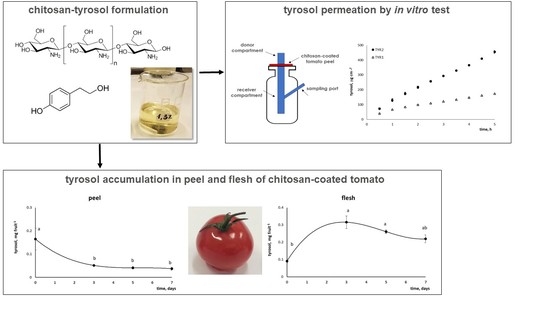
Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food
Abstract
:1. Introduction
- (i)
- the effectiveness of chitosan coating to transfer bioactive molecules across tomato peel through in vitro tests;
- (ii)
- the possibility of producing tyrosol-enriched tomatoes by post-harvest application of a tyrosol-chitosan coating; and
- (iii)
- the ability of tyrosol to cross the tomato peel also in vivo by measuring the tyrosol content in the fruit peel and flesh separately.
2. Materials and Methods
2.1. Materials
2.2. Chemicals and Reagents
2.3. Preparation of the Formulation
2.4. In Vitro Permeation Study
2.5. Improvement of Bioactivity of Tomato Fruit
2.5.1. Freeze Drying Process
2.5.2. Extraction and Quantification of Tyrosol Amount Penetrated inside Tomatoes
2.5.3. Carotenoid Extraction and Quantification
2.5.4. Phenolics and Flavonoid Quantification
2.5.5. ABTS Radical Scavenging Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Permeation Study
3.2. Freeze Drying Process
3.3. Tyrosol Content in Tomato Fruit
3.4. Concentration of Carotenoids, Phenolics and Flavonoids in Peel and Flesh of Tyrosol-Enriched Tomatoes
3.5. Antioxidant Activity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Przyby lska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [Green Version]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jianglian, D. Application of Chitosan Based Coating in Fruit and Vegetable Preservation: A Review. J. Food Process. Technol. 2013, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta BBA Biomembr. 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, D.H.; Köhle, H.; Kauss, H. Effect of Chitosan on Membrane Permeability of Suspension-Cultured Glycine max and Phaseolus vulgaris Cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef] [Green Version]
- Mertins, O.; Dimova, R. Insights on the Interactions of Chitosan with Phospholipid Vesicles. Part II: Membrane Stiffening and Pore Formation. Langmuir 2013, 29, 14552–14559. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- Larrosa, M.; Espín, J.C.; Tomás-Barberán, F.A. Antioxidant capacity of tomato juice functionalised with enzymatically synthesised hydroxytyrosol. J. Sci. Food Agric. 2003, 83, 658–666. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Trevisan, M.; Lucini, L. Untargeted screening of the bound/free phenolic composition in tomato cultivars for industrial transformation. J. Sci. Food Agric. 2019, 99, 6173–6181. [Google Scholar] [CrossRef]
- Pagno, C.H.; Castagna, A.; Trivellini, A.; Mensuali-Sodi, A.; Ranieri, A.; Ferreira, E.A.; Rios, A.D.O.; Flôres, S.H. The nutraceutical quality of tomato fruit during domestic storage is affected by chitosan coating. J. Food Process. Preserv. 2017, 42, e13326. [Google Scholar] [CrossRef] [Green Version]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F.; Caserini, M.; Monti, D. Topical Formulations Containing Finasteride. Part II: Determination of Finasteride Penetration into Hair Follicles using the Differential Stripping Technique. J. Pharm. Sci. 2014, 103, 2323–2329. [Google Scholar] [CrossRef]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Centini, M.; Anselmi, C. 4-Methylbenzylidene camphor microspheres: Reconstituted epidermis (Skinethic®) permeation and distribution. Int. J. Cosmet. Sci. 2015, 37, 298–305. [Google Scholar] [CrossRef]
- Gerardi, C.; Tommasi, N.; Albano, C.; Blando, F.; Rescio, L.; Pinthus, E.; Mita, G. Prunus mahaleb L. fruit extracts: A novel source for natural food pigments. Eur. Food Res. Technol. 2015, 241, 683–695. [Google Scholar] [CrossRef]
- Heredia, A.; Peinado, I.; Rosa, E.; Andrés, A. Effect of osmotic pre-treatment and microwave heating on lycopene degradation and isomerization in cherry tomato. Food Chem. 2010, 123, 92–98. [Google Scholar] [CrossRef]
- Castagna, A.; Dall’Asta, C.; Chiavaro, E.; Galaverna, G.; Ranieri, A. Effect of Post-harvest UV-B Irradiation on Polyphenol Profile and Antioxidant Activity in Flesh and Peel of Tomato Fruits. Food Bioprocess Technol. 2013, 7, 2241–2250. [Google Scholar] [CrossRef] [Green Version]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, A.O.; Oludare, G.O.; Aderinto, O.T.; Tasdup, A. Antioxidant and free radical scavenging activities of acqueous and ethanol extracts of Zingiber officinale. Biol. Med. 2012, 3, 25–30. [Google Scholar]
- Shalaby, A.; Shanab, S.M.M. Antioxidant compounds, assays of determination and mode of action. Afr. J. Pharm. Pharmacol. 2013, 7, 528–539. [Google Scholar] [CrossRef]
- Leide, J.; Hildebrandt, U.; Reussing, K.; Riederer, M.; Vogg, G. The Developmental Pattern of Tomato Fruit Wax Accumulation and Its Impact on Cuticular Transpiration Barrier Properties: Effects of a Deficiency in a β-Ketoacyl-Coenzyme A Synthase (LeCER6). Plant Physiol. 2007, 144, 1667–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SINU; Società Italiana di Nutrizione Umana. Livelli di Assunzione di Riferimento di Nutrienti ed Energia Per la Popolazione Italiana; IV Revisione; Coordinamento editoriale SINU-INRAN; SICS: Milan, Italy, 2014. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 4728. [Google Scholar] [CrossRef]
- Kailis, S.G.; Kiritsakis, A. Table olives: Processing, nutritional and health implications. In Olives and Olive Oil as Functional Foods: Bioactivity, Chemistry and Processing; Shahidi, F., Kiritsakis, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 295–324. [Google Scholar]
- Vaz Almeida, M.D.; Parisi, S.; Delgado, A.M. Olive oil and table olives. In Chemistry of the Mediterranean Diet; Delgado, A.M., Vaz Almeida, M.D., Parisi, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 33–57. [Google Scholar]
- Han, C.; Zhao, Y.; Leonard, S.; Traber, M. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Khalid, M.; Rahman, S.U.; Bilal, M.; Huang, D. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]




| Formulation | J (µg cm−2 h−1) | Q%5h |
|---|---|---|
| TYR1 | 27.88 ± 0.12 b | 2.73 ± 0.38 |
| TYR2 | 81.57 ± 3.51a | 3.51 ± 0.30 |
| Sample | Entire Tomato (g) | Minced Tomato (g) | Freeze-Dried (g) | Water (%) | |
|---|---|---|---|---|---|
| T0 | Control | 25.48 ± 3.00 | 25.41 ± 2.99 | 1.91 ± 0.30 | 92.62 ± 0.41 |
| Treated | 20.93 ± 1.41 | 20.90 ± 1.41 | 1.74 ± 0.08 | 91.60 ± 0.25 | |
| T3 | Control | 26.27 ± 1.90 | 26.24 ± 1.89 | 2.07 ± 0.18 | 92.12 ± 0.34 |
| Treated | 22.63 ± 1.46 | 22.57 ± 1.45 | 1.82 ± 0.08 | 91.90 ± 0.27 | |
| T5 | Control | 24.98 ± 3.03 | 24.92 ± 3.03 | 1.93 ± 0.19 | 92.07 ± 0.62 |
| Treated | 23.98 ± 2.32 | 23.90 ± 2.31 | 1.99 ± 0.21 | 91.66 ± 0.28 | |
| T7 | Control | 24.52 ± 2.08 | 24.48 ± 2.07 | 1.97 ± 0.15 | 91.92 ± 0.28 |
| Treated | 24.27 ± 2.60 | 24.23 ± 2.61 | 1.87 ± 0.25 | 92.30 ± 0.31 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tampucci, S.; Castagna, A.; Monti, D.; Manera, C.; Saccomanni, G.; Chetoni, P.; Zucchetti, E.; Barbagallo, M.; Fazio, L.; Santin, M.; et al. Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food. Foods 2021, 10, 335. https://doi.org/10.3390/foods10020335
Tampucci S, Castagna A, Monti D, Manera C, Saccomanni G, Chetoni P, Zucchetti E, Barbagallo M, Fazio L, Santin M, et al. Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food. Foods. 2021; 10(2):335. https://doi.org/10.3390/foods10020335
Chicago/Turabian StyleTampucci, Silvia, Antonella Castagna, Daniela Monti, Clementina Manera, Giuseppe Saccomanni, Patrizia Chetoni, Erica Zucchetti, Mariacristina Barbagallo, Laura Fazio, Marco Santin, and et al. 2021. "Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food" Foods 10, no. 2: 335. https://doi.org/10.3390/foods10020335
APA StyleTampucci, S., Castagna, A., Monti, D., Manera, C., Saccomanni, G., Chetoni, P., Zucchetti, E., Barbagallo, M., Fazio, L., Santin, M., & Ranieri, A. (2021). Tyrosol-Enriched Tomatoes by Diffusion across the Fruit Peel from a Chitosan Coating: A Proposal of Functional Food. Foods, 10(2), 335. https://doi.org/10.3390/foods10020335