Impact of the Simulated Gastric Digestion Methodology on the In Vitro Intestinal Proteolysis and Lipolysis of Emulsion Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Emulsion Gels
2.2. Characterization of Particle Size of the Emulsions
2.3. Textural Characterization of the Emulsion Gels
2.4. In Vitro Digestion Assays of Emulsion Gels
2.4.1. Preparation of Simulated Digestion Fluids
2.4.2. In Vitro Oral Digestion
2.4.3. In Vitro Gastric Digestion
2.4.4. In Vitro Intestinal Digestion
2.5. Quantification of the Intestinal Proteolysis and Lipolysis of Emulsion Gels
2.5.1. Intestinal Proteolysis
2.5.2. Intestinal Lipolysis
2.6. Statistical Analysis of Data
3. Results and Discussion
3.1. Characterization of Emulsions and Emulsion Gels
3.2. In Vitro Digestibility of Emulsion Gels
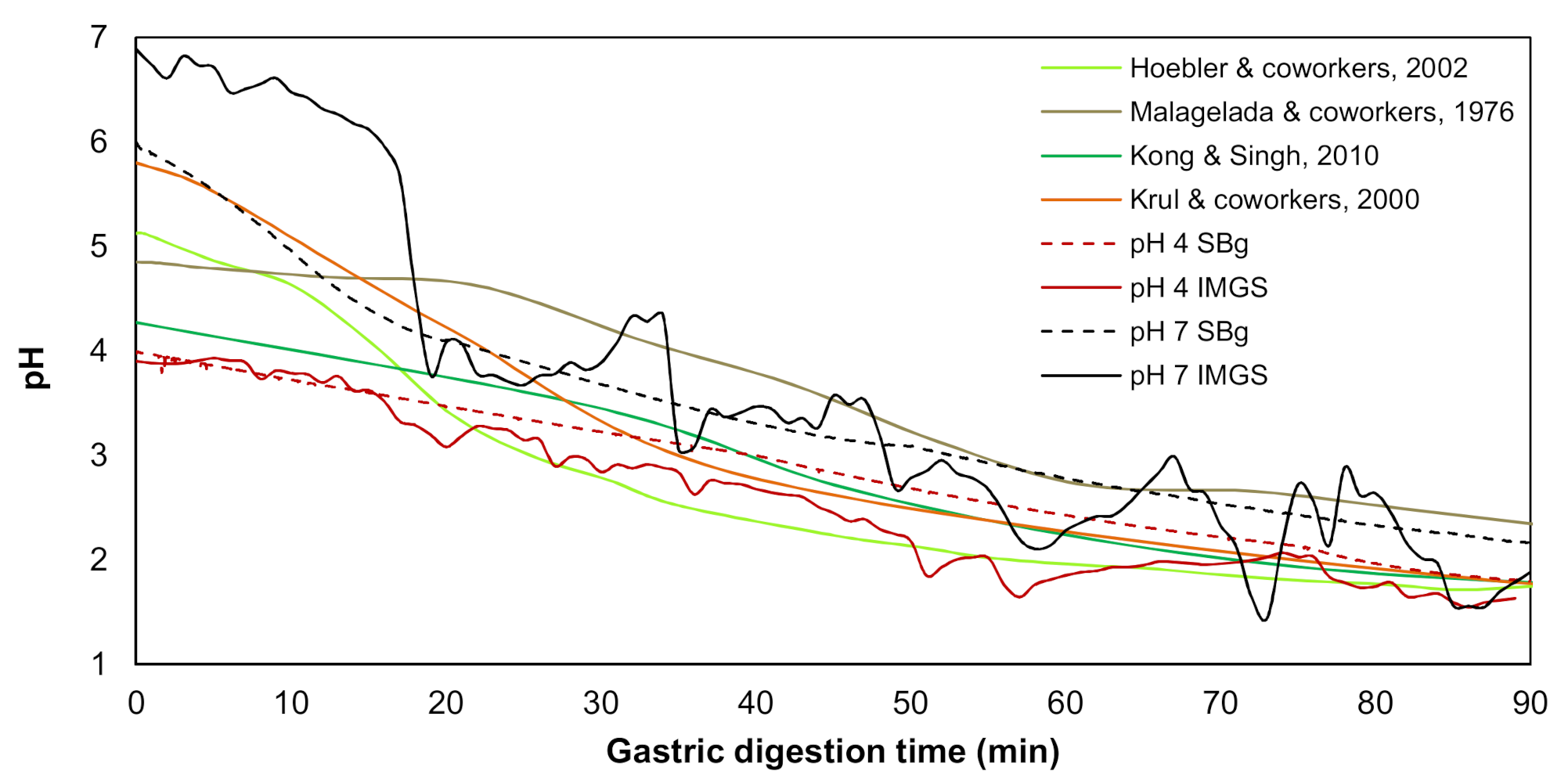
3.2.1. Gastric pH Curves
3.2.2. Impact of the Type of In Vitro Gastric Digestion of Emulsion Gels on the Degree of Intestinal Proteolysis
3.2.3. Influence of the pH of the Emulsion Gels on Intestinal Proteolysis
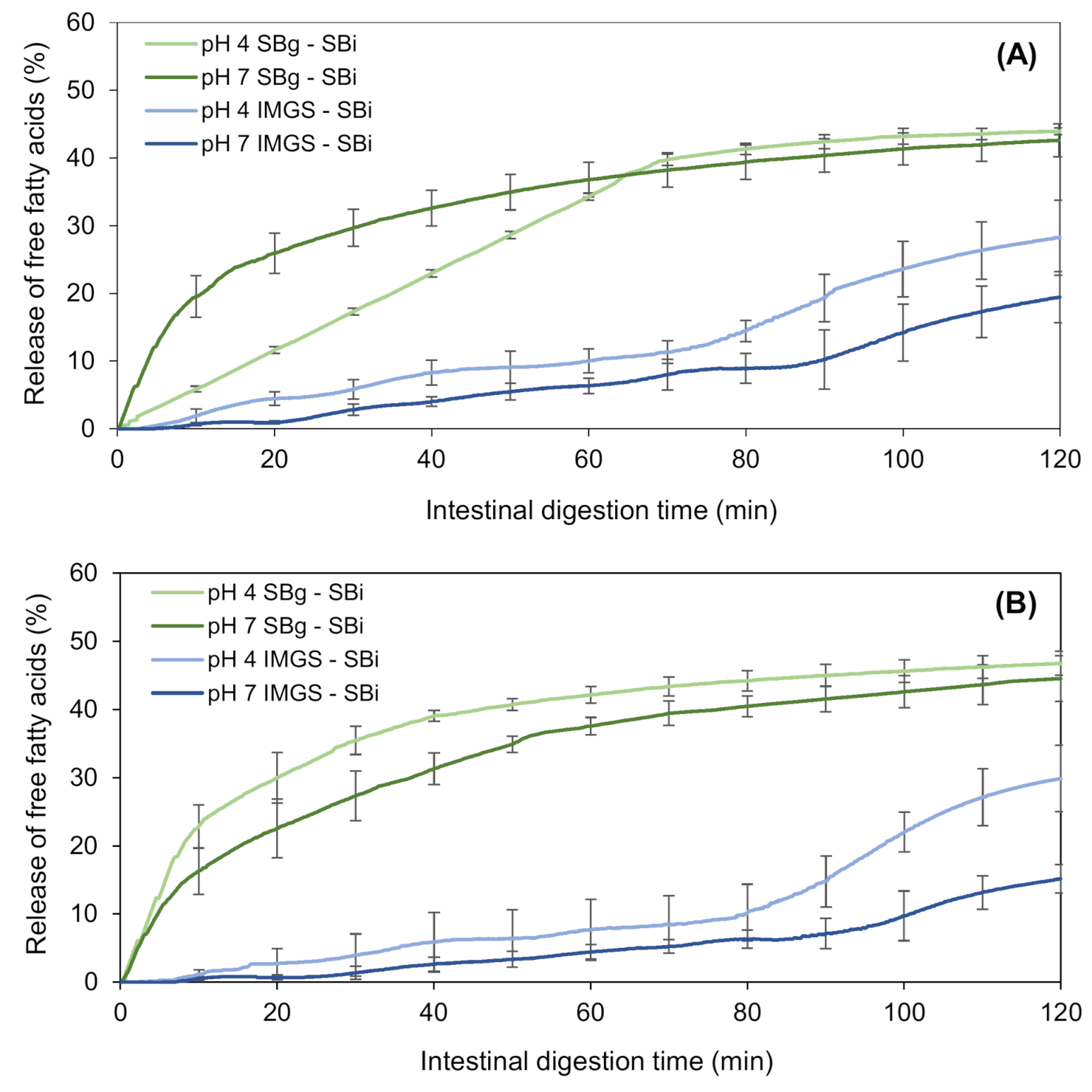
3.2.4. Effect of the Type of In Vitro Gastric Digestion of Emulsion Gels on the Intestinal Lipolysis
3.2.5. Influence of pH of Emulsion Gels on Lipid Digestion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Future foods: A manifesto for research priorities in structural design of foods. Food Funct. 2020, 11, 1933–1945. [Google Scholar] [CrossRef]
- Singh, H.; Gallier, S. Processing of food structures in the gastrointestinal tract and physiological responses. In Food Structures, Digestion and Health; Boland, M., Golding, M., Singh, H., Eds.; Elsevier Inc.: London, UK, 2014; pp. 51–75. [Google Scholar]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef] [Green Version]
- Ménard, O.; Cattenoz, T.; Guillemin, H.; Souchon, I.; Deglaire, A.; Dupont, D.; Picque, D. Validation of a new in vitro dynamic system to simulate infant digestion. Food Chem. 2014, 145, 1039–1045. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. A human gastric simulator (HGS) to study food digestion in human stomach. J. Food Sci. 2010, 75, E627–E635. [Google Scholar] [CrossRef]
- Minekus, M. The TNO gastro-intestinal model (TIM). In The Impact of Food Bioactives and Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing AG: Cham, Switzerland, 2015; pp. 37–45. [Google Scholar]
- Venema, K.; Havenaar, R.; Minekus, M. Improving in vitro simulation of the stomach and intestines. In Designing Functional Foods: Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; McClements, D.J., Decker, E.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 314–339. [Google Scholar]
- Verwei, M.; Minekus, M.; Zeijdner, E.; Schilderink, R.; Havenaar, R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccesibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016, 498, 178–186. [Google Scholar] [CrossRef]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The design, operation, and application of a Dynamic Gastric Model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Kozu, H.; Nakata, Y.; Nakajima, M.; Neves, M.; Uemura, K.; Sato, S.; Kobayashi, I.; Ichikawa, S. Development of a human gastric digestion simulator equipped with peristalsis function for the direct observation and analysis of the food digestion process. Food Sci. Technol. Res. 2014, 20, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xu, Y.; Fan, T.; Liao, Z.; Wu, P.; Wu, X.; Chen, X.D. Gastric emptying and morphology of a “near real” in vitro human stomach model (RD-IV-HSM). J. Food Eng. 2016, 183, 1–8. [Google Scholar] [CrossRef]
- van Aken, G. Relating food emulsion structure and composition to the way it is processed in the gastrointestinal tract and physiological responses: What are the opportunities? Food Biophys. 2010, 5, 258–283. [Google Scholar] [CrossRef]
- Barros, L.; Retamal, C.; Torres, H.; Zúñiga, R.; Troncoso, E. Development of an in vitro mechanical gastric system (IMGS) with realistic peristalsis to assess lipid digestibility. Food Res. Int. 2016, 90, 216–225. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplet. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Sala, G.; van de Velde, F.; Cohen Stuart, M.A.; van Aken, G.A. Oil droplet release from emulsion-filled gels in relation to sensory perception. Food Hydrocoll. 2007, 21, 977–985. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Fraser, R.J.; Bryant, L.K.; Holloway, R.H. Functional association between proximal and distal gastric motility during fasting and duodenal nutrient stimulation in humans. Neurogastroenterol. Motil. 2007, 19, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Borel, P.; Pasquier, B.; Dubois, C.; Senft, M.; Andre, M.; Peyrot, J.; Salducci, J.; Lairon, D. Physicochemical characteristics of emulsions during fat digestion in human stomach and duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 1996, 271, G172–G183. [Google Scholar] [CrossRef]
- Armand, M. Lipases and lipolysis in the human digestive tract: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 156–164. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Singh, R.P. Gastric digestion in vivo and in vitro: How the structural aspects of food influence the digestion process. Annu Rev. Food Sci Technol. 2014, 5, 111–132. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. Effect of gel structure on the gastric digestion of whey protein emulsion gels. Soft Matter 2014, 10, 1214–1223. [Google Scholar] [CrossRef]
- Mat, D.J.L.; Souchon, I.; Michon, C.; Le Feunteun, S. Gastro-intestinal in vitro digestions of protein emulsions monitored by pHstat: Influence of structural properties and interplay between proteolysis and lipolysis. Food Chem. 2020, 311, 125946. [Google Scholar] [CrossRef]
- Gosal, W.S.; Ross-Murphy, S.B. Globular protein gelation. Curr. Opin. Colloid Interface Sci. 2000, 5, 188–194. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Rademacher, B. Protein gels. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; pp. 468–482. [Google Scholar]
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Civille, G.V.; Szczesniak, A.S. Guidelines to training a texture profile panel. J. Texture Stud. 1973, 4, 204–223. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Kulozik, U.; Aguilera, J.M. Ultrasonic generation of aerated gelatin gels stabilized by whey protein β-lactoglobulin. Food Hydrocoll. 2011, 25, 958–967. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriére, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standarised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.; Cotter, P.; López, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives and Health: In Vitro and Ex Vivo Models; Springer International Publishing AG: Cham, Switzerland, 2015. [Google Scholar]
- Arancibia, C.; Miranda, M.; Matiacevich, S.; Troncoso, E. Physical properties and lipid bioavailability of nanoemulsion-based matrices with different thickening agents. Food Hydrocoll. 2017, 73, 243–254. [Google Scholar] [CrossRef]
- Riquelme, N.; Robert, P.; Troncoso, E.; Arancibia, C. Influence of the particle size and hydrocolloid type on lipid digestion of thickened emulsions. Food Funct. 2020, 1, 5955–5964. [Google Scholar] [CrossRef]
- Hoebler, C.; Lecannu, G.; Belleville, C.; Devauz, M.-F.; Popineau, Y.; Barry, J.-L. Development of an in vitro system simulating bucco-gastric digestion to assess the physical and chemical changes of food. Int. J. Food Sci. Nutr. 2002, 53, 389–402. [Google Scholar] [CrossRef]
- Malagelada, J.R.; Longstreth, G.F.; Summerskill, W.H.J.; Go, V.L.W. Measurement of gastric functions during digestion of ordinary solid meals in man. Gastroenterology 1976, 70, 203–210. [Google Scholar] [CrossRef]
- Krul, C.; Luiten-Schuite, A.; Baan, R.; Verhagen, H.; Mohn, G.; Feron, V.; Havenaar, R. Application of a dynamic in vitro gastrointestinal tract model to study the availability of food mutagens, using heterocyclic aromatic amines as model compounds. Food Chem. Toxicol. 2000, 38, 783–792. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Van de Kamp, E.; Rompelberg, C.J.M. Development of an In Vitro Digestion Model to Determine the Bioaccessibility of Contaminants from Food. RIVM Report 320102002/2004. National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2004. Available online: http:/www.rivm.nl/en/ (accessed on 2 February 2021).
- Marciani, L.; Gowland, P.A.; Fillery-Travis, A.; Manoj, P.; Wright, J.; Smith, A.; Young, P.; Moore, R.; Spiller, R.C. Assessment of antral grinding of a model solid meal with echo-planar imaging. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G844–G849. [Google Scholar] [CrossRef] [Green Version]
- Schulze, K. Imaging and modeling of digestion in the stomach and the duodenum. Neurogastroenterol. Motil. 2006, 18, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Singh, R.P. Disintegration of solid foods in human stomach. J. Food Sci. 2008, 73, R67–R80. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.J.; Camilleri, M.; Prather, C.M.; Hanson, R.B.; Thomforde, G.M. Measurement of axial forces during emptying from the human stomach. Am. J. Physiol. Gastrointest. Liver Physiol. 1992, 263, G230–G239. [Google Scholar] [CrossRef] [PubMed]
- Elmslie, R.G.; White, T.T.; Magee, D.F. Observation on pancreatic function in eight patients with controlled pancreatic fistulas including a review of the literature. Ann. Surg. 1964, 160, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Perez de la Cruz Moreno, M.; Oth, M.; Deferme, S.; Lammert, F.; Tack, J.; Dressman, J.; Augustijns, P. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J. Pharm. Pharmacol. 2006, 58, 1079–1089. [Google Scholar] [CrossRef]
- Ulleberg, E.K.; Comi, I.; Holm, H.; Herud, E.B.; Jacobsen, M.; Vegarud, G.E. Human gastrointestinal juices intended for use in in vitro digestion models. Food Dig. 2011, 2, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Mat, D.J.L.; Le Feunteun, S.; Michon, C.; Souchon, I. In vitro digestion of foods using pH-stat and the INFOGEST protocol: Impact of matrix structure on digestion kinetics of macronutrients, proteins and lipids. Food Res. Int. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: London, UK, 1986; pp. 365–404. [Google Scholar]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Márquez, M.C.; Vázquez, M.A. Modeling of enzymatic protein hydrolysis. Process. Biochem. 1999, 35, 111–117. [Google Scholar] [CrossRef]
- Cheng, Q.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hidrocoll. 2011, 25, 1000–1008. [Google Scholar]
- Berton-Carabin, C.C.; Sagis, L.; Schroën, K. Formation, structure, and functionality of interfacial layers in food emulsions. Annu Rev. Food Sci. Technol. 2018, 9, 551–587. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Food emulsions-Their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- McClements, D. Food Emulsions: Principles, Practices and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 245–372. [Google Scholar]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Malcata, F.X.; Vicente, A.A. Design of whey protein nanostructures for incorporation and reléase of nutraceutical compounds in foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.N.; Zhong, Q.; Foegeding, E.A. Use of whey protein soluble aggregates for thermal stability-A hypothesis paper. J. Food Sci. 2013, 78, R1105–R1115. [Google Scholar] [CrossRef]
- Tang, Q.; McCarthy, O.J.; Munro, P.A. Effect of pH on whey protein concentrate gel properties: Comparisons between small deformation dynamic) and large deformation (failure) testing. J. Texture Stud. 1995, 26, 255–272. [Google Scholar] [CrossRef]
- Langton, M.; Hermansson, A.M. Fine-stranded and particulate gels of β-lactoglobulin and whey protein at varying pH. Food Hydrocoll. 1992, 5, 523–539. [Google Scholar] [CrossRef]
- Bansal, N.; Drake, M.A.; Piraino, P.; Broe, M.L.; Harboe, M.; Fox, P.F.; McSweeney, P.L.H. Suitability of recombinant camel (Camelus dromedarius) chymosin as a coagulant for Cheddar cheese. Int. Dairy J. 2009, 19, 510–517. [Google Scholar] [CrossRef]
- Dangin, M.; Boirie, Y.; Guillet, C.; Beaufreère, B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J. Nutr. 2002, 132, 3228S–3233S. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, O.A.; Zapata, J.E.; Gutiérrez, G.A. Modeling of the kinetics of enzymatic hydrolysis of bovine plasma proteins. Revista EIA 2012, 17, 71–84. [Google Scholar]
- Qi, W.; He, Z. Enzymatic hydrolysis of protein: Mechanism and kinetic model. Front. Chem. China 2006, 3, 308–314. [Google Scholar] [CrossRef]
- González-Tello, P.; Camacho, F.; Jurado, E.; Páez, M.P.; Guadix, E.M. Enzymatic hydrolysis of whey proteins. I. Kinetic model. Biotechnol. Bioeng. 1994, 44, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Ye, A.; Lad, M.; Ferrua, M.; Dalgleish, D.; Singh, H. Disintegation kinetics of food gels during gastric digestion and its role on gastric emptying: And in vitro analysis. Food Funct. 2015, 6, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Vardhanabhuti, B. Effect of initial protein concentration and pH on in vitro gastric digestion of heated whey proteins. Food Chem. 2014, 145, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Lundin, L.; Golding, M.; Wooster, T.J. Understanding food structure and function in developing food for appetite control. Nutr. Diet. 2008, 65, S79–S85. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Lad, M.; Dalgleish, D.; Singh, H. Impact of colloidal structure of gastric digesta on in-vitro intestinal digestion of whey protein emulsion gels. Food Hydrocoll. 2016, 54, 255–265. [Google Scholar] [CrossRef]
- Ye, Z.; Li, R.; Cao, C.; Xu, Y.J.; Cao, P.; Li, Q.; Liu, Y. Fatty acid profiles of typical dietary lipids after gastrointestinal digestion and absorbtion: A combination study between in-vitro and in-vivo. Food. Chem. 2019, 280, 34–44. [Google Scholar] [CrossRef]
- Ye, A.; Wang, X.; Lin, Q.; Han, J.; Singh, H. Dynamic gastric stability and in vitro lipid digestion of whey-protein-stabilised emulsions: Effect of heat treatment. Food Chem. 2020, 318, 126463. [Google Scholar] [CrossRef]





| Constituent | Stock Concentration (mol/L) | Concentration in Simulated Salivary Fluid, SSF (mmol/L) | Concentration in Simulated Gastric Fluid, SGF (mmol/L) | Concentration in Simulated Intestinal Fluid, SIF (mmol/L) |
|---|---|---|---|---|
| KCl | 0.5 | 15.1 | 6.9 | 6.8 |
| KH2PO4 | 0.5 | 3.7 | 0.9 | 0.8 |
| NaHCO3 | 1.0 | 13.6 | 25.0 | 85.0 |
| NaCl | 2.0 | - | 47.2 | 38.4 |
| MgCl2(H2O)6 | 0.15 | 0.15 | 0.12 | 0.33 |
| (NH4)2CO3 | 0.5 | 0.06 | 0.5 | - |
| HCl | 6.0 | 1.1 | 15.6 | 8.4 |
| pH | Homogenization Pressure (bar) | Mean Oil Droplet Diameter (nm) | Pdi |
|---|---|---|---|
| 4 | 500 | 327.8 ± 22.9 aA | 0.20 ± 0.04 aA |
| 1000 | 301.8 ± 7.1 aA | 0.18 ± 0.02 aA | |
| 7 | 500 | 260.4 ± 21.7 bA | 0.10 ± 0.03 bA |
| 1000 | 270.8 ± 0.7 bA | 0.17 ± 0.01 bA |
| pH | Pressure (bar) | Texture Profile Analysis (TPA) | Compression Analysis | |||
|---|---|---|---|---|---|---|
| Hardness (N) | Cohesiveness (Dimensionless) | Chewiness (N) | Stress at Break (kPa) | Strain at Break (Dimensionless) | ||
| 4 | 500 | 10.81 ± 0.55 aA | 0.44 ± 0.01 aA | 4.57 ± 0.10 aA | 21.70 ± 0.83 aA | 0.21 ± 0.04 aA |
| 1000 | 8.97 ± 0.34 aA | 0.46 ± 0.02 aA | 3.77 ± 0.53 aB | 23.43 ± 1.49 aB | 0.24 ± 0.01 aA | |
| 7 | 500 | 11.31 ± 0.12 bA | 0.89 ± 0.02 bA | 8.52 ± 0.38 bA | 76.21 ± 2.34 bA | 0.75 ± 0.12 bA |
| 1000 | 13.27 ± 0.28 bA | 0.86 ± 0.01 bA | 7.40 ± 0.40 bB | 94.96 ± 2.51 bB | 0.76 ± 0.21 bA | |
| Fabrication Conditions of the Emulsion Gels | Methodology of In Vitro Digestion (Gastric–Intestinal) | Final Extent of Intestinal Digestion | ||
|---|---|---|---|---|
| pH | Pressure (bar) | Lipolysis (Free Fatty Acids Released %) | Proteolysis (Protein Hydrolysis %) | |
| 4.0 | 500 | SBg–SBi | 43.92 ± 0.48 a,A | 3.01 ± 0.22 b,A |
| 1000 | 46.74 ± 1.74 a,A | 4.78 ± 0.36 b,A | ||
| 7.0 | 500 | 42.59 ± 2.43 a,A | 2.39 ± 0.19 a,B | |
| 1000 | 44.51 ± 3.35 a,A | 3.93 ± 0.30 a,B | ||
| 4.0 | 500 | IMGS–SBi | 28.24 ± 5.53 b,A | 6.97 ± 0.60 a,A |
| 1000 | 29.85 ± 4.87 b,A | 13.43 ± 0.83 a,B | ||
| 7.0 | 500 | 19.41 ± 3.76 a,A | 14.95 ± 1.29 b,A | |
| 1000 | 15.16 ± 2.09 a,A | 21.54 ± 0.26 b,B | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mella, C.; Quilaqueo, M.; Zúñiga, R.N.; Troncoso, E. Impact of the Simulated Gastric Digestion Methodology on the In Vitro Intestinal Proteolysis and Lipolysis of Emulsion Gels. Foods 2021, 10, 321. https://doi.org/10.3390/foods10020321
Mella C, Quilaqueo M, Zúñiga RN, Troncoso E. Impact of the Simulated Gastric Digestion Methodology on the In Vitro Intestinal Proteolysis and Lipolysis of Emulsion Gels. Foods. 2021; 10(2):321. https://doi.org/10.3390/foods10020321
Chicago/Turabian StyleMella, Camila, Michelle Quilaqueo, Rommy N. Zúñiga, and Elizabeth Troncoso. 2021. "Impact of the Simulated Gastric Digestion Methodology on the In Vitro Intestinal Proteolysis and Lipolysis of Emulsion Gels" Foods 10, no. 2: 321. https://doi.org/10.3390/foods10020321
APA StyleMella, C., Quilaqueo, M., Zúñiga, R. N., & Troncoso, E. (2021). Impact of the Simulated Gastric Digestion Methodology on the In Vitro Intestinal Proteolysis and Lipolysis of Emulsion Gels. Foods, 10(2), 321. https://doi.org/10.3390/foods10020321