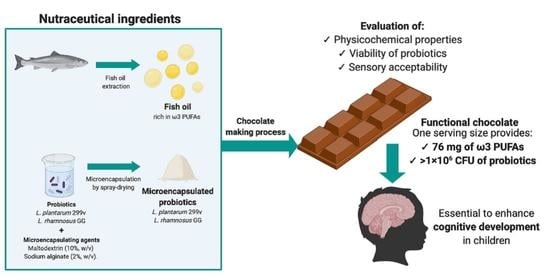
Physicochemical Properties and Sensory Acceptability of a Next-Generation Functional Chocolate Added with Omega-3 Polyunsaturated Fatty Acids and Probiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Active Ingredients
2.2. Probiotic Microencapsulation and Viability Determination
2.3. Milk Chocolate Production and Determination of Probiotics Viability
2.4. Fatty Acid Profile
2.5. Rheological Analysis
2.6. Texture Analysis, Surface Color, and Water Activity Determinations
2.7. Sensory Acceptability Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Viability of Probiotics during Microencapsulation and Chocolate-Making Process
3.2. Fatty Acids Profile
3.3. Rheological Analysis
3.4. Surface Color, Water Activity, and Texture Analysis
3.5. Sensory Acceptability Test of Chocolates Added with Fish Oil and Probiotics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. A practical guide for designing effective nutraceutical combinations in the form of foods, beverages, and dietary supplements against chronic degenerative diseases. Trends Food Sci. Technol. 2019, 88, 179–193. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Santana-Gálvez, J.; Cisneros-Zevallos, L. Designing next-generation functional food and beverages: Combining nonthermal processing technologies and postharvest abiotic stresses. Food Eng. Rev. 2020, in press. [Google Scholar]
- Fernandes, S.S.; Coelho, M.S.; de las Mercedes Salas-Mellado, M. Bioactive compounds as ingredients of functional foods: Polyphenols, carotenoids, peptides from animal and plant sources new. In Bioactive Compounds: Health Benefits and Potential Applications, 1st ed.; Segura-Campos, M.R., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 129–142. [Google Scholar]
- Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, prebiotic, and brain development. Nutrients 2017, 9, 1247. [Google Scholar] [CrossRef]
- Spencer, S.J.; Korosi, A.; Layé, S.; Shukitt-Hale, B.; Barrientos, R.M. Food for thought: How nutrition impacts cognition and emotion. npj Sci. Food 2017, 1, 7. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Impact of fermented foods on human cognitive function—A review of outcome of clinical trials. Sci. Pharm. 2018, 86, 22. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Shen, Y.-E.; Wang, X.; Fu, S.; Zhang, X.; Zhang, Y.-N.; Wang, R.-T. Concomitant memantine and Lactobacillus plantarum treatment attenuates cognitive impairments in APP/PS1 mice. Aging (Albany NY) 2020, 12, 628–649. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rianda, D.; Agustina, R.; Setiawan, E.A.; Manikam, N.R.M. Effect of probiotic supplementation on cognitive function in children and adolescents: A systematic review of randomized trials. Benef. Microbes 2019, 10, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Ackar, D.; Valek Lendić, K.; Valek, M.; Šubarić, D.; Miličević, B.; Babić, J.; Nedi, I. Cocoa polyphenols: Can we consider cocoa and chocolate as potential functional food? J. Chem. 2013, 289392. [Google Scholar] [CrossRef]
- Annunziata, A.; Vecchio, R.; Kraus, A. Factors affecting parents’ choices of functional foods targeted for children. Int. J. Consum. Stud. 2016, 40, 527–535. [Google Scholar] [CrossRef]
- Konar, N.; Toker, O.S.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, İ.; Polyrazoglu, E.S.; Sagdic, O. Enrichment of milk chocolate by using EPA and DHA originated from various origins: Effects on product quality. Sugar Tech. 2018, 20, 745–755. [Google Scholar] [CrossRef]
- Foong, Y.J.; Lee, S.T.; Ramli, N.; Tan, Y.N.; Ayob, M.K. Incorporation of potential probiotic Lactobacillus plantarum isolated from fermented cocoa beans into dark chocolate: Bacterial viability and physicochemical properties analysis. J. Food Qual. 2013, 36, 164–171. [Google Scholar] [CrossRef]
- Tan, J.; Kerr, W. Determination of chocolate melting properties by capacitance based thermal analysis (CTA). J. Food Meas. Charact. 2018, 12, 641–649. [Google Scholar] [CrossRef]
- Gonçalves, E.V.; da Silva Lannes, S.C. Chocolate rheology. Food Sci. Technol. 2010, 30, 845–851. [Google Scholar]
- AOAC. AOAC official method 948.22, Soxhlet fat extraction method. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Rockville, MD, USA, 1995. [Google Scholar]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [CrossRef]
- Castillo, E.C.; Elizondo-Montemayor, L.; Hernández-Brenes, C.; Rodríguez-Sánchez, D.G.; Silva-Platas, C.; Marín-Obispo, L.M.; Rodríguez-Gutierrez, N.A.; Treviño, V.; García-Rivas, G. Integrative analysis of lipid profiles in plasma allows cardiometabolic risk factor clustering in children with metabolically unhealthy obesity. Oxidative Med. Cell. Longev. 2020, 2020, 2935278. [Google Scholar] [CrossRef]
- AOAC. AOAC official method 996.06, fat (total, saturated, and unsaturated) in foods. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000; pp. 20–24. [Google Scholar]
- Kiumarsi, M.; Rafe, A.; Yeganehzad, S. Effect of different bulk sweeteners on the dynamic oscillatory and shear rheology of chocolate. Appl. Rheol. 2017, 27, 1–9. [Google Scholar]
- Secretaría de Gobierno (SEGOB). Norma Oficial Mexicana NOM-186-SSA1/SCFI-2013, Cacao, Chocolate y Productos Similares, y Derivados del Cacao. 2014. Available online: https://www.dof.gob.mx/nota_detalle.php?Codigo=5332832&fecha=17/02/2014 (accessed on 22 November 2020).
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Chen, K.N.; Chen, M.J.; Liu, J.R.; Lin, C.W.; Chiu, H.Y. Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J. Food Sci. 2005, 70, 260–266. [Google Scholar] [CrossRef]
- Tárrega, A.; Rocafull, A.; Costell, E. Effect of blends of short and long-chain inulin on the rheological and sensory properties of prebiotic low-fat custards. LWT Food Sci. Technol. 2010, 43, 556–562. [Google Scholar] [CrossRef]
- Chakraborty, R.; Ganguly, R.; Hore, P.; Nath, S.; Sarkar, K. Maltodextrin: A prebiotic of choice for Lactobacillus plantarum, but not for Lactobacillus casei in combination with antibiotics. Int. J. Probiotics Prebiotics 2018, 13, 19–24. [Google Scholar]
- Hernández-Carranza, P.; López-Malo, A.; Jiménez, T. Microencapsulation quality and efficiency of Lactobacillus casei by spray drying using maltodextrin and vegetable extracts. J. Food Res. 2013, 3, 61–69. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Louesdon, S.; Charlot-Rougé, S.; Tourdot-Maréchal, R.; Bouix, M.; Béal, C. Membrane fatty acid composition and fluidity are involved in the resistance to freezing of Lactobacillus buchneri R1102 and Bifidobacterium longum R0175. Microb. Biotechnol. 2015, 8, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Sinha, P.R. Production of free fatty acids and conjugated linoleic acid in probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei during fermentation and storage. Int. Dairy J. 2007, 17, 1006–1010. [Google Scholar] [CrossRef]
- Collins, Y.F.; McSweeney, P.L.H.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 polyunsaturated fatty acids: Benefits and endpoints in sport. Nutrients 2019, 11, 46. [Google Scholar] [CrossRef]
- Kankaanpää, P.; Yang, B.; Kallio, H.; Isolauri, E.; Salminen, S. Effects of polyunsaturated fatty acids in growth medium on lipid composition and on physicochemical surface properties of lactobacilli. Appl. Environ. Microbiol. 2004, 70, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Shama, S.; Liu, W. Omega-3 fatty acids and gut microbiota: A reciprocal interaction in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2020, 65, 906–910. [Google Scholar] [CrossRef]
- Sumarno, L.; Mangunwidjaja, D.; Fauzi, A.; Syamsu, K.; Indrasti, N.S.; Prasetya, B. Production of omega 6 probiotic by isolation and fermentation of Lactobacillus plantarum JR64. Int. J. Waste Resour. 2012, 2, 11–16. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Gu, Z.; Tian, F.; Ross, R.P.; Stanton, C.; Chen, W.; Zhang, H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 2014, 117, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). GLOBEFISH, a Simple Overview of Omega-3. Available online: http://www.fao.org/in-action/globefish/fishery-information/resource-detail/en/c/1052098/ (accessed on 22 November 2020).
- Keijbets, E.; Chen, J.; Vieira, J. Chocolate demoulding and effects of processing conditions. J. Food Eng. 2010, 98, 133–140. [Google Scholar] [CrossRef]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of marine omega-3 supplements and human health. BioMed Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O. Chocolate Science and Technology, 1st ed.; Wiley-Blackwell Publishers: Oxford, UK, 2016; pp. 54–101. [Google Scholar]
- Glicerina, V.; Balestra, F.; Dalla Rosa, M.; Romani, S. Effect of manufacturing process on the microstructural and rheological properties of milk chocolate. J. Food Eng. 2015, 145, 45–50. [Google Scholar] [CrossRef]
- Taghizadeh, G.; Jahadi, M.; Abbasi, H. Physicochemical properties of probiotic soy milk chocolate mousse during refrigerated storage. Appl. Food Biotechnol. 2018, 5, 79–86. [Google Scholar]
- Hussain, N.; Agus, B.A.P.; Rahim, S.N.F.; Halim, H.S.A. Comparison of quality characteristics between compound and pure milk chocolate. MOJ Food Process. Technol. 2018, 6, 292–296. [Google Scholar] [CrossRef]
- Wilms, L.; Oberfeld, D. Color and emotion: Effects of hue, saturation, and brightness. Psychol. Res. 2018, 82, 896–914. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Palabiyik, I.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Poyrazoglu, E.S.; Sagdic, O. Formulation of dark chocolate as a carrier to deliver eicosapentaenoic and docosahexaenoic acids: Effects on product quality. Food Chem. 2018, 254, 224–231. [Google Scholar] [CrossRef]
- Toker, O.S.; Konar, N.; Pirouzian, H.R.; Oba, S.; Polat, D.G.; Palabiyik, I.; Poyrazoglu, E.S.; Sagdic, O. Developing functional white chocolate by incorporating different forms of EPA and DHA—Effects on product quality. LWT Food Sci. Technol. 2018, 87, 177–185. [Google Scholar] [CrossRef]
- Hodge, S.; Rousseau, D. Fat bloom formation and characterization in milk chocolate observed by atomic force microscopy. J. Am. Oil Chem. Soc. 2002, 79, 1115–1121. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Marinho, J.F.U.; Mazzocato, M.C.; De Martinis, E.C.P.; Luccas, V.; Favaro-Trindade, C.S. Semisweet chocolate as a vehicle for the probiotics Lactobacillus acidophilus LA3 and Bifidobacterium animalis subsp. Lactis BLC1: Evaluation of chocolate stability and probiotic survival under in vitro simulated gastrointestinal conditions. LWT Food Sci. Technol. 2017, 75, 640–647. [Google Scholar] [CrossRef]
- Mohos, F.A. Water activity, shelf life and storage. In Confectionery and Chocolate Engineering; Mohos, F.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 579–603. [Google Scholar]
- Da Silva E Silva, N.; Pino Hernández, E.J.G.; Da Silva Araújo, C.; Peixoto Joele, M.R.S.; Lourenço, L.D.F.H. Development and optimization of biodegradable fish gelatin composite film added with buritic oil. CyTA J. Food 2018, 16, 340–349. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, M.; Mujumdar, A.S.; Xiao, G.; Sun, J. Study on reduction of water activity and storage stability for dehydrated Brassica parachinensis with intermediate moisture. Drying Technol. 2007, 25, 669–674. [Google Scholar] [CrossRef]
- Henney, J.E.; Taylar, C.L.; Boon, C.S. Strategies to Reduce Sodium Intake in the United States; The National Academies Press: Washington, DC, USA, 2010; pp. 1–317. [Google Scholar]
- Skytte, U.P.; Kaylegian, K.E. Ingredients from milk. In Beckett’s Industrial Chocolate Manufacture and Use; Beckett, S.T., Fowler, M.S., Ziegler, G.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 102–134. [Google Scholar]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest wounding stress in horticultural crops as a tool for designing novel functional foods and beverages with enhanced nutraceutical content: Carrot juice as a case study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]



| Fatty Acid | Fish Oil | Chocolate Samples (mg Fatty Acid per 100 g) | |||||
|---|---|---|---|---|---|---|---|
| Control | Prob | FO1 | FO2 | Prob + FO1 | Prob + FO2 | ||
| Octanoic Acid (C8:0) | 183.6 ± 14.6 | 93.7 ± 9.9 ab | 91.2 ± 3.1 ab | 87.3 ± 6.5 b | 89.5 ± 5.2 ab | 109.6 ± 4.9 a | 96.2 ± 8.8 ab |
| Decanoic acid (C10:0) | N.D. | 128.9 ± 1.4 abc | 128.1 ± 5.6 abc | 122.7 ± 1.9 bc | 115.7 ± 1.9 bc | 135.0 ± 4.8 ab | 140.2 ± 9.3 a |
| Lauric acid (C12:0) | 98.3 ± 1.7 | 116.5 ± 4.2 ab | 120.5 ± 5.4 a | 117.0 ± 2.3 ab | 107.1 ± 2.4 b | 126.4 ± 4.4 a | 129.3 ± 5.3 a |
| Myristic acid (C14:0) | 8375.8 ± 22.2 | 395.2 ± 11.5 c | 437.6 ± 29.2 c | 549.0 ± 17.4 b | 770.7 ± 16.0 a | 565.5 ± 24.1 b | 771.3 ± 40.4 a |
| Pentadecanoic acid (C15:0) | 759.4 ± 6.9 | 48.8 ± 1.7 d | 51.8 ± 2.0 cd | 60.0 ± 2.1 bc | 78.0 ± 1.6 a | 63.7 ± 2.5 b | 82.8 ± 5.5 a |
| Palmitic acid (C16:0) | 16,842.4 ± 62.0 | 5541.8 ± 167.0 c | 5911.3 ± 298.2 bc | 5887.6 ± 118.2 bc | 5767.2 ± 128.5 bc | 6312.8 ± 280.5 ab | 6691.5 ± 285.9 a |
| Heptadecanoic acid (C17:0) | 683.0 ± 6.3 | 62.0 ± 5.4 d | 68.8 ± 3.4 cd | 77.1 ± 2.5 bc | 88.4 ± 1.9 ab | 79.4 ± 3.8 bc | 95.4 ± 4.6 a |
| Stearic acid (C18:0) | 3283.4 ± 9.5 | 6281.7 ± 193.1 b | 6658.4 ± 327.7 a | 6414.8 ± 115.8 ab | 5743.6 ± 137.0 b | 6919.3 ± 316.2 a | 6946.7 ± 277.7 a |
| Arachidic acid (C20:0) | 178.6 ± 2.7 | 188.7 ± 5.5 bc | 200.0 ± 10.2 ab | 194.0 ± 3.5 abc | 176.1 ± 4.4 c | 208.8 ± 9.5 ab | 212.1 ± 8.6 a |
| Behenic acid (C22:0) | 149.8 ± 3.0 | 30.7 ± 1.0 c | 33.6 ± 2.1 bc | 32.8 ± 0.8 bc | 35.5 ± 0.8 abc | 37.5 ± 2.4 ab | 40.2 ± 3.8 a |
| Lignoceric acid (C24:0) | 165.9 ± 1.8 | 21.4 ± 1.0 c | 23.7 ± 0.6 bc | 21.2 ± 1.1 c | 25.1 ± 1.1 b | 25.5 ± 0.6 b | 29.9 ± 1.8 a |
| Myristoleic acid (C14:1) | 59.0 ± 2.2 | 35.4 ± 1.5 bc | 36.7 ± 0.6 abc | 36.0 ± 1.2 bc | 34.0 ± 1.2 c | 39.4 ± 2.3 ab | 41.9 ± 2.6 a |
| Palmitoleic acid (C16:1) | 11,325.6 ± 19.1 | 109.3 ± 3.1 d | 139.3 ± 19.1 d | 311.0 ± 18.8 c | 649.5 ± 12.2 a | 300.1 ± 14.3 c | 565.7 ± 34.4 b |
| Oleic Acid (C18:1) | 4907.2 ± 26.6 | 5845.3 ± 148.1 ab | 6160.2 ± 347.3 ab | 6065.5 ± 100.7 ab | 5496.3 ± 113.1 b | 6452.7 ± 277.8 a | 6522.1 ± 250.1 a |
| Vaccenic acid (C18:1) | 2906.6 ± 12.9 | 73.8 ± 1.4 d | 83.0 ± 6.5 d | 122.4 ± 4.7 c | 206.6 ± 4.2 a | 123.6 ± 6.0 c | 186.6 ± 8.2 b |
| Eicosenoic acid (C20:1) | 498.5 ± 3.2 | N.D. | N.D. | 16.0 ± 1.6 c | 30.6 ± 0.6 a | 17.6 ± 1.6 c | 26.1 ± 2.1 b |
| Nervonic acid (C24:1) | 399.5 ± 1.6 | N.D. | N.D. | N.D. | 21.5 ± 2.7 a | N.D. | 16.7 ± 0.7 b |
| Linoleic acid (C18:2) | 1842.2 ± 47.9 | 622.5 ± 17.4 c | 635.3 ± 31.6 bc | 652.9 ± 11.7 bc | 625.8 ± 16.3 c | 704.8 ± 31.1 ab | 732.6 ± 27.7 a |
| Gamma Linolenic acid (C18:3) | 268.3 ± 5.0 | N.D. | N.D. | N.D. | 15.2 ± 0.6 a | N.D. | 13.5 ± 0.5 b |
| Alpha Linolenic acid (C18:3) | 2009.1 ± 6.7 | 46.0 ± 2.5 c | 49.3 ± 2.4 c | 81.0 ± 3.4 b | 134.2 ± 4.1 a | 83.6 ± 5.7 b | 125.1 ± 6.5 a |
| Stearidionic acid (C18:4) | 3458.1 ± 18.5 | N.D. | N.D. | 53.3 ± 3.9 c | 181.0 ± 17.5 a | 54.9 ± 3.3 c | 131.3 ± 6.7 b |
| Eicosadienoic acid (C20:2) | 458.7 ± 1.8 | N.D. | N.D. | 8.3 ± 0.2 b | 23.4 ± 4.8 a | 10.5 ± 0.5 b | 19.5 ± 1.4 a |
| Homo-gamma-linolenic acid (C20:3) | 282.5 ± 1.4 | N.D. | N.D. | 8.4 ± 0.4 b | 16.2 ± 0.8 a | 10.1 ± 0.7 b | 17.0 ± 1.0 a |
| Dihomogamma linolenic acid (C20:3) | 372.9 ± 22.5 | N.D. | N.D. | 11.0 ± 1.3 b | 21.0 ± 1.8 a | 10.8 ± 1.1 b | 18.4 ± 1.7 a |
| Arachidonic acid (C20:4) | 851.2 ± 9.1 | N.D. | N.D. | 31.7 ± 4.0 b | 44.6 ± 2.4 a | 21.3 ± 2.1 c | 41.7 ± 1.9 a |
| Eicosapentaenoic acid (C20:5) | 12,862.1 ± 17.8 | N.D. | 12.6 ± 3.0 d | 214.5 ± 19.2 c | 571.0 ± 16.9 a | 199.9 ± 8.9 c | 460.4 ± 22.4 b |
| Docosapentaenoic acid n-6 (C22:5) | 331.1 ± 21.1 | N.D. | N.D. | N.D. | 18.8 ± 0.8 a | N.D. | 20.5 ± 1.9 a |
| Docosapentaenoic acid n-3 (C22:5) | 1888.1 ± 25.5 | N.D. | N.D. | 36.8 ± 1.4 c | 85.0 ± 1.9 b | 41.8 ± 5.0 c | 104.0 ± 11.9 a |
| Docosahexaenoic acid (C22:6) | 14,122.2 ± 27.0 | N.D. | 9.9 ± 1.4 d | 237.2 ± 22.1 c | 640.0 ± 18.8 a | 208.6 ± 22.0 c | 521.0 ± 26.0 b |
| Total ω-3 | 34,712.6 ± 57.3 | 46.0 ± 2.5 d | 71.8 ± 5.2 d | 633.8 ± 43.4 c | 1632.2 ± 54.6 a | 599.6 ± 41.5 c | 1360.3 ± 72.5 b |
| Total ω-6 | 4034.0 ± 29.6 | 622.5 ± 17.4 c | 635.3 ± 31.6 c | 701.2 ± 13.5 bc | 744.0 ± 22.5 b | 746.7 ± 34.3 b | 844.8 ± 33.5 a |
| Saturated fatty acids (SFA) | 30,720.2 ± 103.6 | 12,909.4 ± 400.6 c | 13,724.9 ± 675.2 abc | 13,563.7 ± 264.9 bc | 12,997.0 ± 298.1 bc | 14,583.4 ± 652.9 ab | 15,235.6 ± 651.0 a |
| Monounsaturated fatty acids (MUFA) | 20,096.3 ± 55.2 | 6063.7 ± 153.8 c | 6419.1 ± 359.7 bc | 6550.9 ± 124.4 bc | 6438.3 ± 131.4 bc | 6933.4 ± 301.8 ab | 7359.1 ± 296.8 a |
| Polyunsaturated fatty acids (PUFA) | 38,746.6 ± 45.8 | 668.5 ± 19.6 c | 707.1 ± 34.0 c | 1335.1 ± 51.9 b | 2376.1 ± 74.4 a | 1346.3 ± 72.1 b | 2205.0 ± 105.5 a |
| Total fatty acids | 89,563.1 ± 189.0 | 19,641.6 ± 574.0 c | 20,851.2 ± 1064.8 bc | 21,449.7 ± 432.9 bc | 21,811.5 ± 502.5 bc | 22,863.2 ± 1022.2 ab | 24,799.7 ± 1052.5 a |
| Treatments | Color Properties | aw | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | ΔE* | WI | ||
| Control | 19.1 ± 2.12 a | 4.60 ± 0.04 a | 4.42 ± 0.04 a | 6.38 ± 0.01 a | 0.77 ± 0.01 b | 0 | 18.85 ± 2.11 a | 0.50 ± 0.03 a |
| Prob | 12.5 ± 1.25 bc | 4.12 ± 0.24 ab | 4.36 ± 0.29 a | 6.00 ± 0.35 a | 0.81 ± 0.02 a | 6.68 ± 1.25 abc | 12.25 ± 1.25 bc | 0.45 ± 0.00 b |
| FO1 | 13.3 ± 0.33 bc | 4.26 ± 0.22 ab | 4.54 ± 0.41 a | 6.23 ± 0.45 a | 0.81 ± 0.02 a | 5.90 ± 0.32 bc | 13.03 ± 0.35 bc | 0.44 ± 0.00 b |
| FO2 | 9.98 ± 0.67 c | 4.22 ± 0.05 ab | 4.72 ± 0.09 a | 6.33 ± 0.10 a | 0.84 ± 0.00 a | 9.13 ± 0.67 a | 9.76 ± 0.68 c | 0.46 ± 0.00 b |
| Prob + FO1 | 13.83 ± 0.61 b | 3.93 ± 0.25 b | 4.09 ± 0.33 a | 5.67 ± 0.41 a | 0.80 ± 0.01 ab | 5.35 ± 0.64 c | 13.64 ± 0.59 b | 0.44 ± 0.00 b |
| Prob + FO2 | 11.20 ± 0.87 bc | 4.31 ± 0.28 ab | 4.87 ± 0.41 a | 6.50 ± 0.49 a | 0.85 ± 0.01 a | 7.94 ± 0.89 ab | 10.96 ± 0.90 bc | 0.47 ± 0.00 ab |
| Treatment | Hardness (N) | Work of Penetration (N) |
|---|---|---|
| Control | 2959.2 ± 232.7 a | 3513 ± 245.1 a |
| Prob | 3095.8 ± 136.1 a | 3147.6 ± 143.0 ab |
| FO1 | 2271.4 ± 85.6 b | 2906.6 ± 141.2 b |
| FO2 | 1430.8 ± 75.5 c | 1903.8 ± 104.3 c |
| Prob + FO1 | 2049.8 ± 155.1 b | 3069.6 ± 345.1 ab |
| Prob + FO2 | 925.8 ± 49.5 d | 1475 ± 102.4 c |
| Parameters | Treatments | |||||
|---|---|---|---|---|---|---|
| Control | Prob | FO1 | FO2 | Prob + FO1 | Prob + FO2 | |
| Appearance | 7.54 ± 0.11 a | 7.46 ± 0.16 a | 7.34 ± 0.12 abc | 7.03 ± 0.13 bc | 7.37 ± 0.12 ab | 6.92 ± 0.17 c |
| Flavor | 8.68 ± 0.11 a | 7.58 ± 0.22 ab | 7.25 ± 0.09 bc | 5.26 ± 0.16 d | 7.00 ± 0.16 c | 5.04 ± 0.21 d |
| Texture | 7.59 ± 0.13 a | 7.58 ± 0.19 a | 7.57 ± 0.12 a | 6.38 ± 0.14 b | 7.31 ± 0.14 a | 6.06 ± 0.21 b |
| Overall acceptability | 7.83 ± 0.10 a | 7.59 ± 0.21 a | 7.42 ± 0.11 ab | 5.57 ± 0.13 c | 7.09 ± 0.14 b | 5.46 ± 0.20 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faccinetto-Beltrán, P.; Gómez-Fernández, A.R.; Orozco-Sánchez, N.E.; Pérez-Carrillo, E.; Marín-Obispo, L.M.; Hernández-Brenes, C.; Santacruz, A.; Jacobo-Velázquez, D.A. Physicochemical Properties and Sensory Acceptability of a Next-Generation Functional Chocolate Added with Omega-3 Polyunsaturated Fatty Acids and Probiotics. Foods 2021, 10, 333. https://doi.org/10.3390/foods10020333
Faccinetto-Beltrán P, Gómez-Fernández AR, Orozco-Sánchez NE, Pérez-Carrillo E, Marín-Obispo LM, Hernández-Brenes C, Santacruz A, Jacobo-Velázquez DA. Physicochemical Properties and Sensory Acceptability of a Next-Generation Functional Chocolate Added with Omega-3 Polyunsaturated Fatty Acids and Probiotics. Foods. 2021; 10(2):333. https://doi.org/10.3390/foods10020333
Chicago/Turabian StyleFaccinetto-Beltrán, Paulinna, Andrea R. Gómez-Fernández, Norma E. Orozco-Sánchez, Esther Pérez-Carrillo, Luis Martín Marín-Obispo, Carmen Hernández-Brenes, Arlette Santacruz, and Daniel A. Jacobo-Velázquez. 2021. "Physicochemical Properties and Sensory Acceptability of a Next-Generation Functional Chocolate Added with Omega-3 Polyunsaturated Fatty Acids and Probiotics" Foods 10, no. 2: 333. https://doi.org/10.3390/foods10020333
APA StyleFaccinetto-Beltrán, P., Gómez-Fernández, A. R., Orozco-Sánchez, N. E., Pérez-Carrillo, E., Marín-Obispo, L. M., Hernández-Brenes, C., Santacruz, A., & Jacobo-Velázquez, D. A. (2021). Physicochemical Properties and Sensory Acceptability of a Next-Generation Functional Chocolate Added with Omega-3 Polyunsaturated Fatty Acids and Probiotics. Foods, 10(2), 333. https://doi.org/10.3390/foods10020333