Ali, A.; Jahan, A.; Ali, H. Steroidal alkaloids as an emerging therapeutic alternative for investigation of their immunosuppressive and hepatoprotective potential. Front. Pharmacol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Sharma, G.N.; Gupta, G.; Sharma, P. A comprehensive review of free radicals, antioxidants, and their relationship with human ailments. Crit. Rev.™ Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and charac-terization of cellulose from different fruit and vegetable pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Merhan, O. Biochemistry and antioxidant properties of carotenoids. Carotenoids 2017, 5, 51. [Google Scholar]
- Nguyen, V.T.; Scarlett, C.J. Mass proportion, bioactive compounds and antioxidant capacity of carrot peel as affected by various solvents. Technologies 2016, 4, 36. [Google Scholar] [CrossRef]
- Mezzomo, N.; Ferreira, S.R. Carotenoids functionality, sources, and processing by supercritical technology: A review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef]
- Islamian, J.P.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. (Yakhteh) 2015, 16, 386. [Google Scholar]
- Wang, Y.; Chung, S.J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef]
- Cerna, J.; Athari, N.; Robbs, C.; Walk, A.; Edwards, C.; Adamson, B.; Khan, N. Macular Carotenoids, Retinal Morphometry, and Cognitive Function in Multiple Sclerosis (OR05–07–19). Curr. Dev. Nutr. 2019, 3 (Suppl. S1), nzz029-OR05. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.W.; Kim, H. Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 2017, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration during 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Graff, R.E.; Pettersson, A.; Lis, R.T.; Ahearn, T.U.; Markt, S.C.; Wilson, K.M.; Mucci, L.A. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am. J. Clin. Nutr. 2016, 103, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Gul, K.; Tak, A.; Singh, A.K.; Singh, P.; Yousuf, B.; Wani, A.A. Chemistry, encapsulation, and health benefits of β-carotene-A review. Cogent Food Agric. 2015, 1, 1018696. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.; Yang, F.; Zeng, A.; Yang, S.; Luo, Y.; Zhang, Y.; Xie, Y.; Ye, T.; Xia, Y.; et al. The extract from Punica granatum (pomegranate) peel induces apoptosis and impairs metastasis in prostate cancer cells. Biomed. Pharmacother. 2017, 93, 976–984. [Google Scholar] [CrossRef]
- Durgadevi, P.K.S.; Saravanan, A.; Uma, S. Antioxidant Potential and Antitumour Activities of Nendran Banana Peels in Breast Cancer Cell Line. Indian J. Pharm. Sci. 2019, 81, 464–473. [Google Scholar]
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.F.; Fares, N. Long-term intake of phenolic compounds attenuates age-related cardiac remodeling. Aging Cell 2019, 18, e12894. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins, and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Mohammad Nabavi, S.; Fazel Naba, S. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in alzheimer’s and parkinson’s disease. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2017, 16, 387–397. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Moeller, S.M.; Voland, R.; Tinker, L.; Blodi, B.A.; Klein, M.L.; Gehrs, K.M.; Parekh, N. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an ancillary study of the women’s health initiative. Arch. Ophthalmol. 2008, 126, 354–364. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Pallet, V. Poly-phenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef]
- Haskell-Ramsay, C.F.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Carnaroglio, D.; Grillo, G.; Cravotto, G.; Pagliaro, M. High-quality essential oils extracted by an eco-friendly process from different citrus fruits and fruit regions. ACS Sustain. Chem. Eng. 2017, 5, 5578–5587. [Google Scholar] [CrossRef]
- Jadhav, S.S. Value added products from Sapota: A review. Int. J. Food Sci. Nutr. 2018, 3, 114–120. [Google Scholar]
- Baskar, M.; Hemalatha, G.; Muneeshwari, P. Traditional and Med. Importance of Sapota–Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1711–1717. [Google Scholar] [CrossRef]
- Jain, P.K.; Soni, P.; Upmanyu, N.; Shivhare, Y. Evaluation of analgesic activity of Manilkara zapota (Leaves). Eur. J. Exp. Biol. 2011, 1, 14–17. [Google Scholar]
- Rakholiya, K.; Kaneria, M.; Chanda, S. Inhibition of microbial pathogens using fruit and vegeta-ble peel extracts. Int. J. Food Sci. Nutr. 2014, 65, 733–739. [Google Scholar] [CrossRef]
- Srivastava, M.; Hegde, M.; Chiruvella, K.K.; Koroth, J.; Bhattacharya, S.; Choudhary, B.; Raghavan, S.C. Sapodilla plum (Achras sapota) induces apoptosis in cancer cell lines and inhibits tumor progression in mice. Sci. Rep. 2014, 4, 6147. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Ex-traction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Lafarga, T.; Viñas, I.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. Technol. 2018, 47, 412–420. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Manchon, N.; Guillamon, E.; García-Lafuente, A.; Garicochea, A.V.; Martinez, J.A. Methods and techniques for the analysis of isoflavones in foods. In Chromatography Types, Techniques and Methods; Hurst, W.J., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2010; pp. 157–198. [Google Scholar]
- Tambunan, A.P.; Bahtiar, A.; Tjandrawinata, R.R. Influence of extraction parameters on the yield, phytochemical, TLC-densitometric quantification of quercetin, and LC-MS profile, and how to standardize different batches for long term from Ageratum conyoides L. leaves. Pharmacogn. J. 2017, 9, 767–774. [Google Scholar] [CrossRef]
- Su, D.; Wang, Z.; Dong, L.; Huang, F.; Zhang, R.; Jia, X.; Zhang, M. Impact of thermal processing and storage temperature on the phenolic profile and antioxidant activity of different varieties of lychee juice. LWT 2019, 116, 108578. [Google Scholar] [CrossRef]
- Srinivasan, M.; Devipriya, N.; Kalpana, K.B.; Menon, V.P. Lycopene: An antioxidant and radio-protector against γ-radiation-induced cellular damages in cultured human lymphocytes. Toxicology 2009, 262, 43–49. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, E.J.; Newton, C.C.; McCullough, M.L. Lycopene, tomato products and prostate cancer-specific mortality among men diagnosed with nonmetastatic prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Int. J. Cancer 2016, 138, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.; Akbari, S.; Bhatt, H.; Joshi, D.C. Standardization of solvent extraction process for Lycopene extraction from tomato pomace. J. Appl. Biotechnol. BioEng. 2017, 2, 00019. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Niu, H.; Li, D.; Zhang, J. Optimization of operating parameters for supercritical carbon dioxide extraction of lycopene by response surface methodology. J. Food Eng. 2008, 89, 298–302. [Google Scholar] [CrossRef]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Cheng, L.W. Potato peels and their bioactive glycoalkaloids and phenolic compounds inhibit the growth of pathogenic trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Kenny, O.M.; Brunton, N.P.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Cytotoxic and apoptotic potential of potato glycoalkaloids in a number of cancer cell lines. J. Agric. Sci. Appl. 2013, 2, 184–192. [Google Scholar] [CrossRef]
- Lee, K.R.; Kozukue, N.; Han, J.S.; Park, J.H.; Chang, E.Y.; Baek, E.J.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef]
- Ji, X.; Rivers, L.; Zielinski, Z.; Xu, M.; MacDougall, E.; Stephen, J.; Robertson, G.S. Quantitative analysis of phenolic components and glycoalkaloids from 20 potato clones and in vitro evaluation of antioxidant, cholesterol uptake, and neuroprotective activities. Food Chem. 2012, 133, 1177–1187. [Google Scholar] [CrossRef]
- Friedman, M.; Roitman, J.N.; Kozukue, N. Glycoalkaloid and calystegine contents of eight potato cultivars. J. Agric. Food Chem. 2003, 51, 2964–2973. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering Valuable Bioactive Compounds from Potato Peels with Sequential Hydrothermal Extraction. Waste Biomass Valorization 2020, 1–17. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Grootaert, C.; Capanoglu, E.; Ozkan, C.; Smagghe, G.; Raes, K.; Van Camp, J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA. hy926 cells. Mol. Nutr. Food Res. 2017, 61, 1600455. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gur, C.; Cetin, B.; Akay, S.; Gulce-Iz, S.; Yesil-Celiktas, O. Extracts from black carrot tissue culture as potent anticancer agents. Plant Foods Hum. Nutr. 2013, 68, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Ghazala, I.; Sila, A.; Frikha, F.; Driss, D.; Ellouz-Chaabouni, S.; Haddar, A. Antioxidant and antimicrobial properties of water soluble polysaccharide extracted from carrot peels by-products. J. Food Sci. Technol. 2015, 52, 6953–6965. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Sotiroudis, G.; Melliou, E.; Sotiroudis, T.G.; Chinou, I. Chemical analysis, antioxidant and antimicrobial activity of three Greek cucumber (Cucumis sativus) cultivars. J. Food Bio-Chem. 2010, 34, 61–78. [Google Scholar] [CrossRef]
- Dixit, Y.; Kar, A. Protective role of three vegetable peels in alloxan induced diabetes mellitus in male mice. Plant Foods Human Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef]
- Zeyada, N.N.; Zeitoum, M.A.M.; Barbary, O.M. Utilization of some vegetables and fruit waste as natural antioxidants. Alex. J. Food Sci. Technol. 2008, 5, 1–11. [Google Scholar]
- Tadee, P.; Chukiatsiri, K.; Amornlerdpisan, D.; Paserakung, A.; Kittiwan, N. Antimicrobial effect of Japanese pumpkin (Cucurbita maxima) extract on local mastitis pathogen. Vet. Integr. Sci. 2020, 18, 141–152. [Google Scholar]
- Chen, X.; Qian, L.; Wang, B.; Zhang, Z.; Liu, H.; Zhang, Y.; Liu, J. Synergistic hypoglycemic effects of pumpkin polysaccharides and puerarin on type II diabetes mellitus mice. Molecules 2019, 24, 955. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Aires, A.; Dias, C.; Almeida, J.A.; De Vasconcelos, M.C.B.M.; Santos, P.; Rosa, E.A. Evaluation of the potential of squash pumpkin by-products (seeds and shell) as sources of antioxidant and bioactive compounds. J. Food Sci. Technol. 2015, 52, 1008–1015. [Google Scholar] [CrossRef]
- Wu, H.; Luo, T.; Li, Y.M.; Gao, Z.P.; Zhang, K.Q.; Song, J.Y.; Cao, Y.P. Granny Smith apple procyanidin extract upregulates tight junction protein expression and modulates oxidative stress and inflammation in lipopolysaccharide-induced Caco-2 cells. Food Funct. 2018, 9, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a Source of Dietary Phytonutrients: Bioavailability and Evidence of Protective Effects against Human Cardiovascular Disease. Sci. Res. 2014, 5, 1234–1246. [Google Scholar] [CrossRef]
- Lam, C.K.; Zhang, Z.; Yu, H.; Tsang, S.Y.; Huang, Y.; Chen, Z.Y. Apple polyphenols inhibit plasma CETP activity and reduce the ratio of non-HDL to HDL cholesterol. Mol. Nutr. Food Res. 2008, 52, 950–958. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.J.; Gill, C.I.R.; O’brien, G.; Rao, J.R.; McRoberts, W.C.; Hughes, P.; Rowland, I.R. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- Fernández, K.; Labra, J. Simulated digestion of proanthocyanidins in grape skin and seed ex-tracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2013, 139, 196–202. [Google Scholar] [CrossRef]
- Lanzi, C.R.; Perdicaro, D.J.; Antoniolli, A.; Fontana, A.R.; Miatello, R.M.; Bottini, R.; Prieto, M.A.V. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct. 2016, 7, 1544–1553. [Google Scholar] [CrossRef]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.F.; Maroun, R.; Fares, N. The impact of long-term in-take of phenolic compoundsrich grape pomace on rat gut microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; José, M.; Domínguez-perles, R.; Barros, A.I.R.N.A. Industrial Crops & Products Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar]
- Iora, S.R.; Maciel, G.M.; Zielinski, A.A.; da Silva, M.V.; Pontes, P.V.D.A.; Haminiuk, C.W.; Gran-ato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Chongnativisit, W.; Chaikul, P.; Lourith, N. Phenolic-rich Pomegranate Peel Extract: In Vitro, Cellular, and In Vivo Activities for Skin Hyperpigmentation Treatment. Planta Medica 2020, 86, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Bigoniya, P.; Singh, K. Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from Citrus sinensis. Rev. Bras. Farmacogn. 2014, 24, 330–340. [Google Scholar] [CrossRef]
- Rawson, N.E.; Ho, C.T.; Li, S. Efficacious anti-cancer property of flavonoids from citrus peels. Food Sci. Hum. Wellness 2014, 3, 104–109. [Google Scholar] [CrossRef]
- Chen, Z.T.; Chu, H.L.; Chyau, C.C.; Chu, C.C.; Duh, P.D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef]
- Omar, J.; Alonso, I.; Garaikoetxea, A.; Etxebarria, N. Optimization of focused ultrasound extraction (FUSE) and supercritical fluid extraction (SFE) of citrus peel volatile oils and antioxidants. Food Anal. Methods 2013, 6, 1244–1252. [Google Scholar] [CrossRef]
- Jagannath, A.; Biradar, R. Comparative Evaluation of Soxhlet and Ultrasonics on the Structural Morphology and Extraction of Bioactive Compounds of Lemon (Citrus limon L.) Peel. J. Food Chem. NanoTechnol. 2019, 5, 56–64. [Google Scholar] [CrossRef]
- Singanusong, R.; Nipornram, S.; Tochampa, W.; Rattanatraiwong, P. Low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainam-pueng) and lime (Citrus aurantifolia) peels and the antioxidant. Food Anal. Methods 2015, 8, 1112–1123. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; El-Maraghy, N.; Reda, E.; Barakat, W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: Role of adiponectin and TNF-α. Anais Acad. Bra-Sileira Ciênc. 2014, 86, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zheng, D.; Zhong, G.; Hu, Y. Mangiferin mitigates diabetic cardiomyopathy in strepto-zotocin-diabetic rats. Can. J. Physiol. Pharmacol. 2013, 91, 759–763. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.S.; Cho, M.; Choi, H.K.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Jeong, J.J.; Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, D.H. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways. Eur. J. Pharmacol. 2014, 740, 652–661. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Rao, U.P. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Guo, H.; Luo, H.; Yuan, H.; Xia, Y.; Shu, P.; Huang, X.; Deng, J. Litchi seed extracts diminish prostate cancer progression via induction of apoptosis and attenuation of EMT through Akt/GSK-3β signaling. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Wang, J.; Lin, P.; Liu, G.; Lu, Y.; Wei, Y. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol. Appl. Pharmacol. 2006, 215, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.R.; Wang, H.; Sun, J.; Yang, B.; Duan, X.W.; Jiang, Y.M. Pericarp and seed of litchi and longan fruits: Constituent, extraction, bioactive activity, and potential utilization. J. Zhejiang Univ.-Sci. B 2019, 20, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Kunworarath, N.; Rangkadilok, N.; Suriyo, T.; Thiantanawat, A.; Satayavivad, J. Longan (Dimo-carpus longan Lour.) inhibits lipopolysaccharide-stimulated nitric oxide production in macrophages by suppressing NF-κB and AP-1 signaling pathways. J. Ethnopharmacol. 2016, 179, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Liao, X.B.; Li, X.H.; Guo, J.W.; Qu, X.L.; Li, L.M. Effect and mechanism of Litchi semen effective constituents on insulin resistance in rats with type 2 diabetes mellitus. Zhong yao cai= Zhongyaocai= J. Chin. Med. Mater. 2015, 38, 1466. [Google Scholar]
- Panyathep, A.; Chewonarin, T.; Taneyhill, K.; Vinitketkumnuen, U. Antioxidant and anti-matrix metalloproteinases activities of dried longan (Euphoria longana) seed extract. Scienceasia 2013, 39, 12–18. [Google Scholar] [CrossRef][Green Version]
- Sudjaroen, Y.; Hull, W.E.; Erben, G.; Würtele, G.; Changbumrung, S.; Ulrich, C.M.; Owen, R.W. Isolation and characterization of ellagitannins as the major polyphenolic components of Lon-gan (Dimocarpus longan Lour) seeds. Phytochemistry 2012, 77, 226–237. [Google Scholar] [CrossRef]
- Bai, X.; Pan, R.; Li, M.; Li, X.; Zhang, H. HPLC profile of Longan (cv. Shixia) pericarp-sourced phenolics and their antioxidant and cytotoxic effects. Molecules 2019, 24, 619. [Google Scholar] [CrossRef]
- Kamal, A.M.; Taha, M.S.; Mousa, A.M. The Radioprotective and Anticancer Effects of Banana Peels Extract on Male Mice. J. Food Nutr. Res. 2019, 7, 827–835. [Google Scholar] [CrossRef]
- Rattanavichai, W.; Cheng, W. Effects of hot-water extract of banana (Musa acuminata) fruit’s peel on the antibacterial activity, and anti-hypothermal stress, immune responses and disease resistance of the giant freshwater prawn, Macrobrachium rosenbegii. Fish Shellfish. Immunol. 2014, 39, 326–335. [Google Scholar] [CrossRef]
- Yin, X.; Quan, J.; Kanazawa, T. Banana prevents plasma oxidative stress in healthy individuals. Plant Foods Hum. Nutr. 2008, 63, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Bhowmik, D.; Duraivel, S.; Umadevi, M. Traditional and medicinal uses of banana. J. Pharmacogn. Phytochem. 2012, 1, 51–63. [Google Scholar]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of banana (Musa AAA) peel as a source of antioxidant phenolic com-pounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Emmanuel, E.U.; Onagbonfeoana, E.S.; Adanma, O.C.; Precious, O.C.; Faith, A.I.; Ndukaku, O.Y. In vivo and in vitro antioxidant and hypolipidemic activity of methanol extract of pineapple peels in Wistar rats. Int. J. Biosci. 2016, 8, 64–72. [Google Scholar]
- Leipner, J.; Iten, F.; Saller, R. Therapy with proteolytic enzymes in rheumatic disorders. Bio-Drugs 2001, 15, 779–789. [Google Scholar] [CrossRef]
- Kaur, H.; Corscadden, K.; Lott, C.; Elbatarny, H.S.; Othman, M. Bromelain has paradoxical effects on blood coagulability: A study using thromboelastography. Blood Coagul. Fibrinolysis 2016, 27, 745–752. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. Anticancer property of bromelain with therapeutic potential in malignant peritoneal mesothelioma. Cancer Investig. 2013, 31, 241–250. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Visentainer, J.V. Antioxidant activity, phenolics and UPLC–ESI (–)–MS of extracts from different tropical fruits parts and processed peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Bhat, G.P.; Surolia, N. In vitro antimalarial activity of extracts of three plants used in the traditional medicine of India. Am. J. Trop. Med. Hyg. 2001, 65, 304–308. [Google Scholar] [CrossRef]
- Chávez-Quintal, P.; González-Flores, T.; Rodríguez-Buenfil, I.; Gallegos-Tintoré, S. Antifungal activity in ethanolic extracts of Carica papaya L. cv. Maradol leaves and seeds. Indian J. Microbiol. 2011, 51, 54–60. [Google Scholar] [CrossRef]
- Abdulazeez, A.; Ibrahim, S.; Ayo, J. Effect of fermented and unfermented seed extracts of Carica papaya on pre-implantation embryo development in female Wistar rats (Rattus norvegicus). Sci. Res. Essays 2009, 4, 1080–1084. [Google Scholar]
- Nayak, B.S.; Pereira, L.P.; Maharaj, D. Wound healing activity of Carica papaya L. in experimentally induced diabetic rats. Indian J. Exp. Biol. 2007, 45, 739–743. [Google Scholar]
- Aravind, G.; Bhowmik, D.; Duraivel, S.; Harish, G. Traditional and medicinal uses of Carica papaya. J. Med. Plants Stud. 2013, 1, 7–15. [Google Scholar]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of ex-traction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herbal Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.F.; Sauri-Duch, E. Chemical composition of crude oil from the seeds of pumpkin (Cucurbita spp.) and mamey sapota (Pout-eria sapota Jacq.) grown in Yucatan, Mexico. CYTA-J. Food 2013, 11, 324–327. [Google Scholar]
- Banožić, M.; Banjari, I.; Jakovljević, M.; Šubarić, D.; Tomas, S.; Babić, J.; Jokić, S. Optimization of ultrasound-assisted extraction of some bioactive compounds from tobacco waste. Molecules 2019, 24, 1611. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Espinosa-Andrews, H.; Trombotto, S.; Ayora-Talavera, T.; Patrón-Vázquez, J.; Gonzá-lez-Flores, T.; Pacheco, N. Ultrasound-assisted extraction optimization of phenolic compounds from Citrus latifolia waste for chitosan bioactive nanoparticles development. Molecules 2019, 24, 3541. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Ilakya, S.; Anitha, S.L.; Sabarima, S.P.; Priya, B. Ultrasound as-sisted extraction of pectin from waste Artocarpus heterophyllus fruit peel. Ultrason. Sonochem. 2017, 34, 525–530. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical fluid extraction of carotenoids from vegetable waste matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef] [PubMed]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of antioxidants from apple pomace by Supercritical fluid extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Chun, B.S. Optimization of carotenoids and antioxidant activity of oils obtained from a co-extraction of citrus (Yuzu ichandrin) by-products using supercritical carbon diox-ide. Biomass Bioenergy 2017, 106, 1–7. [Google Scholar] [CrossRef]
- Erşan, S.; Üstündağ, Ö.G.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenol-ic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Ko, M.J.; Kwon, H.L.; Chung, M.S. Pilot-scale subcritical water extraction of flavonoids from satsuma mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. Technol. 2016, 38, 175–181. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef]
- Filip, S.; Pavlić, B.; Vidović, S.; Vladić, J.; Zeković, Z. Optimization of microwave-assisted ex-traction of polyphenolic compounds from Ocimum basilicum by response surface methodology. Food Anal. Methods 2017, 10, 2270–2280. [Google Scholar] [CrossRef]
- Kulkarni, V.; Rathod, V. Green process for extraction of mangiferin from Mangifera indica leaves. J. Biol. Act. Prod. Nat. 2016, 6, 406–411. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancement of microwave-assisted extraction of bioactive compounds from cabbage outer leaves via the application of ultra-sonic pretreatment. Sep. Purif. Technol. 2015, 144, 37–45. [Google Scholar] [CrossRef]
- Hossain, M.B.; Aguiló-Aguayo, I.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Effect of pulsed electric field and pulsed light pre-treatment on the extraction of steroidal alkaloids from potato peels. Innov. Food Sci. Emerg. Technol. 2015, 29, 9–14. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blue-berry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, E.J. Microwave and pulsed electric field assisted extractions of polyphenols from defatted canola seed cake. Int. J. Food Sci. Technol. 2015, 50, 1109–1115. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Water and ethanol as co-solvent in supercritical fluid ex-traction of proanthocyanidins from grape marc: A comparison and a proposal. J. Supercrit. Fluids 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Floris, T.; Filippino, G.; Scrugli, S.; Pinna, M.B.; Argiolas, F.; Argiolas, A.; Reverchon, E. Antioxidant compounds recovery from grape residues by a Supercritical antisolvent assisted pro-cess. J. Supercrit. Fluids 2010, 54, 165–170. [Google Scholar] [CrossRef]
- Sosa, M.V.; Rodríguez-Rojo, S.; Mattea, F.; Cismondi, M.; Cocero, M.J. Green tea encapsulation by means of high pressure antisolvent coprecipitation. J. Supercrit. Fluids 2011, 56, 304–311. [Google Scholar] [CrossRef]
- Mezzomo, N.; Comim, S.R.R.; Campos, C.E.; Ferreira, S.R. Nanosizing of sodium ibuprofen by SAS method. Powder Technol. 2015, 270, 378–386. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M.; Xiao, D.; Tian, H.; Zhang, W. Application of supercritical anti-solvent technologies for the synthesis of delivery systems of bioactive food components. Food Biophys. 2008, 3, 186–190. [Google Scholar] [CrossRef]
- Zabot, G.L.; Meireles, M.A.A. On-line process for pressurized ethanol extraction of onion peels extract and particle formation using supercritical antisolvent. J. Supercrit. Fluids 2016, 110, 230–239. [Google Scholar] [CrossRef]
- Czaikoski, K.; Mesomo, M.C.; de Paula Scheer, A.; Dalla Santa, O.R.; Queiroga, C.L.; Corazza, M.L. Kinetics, composition and biological activity of Eupatorium intermedium flower extracts obtained from scCO2 and compressed propane. J. Supercrit. Fluids 2015, 97, 145–153. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Martinez, J.; Martinez-Correa, H.A. Extraction of bioactive compounds from peach palm pulp (Bactris gasipaes) using supercritical CO2. J. Supercrit. Fluids 2014, 93, 2–6. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Benedito, J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, X.; Cong, J.; Zhang, H.; Ma, H.; Duan, Y. Subcritical water extraction, identification and antiproliferation ability on HepG2 of polyphenols from lotus seed epicarp. Ind. Crops Prod. 2019, 129, 472–479. [Google Scholar] [CrossRef]
- Xian, Z.H.U.; Chao, Z.H.U.; Liang, Z.H.A.O.; CHENG, H. Amino acids production from fish proteins hydrolysis in subcritical water. Chin. J. Chem. Eng. 2008, 16, 456–460. [Google Scholar]
- Getachew, A.T.; Chun, B.S. Influence of pretreatment and modifiers on subcritical water liquefaction of spent coffee grounds: A green waste valorization approach. J. Clean. Prod. 2017, 142, 3719–3727. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Vardanega, R.; Santos, D.T.; Meireles, M.A.A. Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation. Pharm. Rev. 2014, 8, 88. [Google Scholar]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Luo, X. Advances in ultra-sound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Alzorqi, I.; Manickam, S. Ultrasonic process intensification for the efficient extraction of nutritionally active ingredients of polysaccharides from bioresources. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2015. [Google Scholar]
- Leong, T.S.; Martin, G.J.; Ashokkumar, M. Ultrasonic encapsulation—A review. Ultrason. Sonochem. 2017, 35, 605–614. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ramachandraiah, K.; Jiang, G.H.; Eun, J.B. Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract. Foods 2020, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Holkar, C.R.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic cavitation as a novel approach for extraction of oil from waste date seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and macera-tion techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Delazar, A.; Nahar, L.; Hamedeyazdan, S.; Sarker, S.D. Microwave-assisted extraction in natural products isolation. Nat. Prod. Isol. 2012, 864, 89–115. [Google Scholar]
- Jaitak, V.; Bandna, B.S.; Kaul, Y.V. An efficient microwave-assisted extraction process of stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni). Phytochem. Anal. 2009, 20, 240–245. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, A.; Prieto, P. Microwave-Assisted Green Organic Synthesis. In Alternative Energy Sources for Green Chemistry; The Royal Society of Chemistry: London, UK, 2016; pp. 1–33. [Google Scholar]
- Liu, Z.; Deng, B.; Li, S.; Zou, Z. Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind. Crops Prod. 2018, 124, 353–362. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. UPB Sci. Bull. Ser. B 2012, 74, 129–142. [Google Scholar]
- Zhang, F.; Chen, B.; Xiao, S.; Yao, S.Z. Optimization and comparison of different extraction techniques for sanguinarine and chelerythrine in fruits of Macleaya cordata (Willd) R. Br. Sep. Purif. Technol. 2005, 42, 283–290. [Google Scholar] [CrossRef]
- De la Guardia, M.; Armenta, S. Greening sample treatments. In Comprehensive Analytical Chemistr; Elsevier: Amsterdam, The Netherlands, 2011; Volume 57, pp. 87–120. [Google Scholar]
- Ciriminna, R.; Carnaroglio, D.; Delisi, R.; Arvati, S.; Tamburino, A.; Pagliaro, M. Industrial feasibility of natural products extraction with microwave technology. Chem. Sel. Rev. 2016, 1, 549–555. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed electric field assisted extraction of bio-active compounds from cocoa bean shell and coffee silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Arnal, Á.J.; Royo, P.; Pataro, G.; Ferrari, G.; Ferreira, V.J.; López-Sabirón, A.M.; Ferreira, G.A. Implementation of PEF treatment at real-scale tomatoes processing considering LCA methodology as an innovation strategy in the agri-food sector. Sustainability 2018, 10, 979. [Google Scholar] [CrossRef]
- Siddeeg, A.; Faisal Manzoor, M.; Haseeb Ahmad, M.; Ahmad, N.; Ahmed, Z.; Kashif Iqbal Khan, M.; Ammar, A.F. Pulsed electric field-assisted ethanolic extraction of date palm fruits: Bioactive compounds, antioxidant activity and physicochemical properties. Processes 2019, 7, 585. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Brito, P.S.; Canacsinh, H.; Mendes, J.P.; Redondo, L.M.; Pereira, M.T. Comparison between monopolar and bipolar microsecond range pulsed electric fields in enhancement of apple juice ex-traction. IEEE Trans. Plasma Sci. 2012, 40, 2348–2354. [Google Scholar] [CrossRef]
- Fincan, M.; DeVito, F.; Dejmek, P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment. J. Food Eng. 2004, 64, 381–388. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Delsart, C.; Ghidossi, R.; Poupot, C.; Cholet, C.; Grimi, N.; Vorobiev, E.; Peuchot, M.M. Enhanced extraction of phenolic compounds from Merlot grapes by pulsed electric field treatment. Am. J. Enol. Vitic. 2012, 63, 205–211. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess. Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Wiktor, A.; Rybak, K.; Miszczykowska, A.; Czyzewski, J.; Tylewicz, U. The impact of pulsed electric field on the extraction of bioactive compounds from beetroot. Foods 2019, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Iaffaioli, R.V.; Falcone, M.; Ferrari, G.; Pataro, G.; Donsì, F. Effect of pulsed electric fields–assisted extraction on anti-inflammatory and cytotoxic activity of brown rice bioactive compounds. Food Res. Int. 2016, 87, 115–124. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.Y.O.; Lima, G.P.P. Phenolic compounds: Functional properties, impact of processing and bioavailability. In Phenolic Compounds Biological Activity; InTech: Rijeka, Croatia, 2017; pp. 1–24. [Google Scholar]
- Echeverria, F.; Jimenez, P.; Castro-Sepulveda, M.; Bustamante, A.; Garcia, P.; Poblete-Aro, C.; Gar-cia-Diaz, D.F. Microencapsulated pomegranate peel extract induces mitochondrial com-plex IV activity and prevents mitochondrial cristae alteration in brown adipose tissue in mice fed on a high-fat diet+. Br. J. Nutr. 2020, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L.; Roy, P.K.; Zothantluanga, J.H.; Ray, S.; Das, S. Encapsulation of Bioactive Compound and Its Therapeutic Potential. In Bioactive Natural Products for Pharmaceutical Applications; Springer: Cham, Swizerland, 2021; pp. 687–714. [Google Scholar]
- Hu, Y.; Li, Y.; Zhang, W.; Kou, G.; Zhou, Z. Physical stability and antioxidant activity of citrus flavonoids in arabic gum-stabilized microcapsules: Modulation of whey protein concentrate. Food Hydrocoll. 2018, 77, 588–597. [Google Scholar] [CrossRef]
- Ramírez, M.J.; Giraldo, G.I.; Orrego, C.E. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015, 277, 89–96. [Google Scholar] [CrossRef]
- Do Carmo, E.L.; Teodoro, R.A.R.; Félix, P.H.C.; de Barros Fernandes, R.V.; de Oliveira, É.R.; Veiga, T.R.L.A.; Borges, S.V.; Botrel, D.A. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018, 249, 51–59. [Google Scholar] [CrossRef]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological application of maltodextrins according to the degree of polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef]
- Souza, A.L.; Hidalgo-Chávez, D.W.; Pontes, S.M.; Gomes, F.S.; Cabral, L.M.; Tonon, R.V. Microencapsulation by spray drying of a lycopene-rich tomato concentrate: Characterization and stability. LWT 2018, 91, 286–292. [Google Scholar] [CrossRef]
- Chen, F.P.; Liu, L.L.; Tang, C.H. Spray-drying Microencapsulation of Curcumin Nanocomplexes with Soy Protein Isolate: Encapsulation, Water Dispersion, Bioaccessibility and Bioactivities of Curcumin. Food Hydrocoll. 2020, 105, 105821. [Google Scholar] [CrossRef]
- Deng, X.X.; Chen, Z.; Huang, Q.; Fu, X.; Tang, C.H. Spray-drying microencapsulation of β-carotene by soy protein isolate and/or OSA-modified starch. J. App. Polym. Sci. 2014, 131, 40399. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Encapsulation of phenolic-rich extract from banana (Musa cavendish) peel. J. Food Sci. Technol. 2020, 57, 2089–2098. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Martin, L.G.P.; Fakhouri, F.M.; de Oliveira, R.A. Influence of spray drying on bioactive compounds of blackberry pulp microencapsulated with arrowroot starch and gum arabic mixture. J. Microencapsul. 2020, 37, 65–76. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Wang, X.; Prakash, S.; Zhao, Y.; Han, J.; Wang, Z. Influences of different carbohydrates as wall material on powder characteristics, encapsulation efficiency, stability and degradation kinetics of microencapsulated lutein by spray drying. Int. J. Food Sci. Technol. 2020, 55, 2872–2882. [Google Scholar] [CrossRef]
- Poyrazoglu, E.S.; Ozat, E.T.; Coksari, G.; Ozat, E.; Konar, N. Effect of various process conditions on efficiency and colour properties of Pistacia terebinthus oil encapsulated by spray drying. Int. J. Food Eng. 2017, 3, 132–135. [Google Scholar] [CrossRef]
- ANTIGO, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of pH on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2018, 38, 72–77. [Google Scholar] [CrossRef]
- Aphibanthammakit, C.; Barbar, R.; Nigen, M.; Sanchez, C.; Chalier, P. Emulsifying properties of Acacia senegal gum: Impact of high molar mass protein-rich AGPs. Food Chem. 2020, 6, 100090. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.P.; Shivhare, U.S. Microencapsulation by complex coacervation using whey protein isolates and gum acacia: An approach to preserve the functionality and controlled release of β-carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Omer, E.A.; AL-Omari, A.A.; Elgamidy, A.H.; Elgamidy, A.A.; Elgamidy, A.M. The emulsifying stability of gum Arabic using the local sesame oil obtained from AL-BAH A area. Int. J. Eng. Res. Technol. 2015, 4, 1172–1175. [Google Scholar]
- Neves, M.I.L.; Desobry-Banon, S.; Perrone, I.T.; Desobry, S.; Petit, J. Encapsulation of curcumin in milk powders by spray-drying: Physicochemistry, rehydration properties, and stability during storage. Powder Technol. 2019, 345, 601–607. [Google Scholar] [CrossRef]
- Gheonea, I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-loaded albumin nanoparticles with prolonged blood circulation and improved biocompatibility for highly effective targeted pancreatic tumor therapy. Nanoscale Res. Lett. 2017, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Barone Lumaga, R.; Ferracane, R.; Radetsky, I.; Mennella, I.; Schettino, R.; Fogliano, V. Curcumin bioavailability from enriched bread: The effect of microencapsulated ingredients. J. Agric. Food Chem. 2012, 60, 3357–3366. [Google Scholar] [CrossRef]
- Tran, N.; Tran, M.; Truong, H.; Le, L. Spray-drying microencapsulation of high concentration of bioactive compounds fragments from Euphorbia hirta L. extract and their effect on diabetes mellitus. Foods 2020, 9, 881. [Google Scholar] [CrossRef]
- Turan, F.T.; Cengiz, A.; Kahyaoglu, T. Evaluation of ultrasonic nozzle with spray-drying as a novel method for the microencapsulation of blueberry’s bioactive compounds. Innov. Food Sci. Emerg. Technol. 2015, 32, 136–145. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef]
- Silva, E.K.; Rosa, M.T.M.; Meireles, M.A.A. Ultrasound-assisted formation of emulsions stabilized by biopolymers. Curr. Opin. Food Sci. 2015, 5, 50–59. [Google Scholar] [CrossRef]
- Silva, E.K.; Azevedo, V.M.; Cunha, R.L.; Hubinger, M.D.; Meireles, M.A.A. Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocoll. 2016, 56, 71–83. [Google Scholar] [CrossRef]
- Shanmugam, A.; Chandrapala, J.; Ashokkumar, M. The effect of ultrasound on the physical and functional properties of skim milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 251–258. [Google Scholar] [CrossRef]
- Shanmugam, A.; Ashokkumar, M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems–physicochemical characterization. Food Hydrocoll. 2014, 39, 151–162. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U. Microencapsulation of bioactive food ingredients and controlled release-A review. MOJ Food Process. Technol. 2016, 2, 00059. [Google Scholar]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Lee, S.J.; Wong, M. Nano-and microencapsulation of phytochemicals. In Nano-and Microencapsulation for Foods; John Wiley & Sons Ltd.: Auckland, New Zealand, 2014; pp. 117–165. [Google Scholar]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild blueberry polyphenol-protein food ingredients produced by three drying methods: Comparative physico-chemical properties, phyto-chemical content, and stability during storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef]
- Sormoli, M.E.; Langrish, T.A. Spray drying bioactive orange-peel extracts produced by Soxhlet extraction: Use of WPI, antioxidant activity and moisture sorption isotherms. LWT-Food Sci. Technol. 2016, 72, 1–8. [Google Scholar] [CrossRef]
- Agudelo, C.; Barros, L.; Santos-Buelga, C.; Martínez-Navarrete, N.; Ferreira, I.C. Phytochemical content and antioxidant activity of grapefruit (Star Ruby): A comparison between fresh freeze-dried fruits and different powder formulations. LWT 2017, 80, 106–112. [Google Scholar] [CrossRef]
- Satpute, M.; Annapure, U. Approaches for delivery of heat sensitive nutrients through food systems for selection of appropriate processing techniques: A review. J. Hyg. Eng. Des. 2013, 4, 71–92. [Google Scholar]
- Bazaria, B.; Kumar, P. Effect of dextrose equivalency of maltodextrin together with Arabic gum on properties of encapsulated beetroot juice. J. Food Meas. Charact. 2017, 11, 156–163. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh, I.; Hajiabbas, M.; Pakzad, H.; Sajadi Dehkordi, S.; Vossoughi, A. Encapsulation of Food Components and Bioactive Ingredients and Targeted Release. Int. J. Eng. 2020, 33, 1–11. [Google Scholar]
- Tulini, F.L.; Souza, V.B.; Echalar-Barrientos, M.A.; Thomazini, M.; Pallone, E.M.; Favaro-Trindade, C.S. Development of solid lipid microparticles loaded with a proanthocyanidin-rich cinna-mon extract (Cinnamomum zeylanicum): Potential for increasing antioxidant content in functional foods for diabetic population. Food Res. Int. 2016, 85, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Tulini, F.L.; Souza, V.B.; Thomazini, M.; Silva, M.P.; Massarioli, A.P.; Alencar, S.M.; Favaro-Trindade, C.S. Evaluation of the release profile, stability and antioxidant activity of a pro-anthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract co-encapsulated with α-tocopherol by spray chilling. Food Res. Int. 2017, 95, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Oriani, V.B.; Alvim, I.D.; Consoli, L.; Molina, G.; Pastore, G.M.; Hubinger, M.D. Solid lipid microparticles produced by spray chilling technique to deliver ginger oleoresin: Structure and com-pound retention. Food Res. Int. 2016, 80, 41–49. [Google Scholar] [CrossRef]
- Pedroso, D.L.; Dogenski, M.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in cocoa butter using spray chilling technology. Braz. J. Microbiol. 2013, 44, 777–783. [Google Scholar] [CrossRef]
- Mazzocato, M.C.; Thomazini, M.; Favaro-Trindade, C.S. Improving stability of vitamin B12 (Cyanocobalamin) using microencapsulation by spray chilling technique. Food Res. Int. 2019, 126, 108663. [Google Scholar] [CrossRef]
- Meiners, J.A. Fluid bed microencapsulation and other coating methods for food ingredient and nutraceutical bioactive compounds. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Woodhead Publishing: Sawston, Cambridge, UK, 2012; pp. 151–176. [Google Scholar]
- Nedović, V.; Kalušević, A.; Manojlović, V.; Petrović, T.; Bugarski, B. Encapsulation systems in the food industry. In Advances in Food Process Engineering Research and Applications; Springer: Boston, MA, USA, 2013; pp. 229–253. [Google Scholar]
- Zuidam, N.J.; Heinrich, J. Encapsulation of aroma. In Encapsulation Technologies for Food Active Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer: London, UK, 2010. [Google Scholar]
- Scala, F. Mass Transfer around Active Particles in Fluidized Beds; INTECH Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive com-pounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Prata, A.S.; Maudhuit, A.; Boillereaux, L.; Poncelet, D. Development of a control system to anticipate agglomeration in fluidised bed coating. Powder Technol. 2012, 224, 168–174. [Google Scholar] [CrossRef]
- Benelli, L.; Oliveira, W.P. Fluidized bed coating of inert cores with a lipid-based system loaded with a polyphenol-rich Rosmarinus officinalis extract. Food Bioprod. Process. 2019, 114, 216–226. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive com-pounds using spray and freeze-drying techniques: A review. Drying Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.D.M.; Martínez-Navarrete, N. The Impact of Freeze-Drying Conditions on the Physico-Chemical Properties and Bioactive Compounds of a Freeze-Dried Orange Puree. Foods 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Stratta, L.; Capozzi, L.C.; Franzino, S.; Pisano, R. Economic Analysis of a Freeze-Drying Cycle. Processes 2020, 8, 1399. [Google Scholar] [CrossRef]
- Charalampia, D.; Koutelidakis, A.E. From Pomegranate Processing By-Products to Innovative value added Func-tional Ingredients and Bio-Based Products with Several Applications in Food Sector. BAOJ Biotech. 2017, 3, 210. [Google Scholar]
- Basanta, M.F.; Rizzo, S.A.; Szerman, N.; Vaudagna, S.R.; Descalzo, A.M.; Gerschenson, L.N.; Pérez, C.D.; Rojas, A.M. Plum (Prunus salicina) peel and pulp microparticles as natural antioxidant additives in breast chicken patties. Food Res. Int. 2018, 106, 1086–1094. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Jridi, M.; Khemakhem, I.; Bouaziz, M.; Attia, H. Storage stability of traditional Tunisian butter enriched with antioxidant extract from tomato processing by-products. Food Chem. 2017, 233, 476–482. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Ghafoor, K.; Al-Juhaimi, F.; Babiker, E.E.; Ahmed, I.A.M. Thermosonication process for optimal functional properties in carrot juice containing orange peel and pulp extracts. Food Chem. 2018, 245, 79–88. [Google Scholar] [CrossRef]
- Natukunda, S.; Muyonga, J.H.; Mukisa, I.M. Effect of tamarind (Tamarindus indica L.) seed on antioxidant activity, phytocompounds, physicochemical characteristics, and sensory acceptability of enriched cookies and mango juice. Food Sci. Nutr. 2016, 4, 494–507. [Google Scholar] [CrossRef]
- Ortiz, L.; Dorta, E.; Lobo, M.G.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of ba-nana (Musa acuminata Colla AAA) peel extract as an antioxidant source in orange juices. Plant Foods Hum. Nutr. 2017, 72, 60–66. [Google Scholar] [CrossRef]
- Ortiz, L.; Dorta, E.; Lobo, M.G.; González-Mendoza, L.A.; Díaz, C.; González, M. Use of ba-nana peel extract to stabilise antioxidant capacity and sensory properties of orange juice during pasteurisation and refrigerated storage. Food Bioprocess Technol. 2017, 10, 1883–1891. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat flour replacement by wine grape pomace powder positively affects physical, functional and sensory properties of cookies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted Technol. and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar] [CrossRef]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Fan, Y. Recent advances in continuous extraction of bioactive ingredients from food-processing wastes by pulsed electric fields. Crit. Rev. Food Sci. Nutr. 2020, 1–13. [Google Scholar] [CrossRef]
- Zin, M.M.; Anucha, C.B.; Bánvölgyi, S.J.F. Recovery of Phytochemicals via Electromagnetic Irradiation (Microwave-Assisted-Extraction): Betalain and Phenolic Compounds in Perspective. Foods 2020, 9, 918. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive com-pounds from plants, food-by-products, seaweeds and microalgae–An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Souza, M.C.; Santos, M.P.; Sumere, B.R.; Silva, L.C.; Cunha, D.T.; Martinez, J.; Rostagno, M.A. Isolation of gallic acid, caffeine and flavonols from black tea by on-line coupling of pressurized liquid extraction with an adsorbent for the production of functional bakery products. LWT 2020, 117, 108661. [Google Scholar] [CrossRef]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends in Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Maoto, M.M.; Beswa, D.; Jideani, A.I. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Ayar, A.; Siçramaz, H.; Öztürk, S.; Öztürk Yilmaz, S. Probiotic properties of ice creams produced with dietary fibres from by-products of the food industry. Int. J. Dairy Technol. 2018, 71, 174–182. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of apple pomace on quality characteristics of brown rice based cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Demirkol, M.; Tarakci, Z. Effect of grape (Vitis labrusca L.) pomace dried by different methods on physicochemical, microbiological and bioactive properties of yoghurt. LWT 2018, 97, 770–777. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physico-chemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Costa, A.S.; Cunha, L.M. Effect of Touri-ga nacional grape extract on characteristics of mechanically deboned chicken meat kept under frozen storage. J. Food Process. Eng. 2017, 40, e12434. [Google Scholar] [CrossRef]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casale, M.; Paini, M.; Casazza, A.A.; Lanteri, S.; Perego, P. Production of a novel fermented milk fortified with natural antioxidants and its analysis by NIR spectroscopy. LWT-Food Sci. Technol. 2015, 62, 376–383. [Google Scholar] [CrossRef]
- Kumar, V.; Kushwaha, R.; Goyal, A.; Tanwar, B.; Kaur, J. Process optimization for the preparation of antioxidant rich ginger candy using beetroot pomace extract. Food Chem. 2018, 245, 168–177. [Google Scholar] [CrossRef]
- De Toledo, N.M.V.; Nunes, L.P.; da Silva, P.P.M.; Spoto, M.H.F.; Canniatti-Brazaca, S.G. Influence of pineapple, apple and melon by-products on cookies: Physicochemical and sensory aspects. Int. J. Food Sci. Technol. 2017, 52, 1185–1192. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viskelis, P.; Bobinas, Č.; Mieželienė, A.; Alenčikienė, G.; Venskutonis, P.R. Raspberry marc extracts increase antioxidative potential, ellagic acid, ellagitannin and anthocyanin concentrations in fruit purees. LWT-Food Sci. Technol. 2016, 66, 460–467. [Google Scholar] [CrossRef]
- Pathak, D.; Majumdar, J.; Raychaudhuri, U.; Chakraborty, R. Characterization of physicochemical properties in whole wheat bread after incorporation of ripe mango peel. J. Food Meas. Charact. 2016, 10, 554–561. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W.J.F.S. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2017, 5, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Jung, J.; Colonna, A.; Hasenbeck, A.; Gouw, V.; Zhao, Y. Quality and Consumer Acceptance of Berry Fruit Pomace–Fortified Specialty Mustard. J. Food Sci. 2018, 83, 1921–1932. [Google Scholar] [CrossRef]
- Ismail, T.; Akhtar, S.; Riaz, M.; Hameed, A.; Afzal, K.; Sattar Sheikh, A. Oxidative and microbial stability of pomegranate peel extracts and bagasse supplemented cookies. J. Food Q. 2016, 39, 658–668. [Google Scholar] [CrossRef]
- Sandhya, S.; Khamrui, K.; Prasad, W.; Kumar, M. Preparation of pomegranate peel extract pow-der and evaluation of its effect on functional properties and shelf life of curd. LWT 2018, 92, 416–421. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of functional flours from pineapple by-products on human intestinal microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Oliveira, V.R.D.; Preto, L.T.; de Oliveira Schmidt, H.; Komeroski, M.; Silva, V.L.D.; de Oliveira Rios, A. Physicochemical and sensory evaluation of cakes made with passion fruit and orange residues. J. Culin. Sci. Technol. 2016, 14, 166–175. [Google Scholar] [CrossRef]
| Bioactive Compounds | Functionality for Processed Foods | Claimed Health Benefits | Parts | Sources |
|---|---|---|---|---|
| Lycopene | Antioxidants, food colorant | Radio protectant [65], anti-cancer agent [66], inhibit neurodegenerative diseases [35], promoter of heart health [67] | Peel- 611.10 mg/100 g DW [68]; Pomace- 28.64 mg/100 g DW [69] | Tomato |
| Polyphenols (gallic, chlorogenic, caffeic, ferulic, syringic, and p coumaric acids); and steroidal alkaloids (α-solanine, α-chaconine, aglycone solanidine) | Antioxidants, thickener | Anti-pathogenic [70], anti-inflammatory [71], anti-carcinogenic activities [71,72], neuroprotective activities [73] | Peel- alkaloids 84–2226 mg/kg [74]; polyphenols 32.87 mg/g DW [75] | Potato |
| Phenols, β-carotene | Antioxidants, pro-vitamin | Anti-inflammatory [76], anti-cancer agent [77], anti-microbial [78] | Peel: β-carotene 20.4 mg GAE/g DW; polyphenols 1371 mg GAE/g DW [79] | Carrot |
| Chlorophyll, caryophyllene, phellandrene, pheophytin | Antioxidants | Antimicrobial [80], antidiabetic [81] | Peel: chlorophyll 3.46 mg/g, caryophyllene 1.49 mg/g, phellandrene 1.21 mg/g, pheophytin 1.95 mg/g [82] | Cucumber |
| p-hydroxybenzoic acid, trans-p-coumaric acid, p-hydroxybenzaldehyde, caffeic acid | Antioxidants, fiber-rich component | Antimicrobial [83], treatment for diabetes mellitus [84] | Seeds: polyphenols 2.34–6.12 mg GAE/g DW; Shells: polyphenols 7.41–10.69 mg GAE/g DW [85] | Pumpkin |
| Anthocyanins, cinnamic acid, dihydrochalcones (phloretin), flavan-3-ol (epicatechin), flavonol (quercitin glycosides) | Antioxidant activity (ROS and RNS), food additive (natural alternative to synthetic antioxidants and anti-microbials) | Reduction of oxidative stress and inflammation properties [86,87], modifications of plasma lipids and lipoprotein levels [88,89], and anti-cancer activity [90] | Wastes (pomace, peel)- Anthocyanins 2.83 g/100 g DW; cinnamic acid 1.06 g/100 g DW; phloretic 569 mg/100 g DW; epicatechin 291 mg/100 g DW; flavonol 768 mg/100 g DW [91] | Apple |
| malvidin-3-O-glucoside, peonidin-3-O-glucoside, gallic acid, p-hydroxybenzoic acid, cinnamic acid, vanillic acid, proanthocyanidins, coumaric acid, chlorogenic acid, engeletin, quercetin, astilbin, resveratrol | Antioxidants (ROS/RNS), natural additive | Cardioprotective effect [92], prevention of metabolic syndrome [93], management of diabetes [94], anti-proliferative [95], anti-microbial/bacterial potential [96,97] | Pomace: anthocyanins 1246.85–2092.93 mg/100 g, total phenolic content 3014.55–5101.82 mg GAE/100 g, total flavonoids 1648.28 to 2983.91 mg CE/100 g, Total anthocyanin 1246.85–2092.93 mg/100 g [98] | Grapes |
| Gallic acid, delphinidin-3,5-diglucoside, cyaniding diglucoside, sinapic acid, α –punicalagin, β –Punicalagin, ellagic acid, hesperidine, quercetrin | Antioxidants, dietary fibers, single-cell protein, industrial enzymes, functional food ingredients, food additives, food lipid stabilizer, and artificial sweetener | Alleviates hypercholesterolemia [99], hyperpigmentation treatment [100], anti-cancer activity [43], dietary supplements | Peels- polyphenols 249.4 mg/g, flavonoids 59.1 mg/g, proanthocyanidins 10.9 mg/g [101] | Pomegranate |
| Naringin, eriocitrin, hesperidin, narirutin, limonin | Thickening and gelling agent, stabilizer, food additive | Mucoprotective agent [102], anti-carcinogenic [103], cytoprotective effect [104], prevention of neurodegenerative diseases [49] | Peel: Total phenolic content- 1259 mg GAE/100 g (orange), 1812 mg GAE/100 g (lemon), 793 mg GAE/100 g (mandarin) [105]; Total Flavonoids: 4.52 mg CE/100 g lemon peel [106], TF: 2539.82 mg QE/100 g mandarin peel [107]; lemon seeds- limonin 8.95 mg/g DW; Valencia orange seeds- limonin 10 mg/g DW [108] | Citrus fruits |
| Gallic acid, anthocyanins, ellagic acid, quercetin, tannins, xanthones, mangiferin and its related compounds, kaempferol | Antioxidants, micronutrient, protein-rich food source, | Modulation of diabetes and dyslipidemia [109], heart-protective effects [46,110], anti-cancer [111,112], anti-inflammatory [113] | Peel- Total polyphenolic content: Raw—90 to 110 mg/g, ripe- 55 to 100 mg/g [114] | Mango |
| Epicatechin-3-gallate, malvidin-3-glucoside, procyanidin B2, gallic acid, procyanidin B4, anthocyanins, naringin, isoscopoletin, coumaric acid | Antioxidant, Food colorant, food additives | Pain reliever & Anti-cancer agent [115,116], tyrosinase inhibitory [117,118], anti-inflammatory [119], immunomodulatory, anti-glycated, anti-diabetics [120], metalloproteinase activity [121] | Seeds: phenolic compounds 80.9 g/kg DW [122], Pericarp: phenolic content 57.8 mg GAE/g DW [123] | Logan/Litchi |
| Gallocatechin, catecholamine, anthocyanins, delphinidin, cyanide, ferulic acid, cinnamic acid, Epicatechin, Procyanidin | Antioxidant, thickening agent, natural bio-colorant, bio-flavors, source of macro & micro-nutrients | Anticancer [44,124], anti-bacterial [125], lower plasma oxidative stress [126], treatment of diarrhea [127] | Peel: phenolic content 29.2 mg GAE/g DW [128] | Banana |
| Ferulic acid, p-coumaric acid, Caffeic acid, bromelain | Prebiotic, single-cell protein, anti-browning agent, texture improver | Manage hyperlipidemia [129], analgesic and anti-inflammatory effects [130], blood coagulation [131], anticancer agent for malignant peritoneal mesothelioma [132] | Peel: phenolics 222–428 mg GAE/100 g DW [133] | Pineapple |
| Carpaine, glucotropacolin, benzylisothiocynate, bemzylthiourea, benzylglucosinolate, sitosterol, hentriacontane, papain, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, and vanillic acid | Rich in digestive enzymes | Antimalarial [134], Antimicrobial/Antifungal [135], abortifacient [136], wound healing [137], treatment of psoriasis & jaundice [138] | Seeds: total phenolic content 0.31–0.77 mg/g [139], Leaf/peel: total polyphenols 28.61–63.59 mg GAE/g, flavonoids 8.36–23.45 mg CE/g, Proanthocyanidins 3–8.89 mg CE/g [140] | Papaya |
| Extraction Techniques | Sources | Compounds | Operating Conditions | Solvent/Co-solvent | Extraction Efficiency, EE (%)/Yield, EY (g/100 g) | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C)/MW Power (W) | Pressure (bar)/Flow Rate of Solvent (ml/min) | Frequency (kHz) | Amplitude (%)/Applied Voltage (kV/cm) | Time (min) | Solid: Solvent | ||||||
| SE | Pomegranate peels | Carotenoids; Punicalagins and ellagic acids | 35; 100 | - | - | - | -;5 | 1:5; 1:5 | Hexane, Isopropanol; Water | EE:85.7; 80.3 & 19.7 | [141,142] |
| Pouteria sapota seeds | Oil | 70 | - | - | - | 360 | 1:7 | Hexane | EE:40 | [143] | |
| UAE | Tobacco waste (midrib, dust, scrap) | Chlorogenic | 50/50 | - | 37 | - | 30 | 1:10 | Ethanol-water | EY:0.35 | [144] |
| Citrus latifolia waste | Catechin and diosmin | 50/130 | - | 20 | 89 | 12.5 | 1:50 | Ethanol | EE:93, 89 | [145] | |
| Pomegranate peels | Carotenoids | 51.5 | - | 20 | 40% | 30 | 1:10 | Vegetable oil | EE:93.8 | [142] | |
| Artocarpus heterophyllus (Jackfruit) peel | Pectin | 60, pH 1.6 | - | - | - | 24 | 1:15 | Water | EE:14.5 | [146] | |
| UAE + PLE | Blackberry, blueberry, and grumixama wastes | Anthocyanin | 80/580 | 100 | 37 | - | 30 | 1:18 | Ethanol/water | EY:9.62–11.66 | [10] |
| SCFE | Vegetable peel wastes (sweet potato, tomato, apricot, peach) | Carotenoids | 59 | 350/15 | - | - | 30 | 1:15.5 | CO2, Ethanol | EE > 90 | [147] |
| Apple pomace | Total phenolic content | 45 | 300/33.3 | - | - | 120 | - | CO2, Ethanol | EY:5.78 | [148] | |
| Citrus peels and seeds | Carotenoids | 41–45 | 250–300/27 | - | - | 120 | 1.5–2.25 | CO2, seed oil | EY:0.198 | [149] | |
| SCWE | Pistachio hulls | Gallotannin & flavonols | 110–190 | 69/4 | - | - | 30–50 | 1:25 | Water | EE > 96 | [150] |
| Mandarin peel | Flavonoids | 130 | 30/1000 | - | - | 15 | 1:34 | Water | EE-96.3 | [151] | |
| MAE | Vine prune residues | Total phenolic content | 120 | - | - | - | 5 | 1:40 | Ethanol -water | EY:2.4 | [152] |
| Ocimum basilicum | Polyphenols | -/442 | - | - | - | 15 | 1:10 | Ethanol | EY:4.3 | [153] | |
| Mangifera indica leaves | Mangiferin | -/272 | - | - | - | 5 | 1:20 | Water | EY:5.5 | [154] | |
| Red grape pomace | Phenolics | 50/200 | - | - | - | 60 | 1:50 | Water-ethanol | EY:23 | [155] | |
| Cabbage leaves | Phenolic content | ~50/100 | - | - | - | 2 | 1:10 | Ethanol | EY:0.86 | [156] | |
| PEF + SLE | Potato peel | Steroidal alkaloids | 15–23 | - | 0.01 | -/0.75 | 200 pulses@ 3 μs, 60 min | 1:5 | Methanol | EY:0.158 | [157] |
| PEF + SE | Blueberry press cake | Total phenolics, anthocyannin | 23 | - | 0.01 | -/1–5 | Pulse width- 1–23 μs, 24 h | 1:6 | Ethanol | EE: >63, >78 | [158] |
| PEF + UAE | Defatted canola seed cake | Polyphenols | 70/200 | - | 0.03 | 900 pulses@ 20 μs, 20 min | 1:10 | Ethanol | EY:2.6 | [159] | |
| Bioactive Compounds | Wall Materials | Advantages of Wall Material | Limitations of Wall Material | Suitable Encapsulation Techniques | References |
|---|---|---|---|---|---|
| Lycopene, Citrus reticulata polyphenol extract | Gum Arabic | Good emulsifying capacity, high solubility | Limited protection to oxidation | Spray drying, freeze-drying | [217,218] |
| Anthocyanin from blackberry by-products, Betanain | Maltodextrin | Low cost, low oxygen permeability, rapid film-forming ability | Poor emulsifying property increase the viscosity | Spray drying | [219,220] |
| Limonene, Lycopene, betalains | Whey protein isolate | Excellent emulsifying abilities provide good emulsion stability | Limited heat and freeze stability | Freeze drying, Spray drying | [219,221] |
| Curcumin, Banana peel extracts, β-carotene | Soy protein isolate | Good emulsifying ability, fast film formation | Soluble in alkaline pH | Freeze drying | [222,223,224] |
| Blackberry pulp | Arrowroot starch and gum arabic | Gelling agent, good emulsifying ability | High viscosity | Spray drying | [225] |
| Chokeberry anthocyannanis extract | Pectin | Gelling agent and colloidal stabilizer | Forms clumps during dispersion, encapsulation depends greatly on methylation degree | Spray drying | [226] |
| Lutein | Inulin | Requires low drying temperature for film formation | Sensitive to environmental conditions | Spray drying | [227,228] |
| Betanins | Xanthan gum | Stabilizes emulsions, protective film against oxidation | High viscosity at low concentration | Spray/freeze drying | [229] |
| β-carotene | Gum acacia | Stabilizes emulsions | High viscosity | Complex coacervation by sonication | [230,231,232] |
| Curcumin | Skim milk powder | Good film forming and emulsifying ability | pH-dependent gel swelling behavior | Spray drying | [233] |
| Lycopene | Whey protein isolate & Gum acacia | Good retention of bioactive compound | Oxidative degradation and mass loss during drying | Complex coacervation, freeze-drying | [234] |
| Type of Study | Encapsulated Bioactive Compound | Dose | Duration | Results | References |
|---|---|---|---|---|---|
| Randomized | Resveratrol | 6 mg | 35 days | Inhibition of cell growth in tumor | [235] |
| Randomized cross-over | Curcumin | 1 g | 3 days | Biotransformation of curcumin are delayed | [236] |
| Controlled | E. hirta powder | 500 mg/kg bw | 15 days | Potential antidiabetic activity | [237] |
| Randomized | Betanin | 60 mg/kg bw | 28 days | Positive effect on regulating hyperglycemia, hyperlipidemia, and oxidative Stress | [238] |
| Randomized | astaxanthin | 100 mg/kg | 72 h | Rate of release and extent of digestion was improved | [239] |
| Raw Material | Waste Part | Extracted/Target Compound | Raw material Processing Method | Value-Added Product | Reported Functional Improvements | References |
|---|---|---|---|---|---|---|
| Apple | Pomace | TPC, TFC, DPPH; TDF | Tray drying | Gluten-free cracker; Ice cream | Rich in antioxidants, dietary fiber, and minerals, specific for coeliac disease patients; dietary fiber-rich products | [287,288] |
| Tamarind | Seed | β-carotene, TPC, TFC, TAA, TCT | Sun drying | Cookies and mango juice | Natural antioxidants enhance nutraceutical properties | [274] |
| Banana | Peel | DPPH, ABTS | Solvent Extraction | Orange juice | Increased antioxidant activity | [275,276] |
| Grapes | Pomace | TPC, DPPH | Freeze-dried; | Yogurt Cheese | Antioxidant properties, anti-inflammatory, anticancer, antimicrobial, and cardiovascular protective properties; | [289,290] |
| TPC, DPPH, FRAP | Solid-phase extraction | Bread | Help in prevention of diseases like atherosclerosis, cancer, cardiovascular disease, and type 2 diabetes; | [291] | ||
| TPC, DPPH, ORAC, ICA | Solvent Extraction | Chicken Meat | Antioxidant properties; | [292] | ||
| TPC, TFC, ABTS, FRAP | Solvent Extraction | Cheese | Improved nutritional properties, sensory attributes like friability and adhesiveness | [293] | ||
| TPC, ARP | Solvent Extraction | Fermented milk | Natural antioxidants | [294] | ||
| Beetroot | Pomace | TPC, AA, Betalain | Solvent Extraction | Candy | Rich in betalain, antioxidant, and phenolics | [277,295] |
| TPC, FRAP, ABTS, Betacyanins | Solvent Extraction with ultrasound | Biscuit | Increased pathogen resistance, anti-inflammatory effect, and antioxidant activities | |||
| Pineapple | Central Axis | TDF | Freeze drying | Cookies | Improved nutritional properties | [296] |
| Apple | Endocarp | |||||
| Melon | Peels | |||||
| Raspberry | Pomace | TPC, TAC, RSC, Free EA, ETs | Solvent Extraction and freeze-dried | Fruit Purees | Antioxidant, antimutagenic, anticarcinogenic, antibacterial, and antiviral properties | [297] |
| Orange | Peel and pulp | TPC, DPPH | Sonication | Carrot juice | Improved functional quality and shelf life | [273] |
| Artichoke | outer bracts, leaves and stems outer bracts, leaves and stems outer bracts, leaves and stems, Outer bracts, leaves, and stems | TPC, AA | Ultrasound-assisted extraction (UAE) | Pasta | Nutraceutical properties, reduction of cholesterol, antioxidant properties | [279] |
| Ripe Mango | Peel | TPC, DPPH | Tray drying | Whole Wheat Bread | Rich in antioxidants, help in the prevention of cardiovascular and neurodegenerative diseases, cancers, etc. | [298] |
| Mango | Seed Kernel | TPC, DPPH | Solvent Extraction | Mango Powder | Natural antibiotic and antifungal properties | [299] |
| Blueberry and Cranberry | Pomace | TPC, RSA | Solvent Extraction | Mustard | Anticancer, antioxidant, and antimicrobial properties | [300] |
| Pomegranate | Peel | TPC, DPPH, ABTS | Solvent Extraction | Curd | Increase the anti-oxidative attributes and shelf life of the product | [301,302] |
| TPC, FRAP, DPPH | Solvent Extraction and freeze-drying | Cookies | Antioxidant, antimicrobial & nutraceutical properties | |||
| Pineapple | Peel and stems | Bromelain (BR) | Polyelectrolyte precipitation | Flour | Enhance the growth of good bacteria in the human microbiota, high antioxidant activity in human gut | [303] |
| Passion fruit and Orange | Albedo | TDF | Oven drying | Cake | Reduce cholesterol, and reduce diabetes risks and obesity | [304] |
| Tomato | Peels and seeds | TPC, RSA, lycopene | Solvent Extraction | Butter | Extended shelf life of butter with antioxidant properties | [272] |
| Cauliflower | Leaves and stem | Isothiocyanates (ITC), TPC, TAA | Ultrasound-assisted extraction (UAE) | Apple juice beverage | Anticarcinogenic properties | [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattnaik, M.; Pandey, P.; Martin, G.J.O.; Mishra, H.N.; Ashokkumar, M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods 2021, 10, 279. https://doi.org/10.3390/foods10020279
Pattnaik M, Pandey P, Martin GJO, Mishra HN, Ashokkumar M. Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods. 2021; 10(2):279. https://doi.org/10.3390/foods10020279
Chicago/Turabian StylePattnaik, Monalisha, Pooja Pandey, Gregory J. O. Martin, Hari Niwas Mishra, and Muthupandian Ashokkumar. 2021. "Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development" Foods 10, no. 2: 279. https://doi.org/10.3390/foods10020279
APA StylePattnaik, M., Pandey, P., Martin, G. J. O., Mishra, H. N., & Ashokkumar, M. (2021). Innovative Technologies for Extraction and Microencapsulation of Bioactives from Plant-Based Food Waste and Their Applications in Functional Food Development. Foods, 10(2), 279. https://doi.org/10.3390/foods10020279









