Effect of Interfacial Ionic Layers on the Food-Grade O/W Emulsion Physical Stability and Astaxanthin Retention during Spray-Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Interfacial Tension (IT)
2.3. Elaboration of O/W Multilayer Emulsions with Encapsulated Astaxanthin
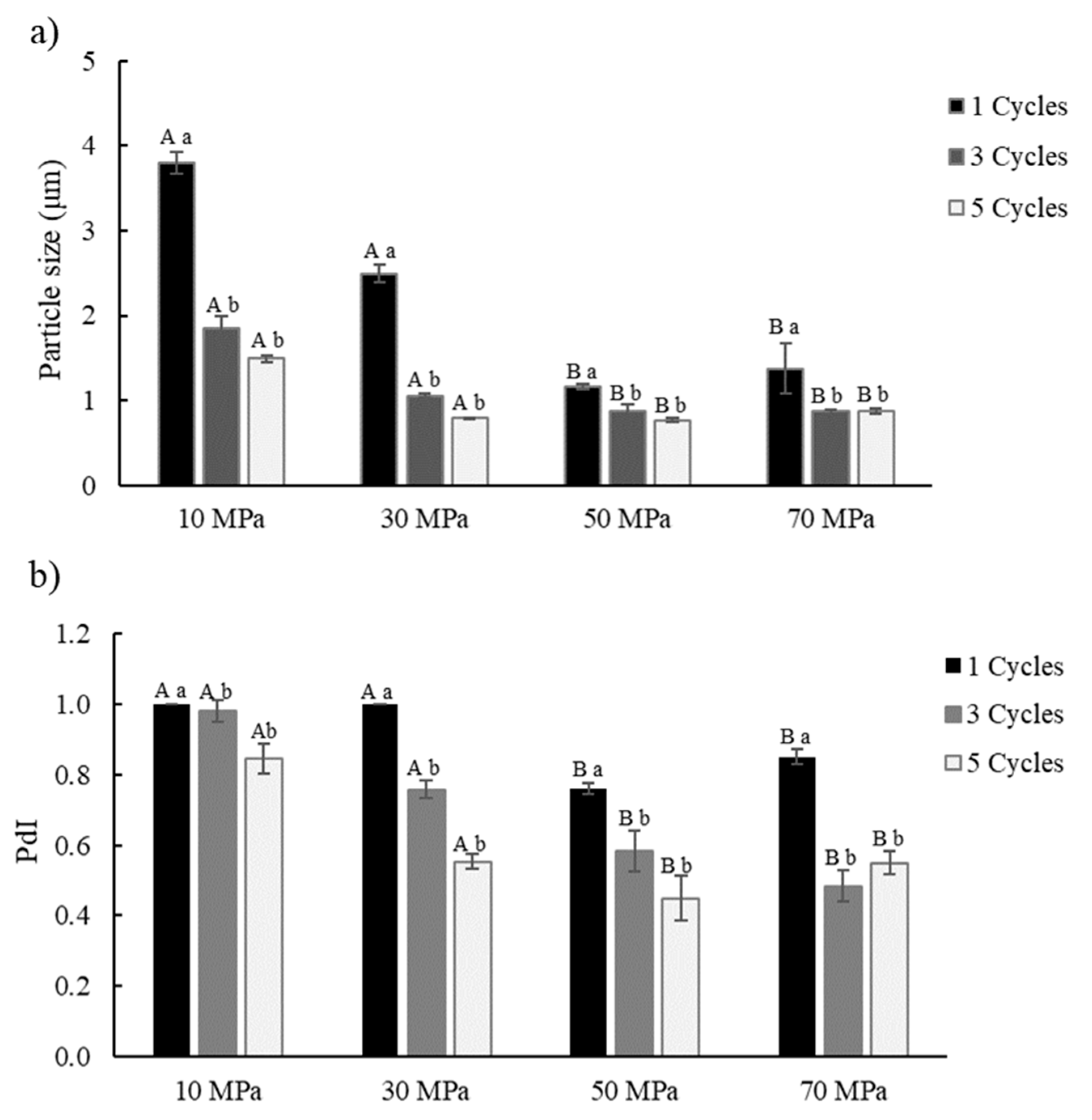
2.4. Mean Size of Particles and Polydispersity Index (PdI) Measurements
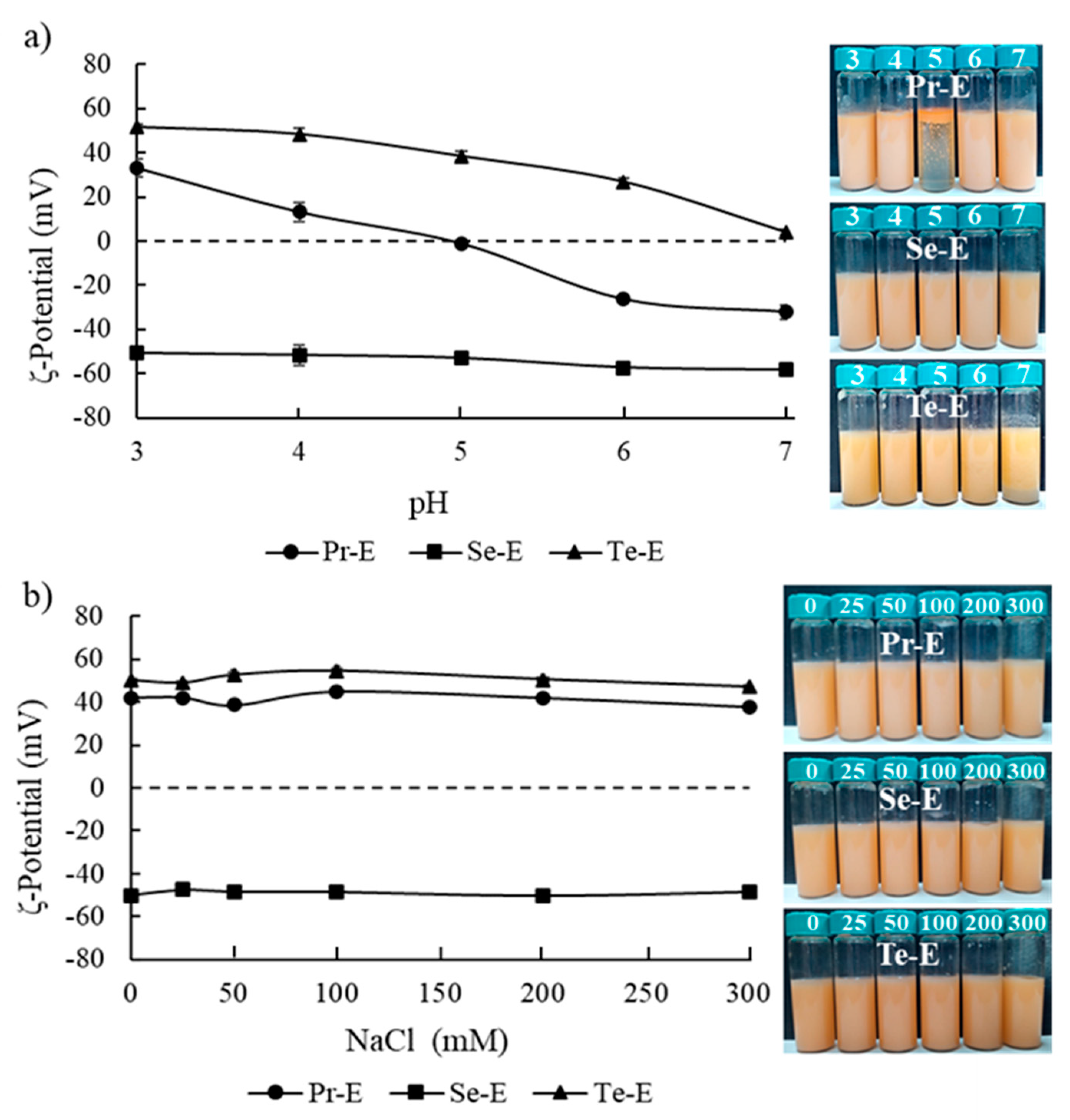
2.5. ζ- Potential Measurements
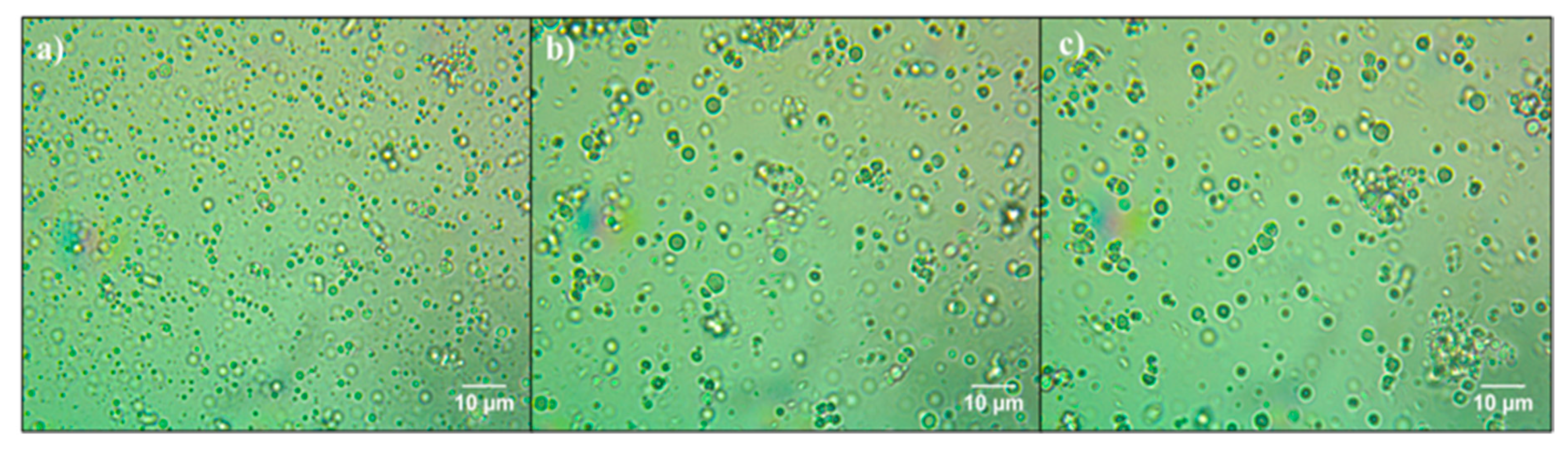
2.6. Optical Microscopy of Emulsions
2.7. Creaming Index (CI) of Emulsions
2.8. Effect of Environmental Stress on the Stability of Emulsions
2.9. Spray-Drying of Emulsions
2.10. Assessment of Astaxanthin Content
2.11. Astaxanthin Retention of Emulsions
2.12. Analysis of Statistics
3. Results and Discussion
3.1. Interfacial Tension (IT) of LPI
3.2. Effect of High-Pressure Homogenization Process Conditions on Pr-E
3.3. Effect of CA and CHI on the Multilayer Emulsion Stability
3.4. Effect of Environmental Stresses on the Emulsion Stability
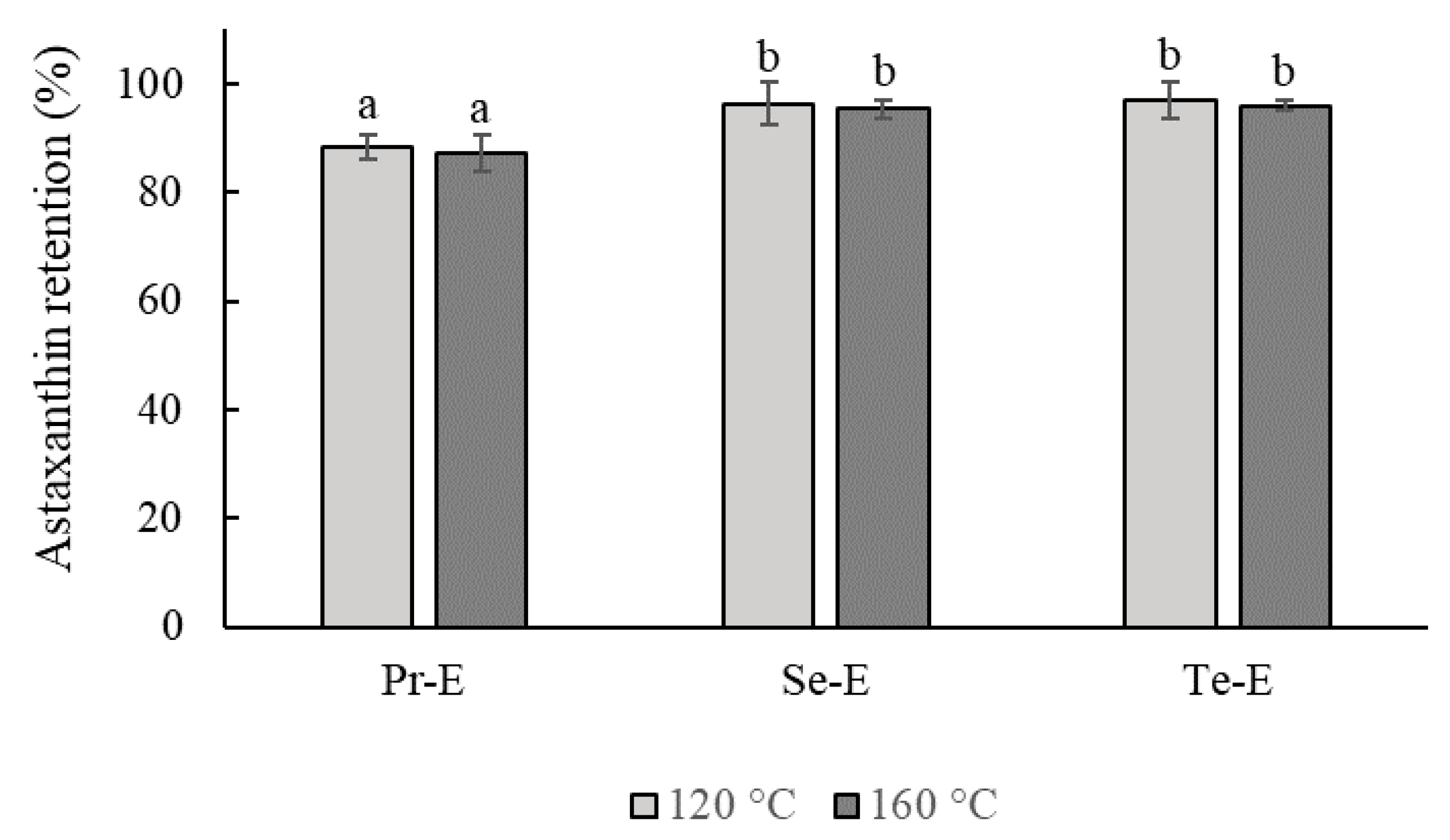
3.5. The Retention of Astaxanthin in the Emulsions during the Spray-Drying Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merefati, A.; Rayner, M.; Timgren, A.; Dejmek, P.; Sjöö, M. Freezing and freeze-drying of pickering emulsions stabilized by starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 512–520. [Google Scholar] [CrossRef]
- Amr, M.B.; Shabbar, A.; Barkat, A.; Hamid, M.; Mohamed, Y.A.; Ahmed, M. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. 2016, 15, 143–182. [Google Scholar]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Int. Food Res. 2020, 134, 109244. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Int. Food Res. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ma, S.; Yang, Z.; Zhou, W.; Du, Z.; Huang, J.; Wang, C. Facile fabrication of poly (L-lactic acid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates. Colloids Surf. B 2016, 140, 382–391. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Gallardo, M.; Morales, E.; Piornos, J.A.; Marqués, A.M.; Rubilar, M. Utilization of proteins from AluProt-CGNA (a novel protein-rich lupin variety) in the development of oil-in-water multilayer emulsion systems. Eur. J. Lipid Sci. Tech. 2016, 118, 1104–1112. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Tavernier, I.; Patel, A.R.; Meeren, P.V.D.; Dewettinck, K. Emulsion-templated liquid oil structuring with soy protein and soy protein: Kappa-carrageenan complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.J.; Adhikari, B. The effect of addition of flaxseed gum on the emulsion properties of soybean protein isolate (SPI). J. Food Eng. 2011, 104, 56–62. [Google Scholar] [CrossRef]
- Xiang, N.; Lyu, Y.; Narsimhan, G. Characterization of fish oil-in-water emulsion produced by layer by layer deposition of soy β-conglycinin and high methoxyl pectin. Food Hydrocoll. 2016, 52, 678–689. [Google Scholar]
- Zhang, C.; Xu, W.; Jin, W.; Shah, B.; Li, Y.; Li, B. Influence of anionic alginate and cationic chitosan on physicochemical stability and carotenoids bioaccessibility of soy protein isolate-stabilized emulsions. Int. Food Res. 2015, 77, 419–425. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Hernández, X.; Wandersleben, T.; Barahona, T.; Medina, C.; Quiroz, A.; Rubilar, M. Influence of multilayer O/W emulsions stabilized by proteins from a novel lupin variety AluProt-CGNA and ionic polysaccharides on d-limonene retention during spray-drying. Colloids Surf. A 2018, 536, 234–241. [Google Scholar] [CrossRef]
- Piornos, J.A.; Burgos-Díaz, C.; Ogura, T.; Morales, E.; Rubilar, M.; Maureira-Butler, I.; Salvo-Garrido, H. Functional and physicochemical properties of a protein isolate from AluProt-CGNA: A novel protein-rich lupin variety (Lupinus luteus). Int. Food Res. 2015, 76, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, F.K.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Xu, Y.; McClements, D.J.; Wang, D. Formation, characterization, and application of chitosan/pectin-stabilized multilayer emulsions as astaxanthin delivery systems. Int. J. Biol. Macromol. 2019, 140, 985–997. [Google Scholar] [CrossRef]
- Wang, D.; Mao, L.; Dai, L.; Yuan, F.; Gao, Y. Characterization of chitosan-ferulic acid conjugates and their application in the design of b-carotene bilayer emulsions with propylene glycol alginate. Food Hydrocoll. 2018, 80, 281–291. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.L.; Medeiros, B.S.; da Silva, L.H.M.; da Silva, M.C.H. Carneiro-da-Cunha, M.G.; Coimbra, M.A.; Vicente, A.A. Interactions between κ-carrageenan and chitosan in nanolayered coatings-Structural and transport properties. Carbohydr. Polym. 2012, 87, 1081–1090. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Moi, S.; Ravi, S.; Gokare, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs. 2014, 12, 128–152. [Google Scholar]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT Food Sci. Technol. 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Wandersleben, T.; Olivos, M.; Lichtin, N.; Bustamante, M.; Solans, C. Food-grade Pickering stabilizers obtained from a protein-rich lupin cultivar (AluProt-CGNA®): Chemical characterization and emulsifying properties. Food Hydrocoll. 2019, 87, 847–857. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Int. Food Res. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.; Fainerman, V.B.; Makievski, A.V.; Krägel, J.; Grigoriev, D.O.; Kazakov, V.N.; Sinyachenko, O.V. Dynamics of protein and mixed protein/surfactant adsorption layers at the water/fluid interface. Adv. Colloid Interface Sci. 2000, 86, 39–82. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Wu, D.; Xu, X.; Wu, C.; Du, M. Physicochemical properties and oil/water interfacial adsorption behavior of cod proteins as affected by high-pressure homogenization. Food Hydrocoll. 2020, 100, 105429. [Google Scholar] [CrossRef]
- Primozic, M.; Duchek, A.; Nickerson, M.; Ghosh, S. Formation, stability, and in vitro digestibility of nanoemulsions stabilized by high-pressure homogenized lentil proteins isolate. Food Hydrocoll. 2018, 77, 126–141. [Google Scholar] [CrossRef]
- Vergara, D.; Shene, C. Encapsulation of lactoferrin into rapeseed phospholipids based liposomes: Optimization and physicochemical characterization. J. Food Eng. 2019, 262, 29–38. [Google Scholar] [CrossRef]
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Julio, L.M.; Copado, C.N.; Diehl, B.W.K.; Vanesa, Y.; Ixtaina, V.Y.; Tomás, M.C. Chia bilayer emulsions with modified sunflower lecithins and chitosan as delivery systems of omega-3 fatty acids. LWT Food Sci. Technol. 2018, 89, 581–590. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating multilayer emulsions by using OSA starch and chitosan suitable for spray drying: Application in the encapsulation of β-carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Shen, Q.; Quek, S.Y. Microencapsulation of astaxanthin with blends of milk protein and fiber by spray drying. J. Food Eng. 2014, 123, 165–171. [Google Scholar] [CrossRef]
- Gu, Y.; Regnier, L.; McClements, D. Influence of environmental stresses on stability of oil-in-water emulsions containing droplets stabilized by beta-lactoglobulin-iota-carrageenan membranes. Adv. Colloid Interface Sci. 2005, 286, 551–558. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, E.; Burgos-Díaz, C.; Zúñiga, R.N.; Jorkowski, J.; Quilaqueo, M.; Rubilar, M. Effect of Interfacial Ionic Layers on the Food-Grade O/W Emulsion Physical Stability and Astaxanthin Retention during Spray-Drying. Foods 2021, 10, 312. https://doi.org/10.3390/foods10020312
Morales E, Burgos-Díaz C, Zúñiga RN, Jorkowski J, Quilaqueo M, Rubilar M. Effect of Interfacial Ionic Layers on the Food-Grade O/W Emulsion Physical Stability and Astaxanthin Retention during Spray-Drying. Foods. 2021; 10(2):312. https://doi.org/10.3390/foods10020312
Chicago/Turabian StyleMorales, Eduardo, César Burgos-Díaz, Rommy N. Zúñiga, Johanna Jorkowski, Marcela Quilaqueo, and Mónica Rubilar. 2021. "Effect of Interfacial Ionic Layers on the Food-Grade O/W Emulsion Physical Stability and Astaxanthin Retention during Spray-Drying" Foods 10, no. 2: 312. https://doi.org/10.3390/foods10020312






