Degradation of Wheat Germ Agglutinin during Sourdough Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sourdough Preparation
2.2. WGA Analysis by Size Exclusion High Performance Liquid Chromatography (SEC-HPLC)
2.3. WGA Quantification with Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
3.1. Microbial Growth and Metabolism in Wheat Sourdoughs
3.2. WGA Analysis Using Size Exclusion Chromatography (SEC)-HPLC
3.3. WGA Quantification Using Enzyme Linked Immunosorbent Assay (ELISA)
4. Discussion
4.1. Proteolysis in Sourdough and Contribution to WGA Degradation
4.2. Fate of WGA during Sourdough Fermentation
4.3. Stability of WGA in Wheat Baking
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arranz-Otaegui, A.; Carretero, L.G.; Ramsey, M.N.; Fuller, D.Q.; Richter, T. Archaeobotanical evidence reveals the origins of bread 14,400 years ago in northeastern Jordan. Proc. Natl. Acad. Sci. USA 2018, 115, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M.U.S. Gluten-Free Products Market Value 2014–2025. Available online: https://www.statista.com/statistics/884086/us%20gluten%20free%0Afood%20market%20value/ (accessed on 23 August 2020).
- Schuppan, D.; Gisbert-Schuppan, K. Wheat Syndromes; Springer Nature: Switzerland, Cham, 2019; ISBN 9783030190224. [Google Scholar]
- Schuppan, D.; Pickert, G.; Ashfaq-Khan, M.; Zevallos, V. Non-celiac wheat sensitivity: Differential diagnosis, triggers and implications. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.D.E.; Walker, M.M.; Talley, N.J. Non-coeliac gluten or wheat sensitivity: Emerging disease or misdiagnosis? Med. J. Aust. 2017, 207, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): An update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017, 152, 1100–1113.e12. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Verdu, E.F. Non-celiac gluten or wheat sensitivity: It’s complicated! Neurogastroenterol. Motil. 2018, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; Gänzle, M.G. Use of sourdough in low FODMAP baking. Foods 2018, 7, 96. [Google Scholar] [CrossRef]
- Li, Q.; Loponen, J.; Gänzle, M.G. Characterization of the extracellular fructanase FruA in Lactobacillus crispatus and its contribution to fructan hydrolysis in breadmaking. J. Agric. Food Chem. 2020, 68, 8637–8647. [Google Scholar] [CrossRef]
- Capuani, A.; Behr, J.; Vogel, R.F. Influence of lactic acid bacteria on redox status and on proteolytic activity of buckwheat (Fagopyrum esculentum Moench) sourdoughs. Int. J. Food Microbiol. 2013, 165, 148–155. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Loponen, J.; Gobbetti, M. Proteolysis in sourdough fermentations: Mechanisms and potential for improved bread quality. Trends Food Sci. Technol. 2008, 19, 513–521. [Google Scholar] [CrossRef]
- James Remington, S. Serine Carboxypeptidase, D. Handb. Proteolytic Enzym. 2013, 3, 3418–3421. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Introduction: Aspartic and glutamic peptidases and their clans. Handb. Proteolytic Enzym. 2013, 1, 3–19. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yonezawa, D. Wheat flour proteases and their action on gluten proteins in dilute acetic acid. Agric. Biol. Chem. 1982, 46, 767–773. [Google Scholar] [CrossRef]
- Bleukx, W.; Delcour, J.A. A second aspartic proteinase associated with wheat gluten. J. Cereal Sci. 2000, 32, 31–42. [Google Scholar] [CrossRef]
- Bleukx, W.; Roels, S.P.; Delcour, J.A. On the presence and activities of proteolytic enzymes in vital wheat gluten. J. Cereal Sci. 1997, 26, 183–193. [Google Scholar] [CrossRef]
- Huang, X.; Schuppan, D.; Rojas Tovar, L.E.; Zevallos, V.F.; Loponen, J.; Gänzle, M. Sourdough fermentation degrades wheat alpha-amylase/trypsin inhibitor (ATI) and reduces pro-inflammatory activity. Foods 2020, 9, 943. [Google Scholar] [CrossRef]
- Jänsch, A.; Korakli, M.; Vogel, R.F.; Gänzle, M.G. Glutathione reductase from Lactobacillus sanfranciscensis DSM20451T: Contribution to oxygen tolerance and thiol exchange reactions in wheat sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4469–4476. [Google Scholar] [CrossRef]
- Loponen, J.; König, K.; Wu, J.; Gänzle, M.G. Influence of thiol metabolism of lactobacilli on egg white proteins in wheat sourdoughs. J. Agric. Food Chem. 2008, 56, 3357–3362. [Google Scholar] [CrossRef]
- Matucci, A.; Veneri, G.; Dalla Pellegrina, C.; Zoccatelli, G.; Vincenzi, S.; Chignola, R.; Peruffo, A.D.B.; Rizzi, C. Temperature-dependent decay of wheat germ agglutinin activity and its implications for food processing and analysis. Food Control 2004, 15, 391–395. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. Lectins; Kluwer Academic Publishers: Amsterdam, The Netherland, 2003; ISBN 9781402066054. [Google Scholar]
- Baieli, M.F.; Urtasun, N.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Efficient wheat germ agglutinin purification with a chitosan-based affinity chromatographic matrix. J. Sep. Sci. 2012, 35, 231–238. [Google Scholar] [CrossRef]
- Wright, C.S.; Raikhel, N. Sequence variability in three wheat germ agglutinin isolectins: Products of multiple genes in polyploid wheat. J. Mol. Evol. 1989, 28, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Burger, M.M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J. Biol. Chem. 1974, 249, 3116–3122. [Google Scholar] [CrossRef]
- Peters, B.P.; Goldstein, I.J.; Flashner, M.; Ebisu, S. Interaction of wheat germ agglutinin with sialic acid. Biochemistry 1979, 18, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Ewen, S.W.B.; Grant, G.; Brown, D.S.; Stewart, J.C.; Peumans, W.J.; Van Damme, E.J.M.; Bardocz, S. Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins. Br. J. Nutr. 1993, 70, 313–321. [Google Scholar] [CrossRef]
- Pellegrina, C.D.; Rizzi, C.; Mosconi, S.; Zoccatelli, G.; Peruffo, A.; Chignola, R. Plant lectins as carriers for oral drugs: Is wheat germ agglutinin a suitable candidate? Toxicol. Appl. Pharmacol. 2005, 207, 170–178. [Google Scholar] [CrossRef]
- Pellegrina, C.D.; Perbellini, O.; Scupoli, M.T.; Tomelleri, C.; Zanetti, C.; Zoccatelli, G.; Fusi, M.; Peruffo, A.; Rizzi, C.; Chignola, R. Effects of wheat germ agglutinin on human gastrointestinal epithelium: Insights from an experimental model of immune/epithelial cell interaction. Toxicol. Appl. Pharmacol. 2009, 237, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Ryva, B.; Zhang, K.; Asthana, A.; Wong, D.; Vicioso, Y.; Parameswaran, R. Wheat germ agglutinin as a potential therapeutic agent for leukemia. Front. Oncol. 2019, 9, 00100. [Google Scholar] [CrossRef]
- Tchernychev, B.; Wilchek, M. Natural human antibodies to dietary lectins. FEBS Lett. 1996, 397, 139–142. [Google Scholar] [CrossRef]
- Gabor, F.; Bogner, E.; Weissenboeck, A.; Wirth, M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 459–480. [Google Scholar] [CrossRef]
- Van Buul, V.J.; Brouns, F.J.P.H. Health effects of wheat lectins: A review. J. Cereal Sci. 2014, 59, 112–117. [Google Scholar] [CrossRef]
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and selection of Lactobacillus sakei strains isolated from traditional dry sausage for their potential use as starter cultures. Food Microbiol. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Vermeulen, N.; Kretzer, J.; Machalitza, H.; Vogel, R.F.; Gänzle, M.G. Influence of redox-reactions catalysed by homo- and hetero-fermentative lactobacilli on gluten in wheat sourdoughs. J. Cereal Sci. 2006, 43, 137–143. [Google Scholar] [CrossRef]
- Hermanson, G.T. Fluorescent Probes. In Bioconjugate Techniques, 3rd ed.; Academic Press: London, UK, 2013; pp. 395–464. ISBN 9780123822390. [Google Scholar]
- Gänzle, M.G.; Zheng, J. Lifestyles of sourdough lactobacilli—Do they matter for microbial ecology and bread quality? Int. J. Food Microbiol. 2019, 302, 15–23. [Google Scholar] [CrossRef]
- Vermeulen, N.; Pavlovic, M.; Ehrmann, M.A.; Gänzle, M.G.; Vogel, R.F. Functional characterization of the proteolytic system of Lactobacillus sanfranciscensis DSM 20451Tduring growth in sourdough. Appl. Environ. Microbiol. 2005, 71, 6260–6266. [Google Scholar] [CrossRef]
- Zheng, J.; Ruan, L.; Sun, M.; Gänzle, M.G. A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl. Environ. Microbiol. 2015, 81, 7233–7243. [Google Scholar] [CrossRef]
- Chaillou, S.; Champomier-Vergès, M.C.; Cornet, M.; Crutz-Le Coq, A.M.; Dudez, A.M.; Martin, V.; Beaufils, S.; Darbon-Rongère, E.; Bossy, R.; Loux, V.; et al. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 2005, 23, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Thiele, C.; Grassl, S.; Gänzle, M. Gluten hydrolysis and depolymerization during sourdough fermentation. J. Agric. Food Chem. 2004, 52, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of glutathione dehydrogenase of Lactobacillus sanfranciscensis on gluten properties and bread volume in type i wheat sourdough bread. J. Agric. Food Chem. 2018, 66, 9770–9776. [Google Scholar] [CrossRef] [PubMed]
- Capuani, A.; Behr, J.; Vogel, R.F. Influence of lactic acid bacteria on the oxidation-reduction potential of buckwheat (Fagopyrum esculentum Moench) sourdoughs. Eur. Food Res. Technol. 2012, 235, 1063–1069. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Thiele, C.; Gänzle, M.G.; Vogel, R.F. Fluorescence labeling of wheat proteins for determination of gluten hydrolysis and depolymerization during dough processing and sourdough fermentation. J. Agric. Food Chem. 2003, 51, 2745–2752. [Google Scholar] [CrossRef]
- Stromeck, A.; Hu, Y.; Chen, L.; Gäzle, M.G. Proteolysis and bioconversion of cereal proteins to glutamate and γ-aminobutyrate (GABA) in rye malt sourdoughs. J. Agric. Food Chem. 2011, 59, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Agriculture and Agri-Food Canada Consumer Trends: Bakery Products in Canada. Available online: https://www.agr.gc.ca/resources/prod/Internet-Internet/MISB-DGSIM/ATS-SEA/PDF/6333-eng.pdf (accessed on 30 August 2020).
- Erni, B.; Boeck, H.D.E.; Loontiens, F.G.; Sharon, N. Wheat germ agglutinin: Effects of limited reduction of the disulfide bonds and carboxymethylation on the properties of the lectin. FEBS Lett. 1980, 120, 149–154. [Google Scholar] [CrossRef]
- Rice, R.H.; Etzler, M.E. Chemical modification and hybridization of wheat germ agglutinins. Biochemistry 1975, 14, 4093–4099. [Google Scholar] [CrossRef]
- Allen, A.K.; Neuberger, A.; Sharon, N. The purification, composition and specificity of wheat-germ agglutinin. Biochem. J. 1973, 131, 155–162. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G.; Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; et al. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Food fermentations for improved digestibility of plant foods—An essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Molognoni, L.; de Sá Ploêncio, L.A.; Costa, F.B.M.; Daguer, H.; Dea Lindner, J. De Use of sourdough fermentation to reducing FODMAPs in breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.M.; Loponen, J.; Poussa, T.; Hillilä, M.; Korpela, R. Randomised clinical trial: Low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.-M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R. Pilot study: Comparison of sourdough wheat bread and yeast-fermented wheat bread in individuals with wheat sensitivity and irritable bowel syndrome. Nutrients 2017, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Therdthai, N.; Zhou, W.; Adamczak, T. Optimisation of the temperature profile in bread baking. J. Food Eng. 2002, 55, 41–48. [Google Scholar] [CrossRef]
- Nijeboer, P.; Bontkes, H.J.; Mulder, C.J.J.; Bouma, G. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J. Gastrointest. Liver Dis. 2013, 22, 435–440. [Google Scholar]
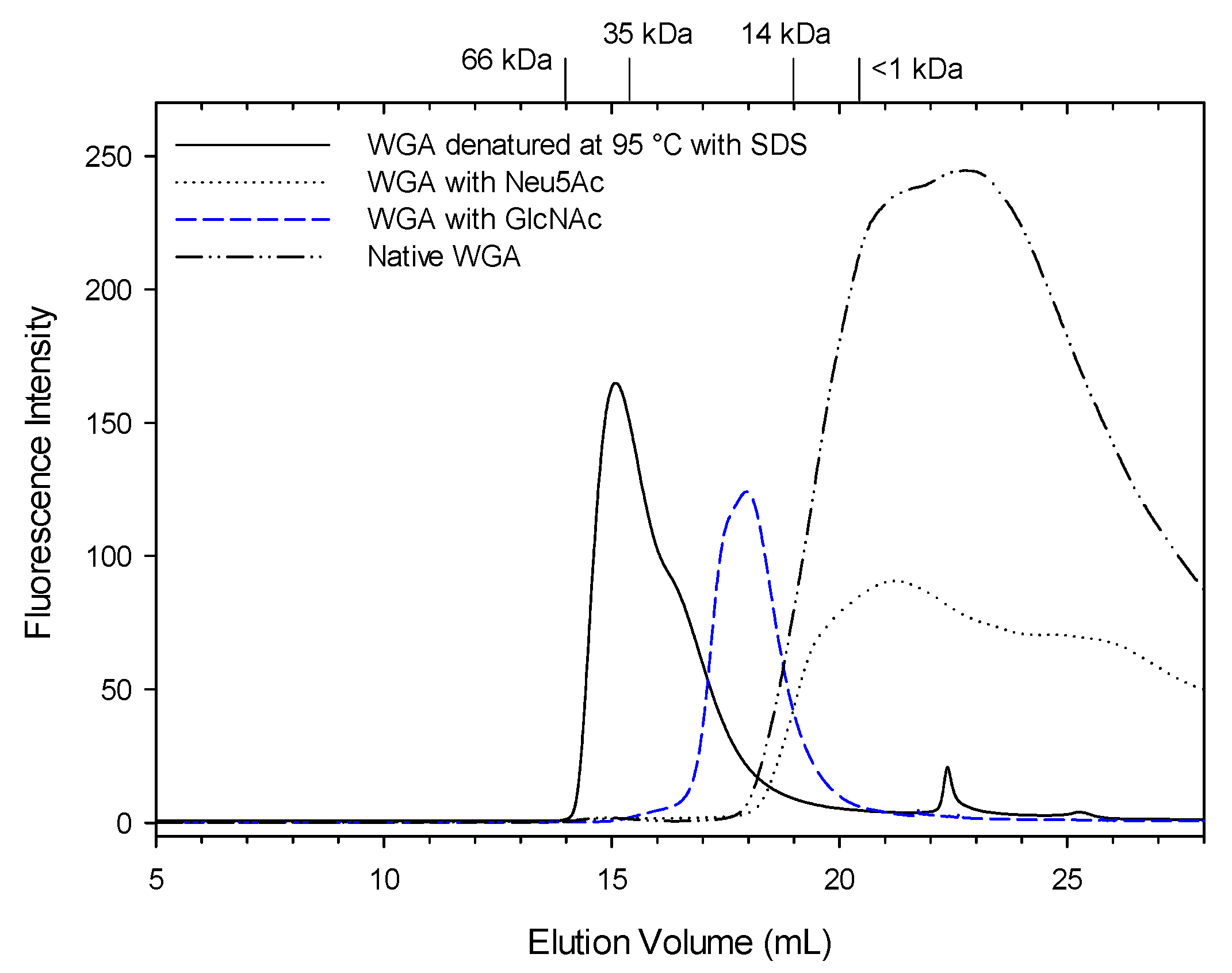

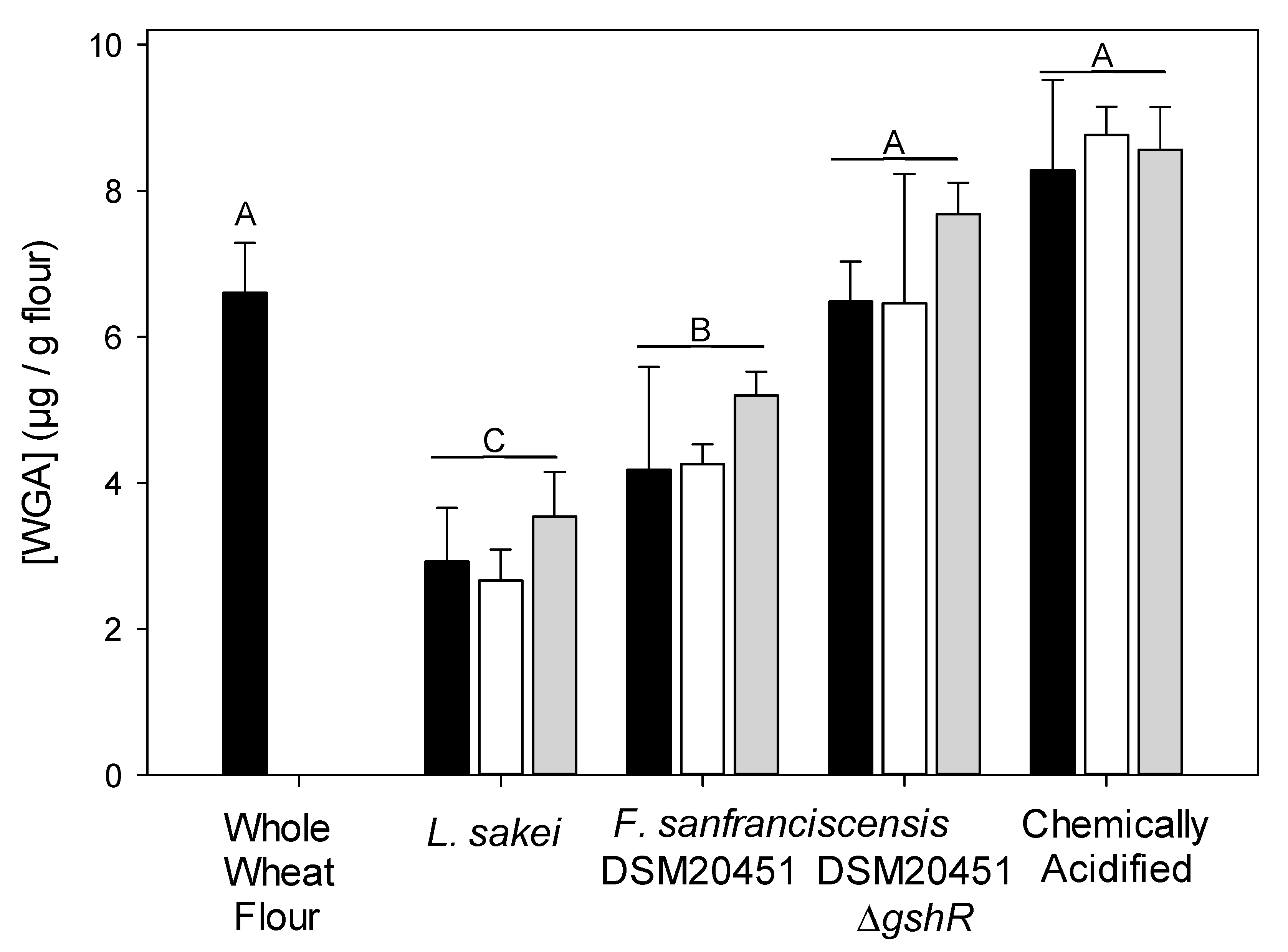
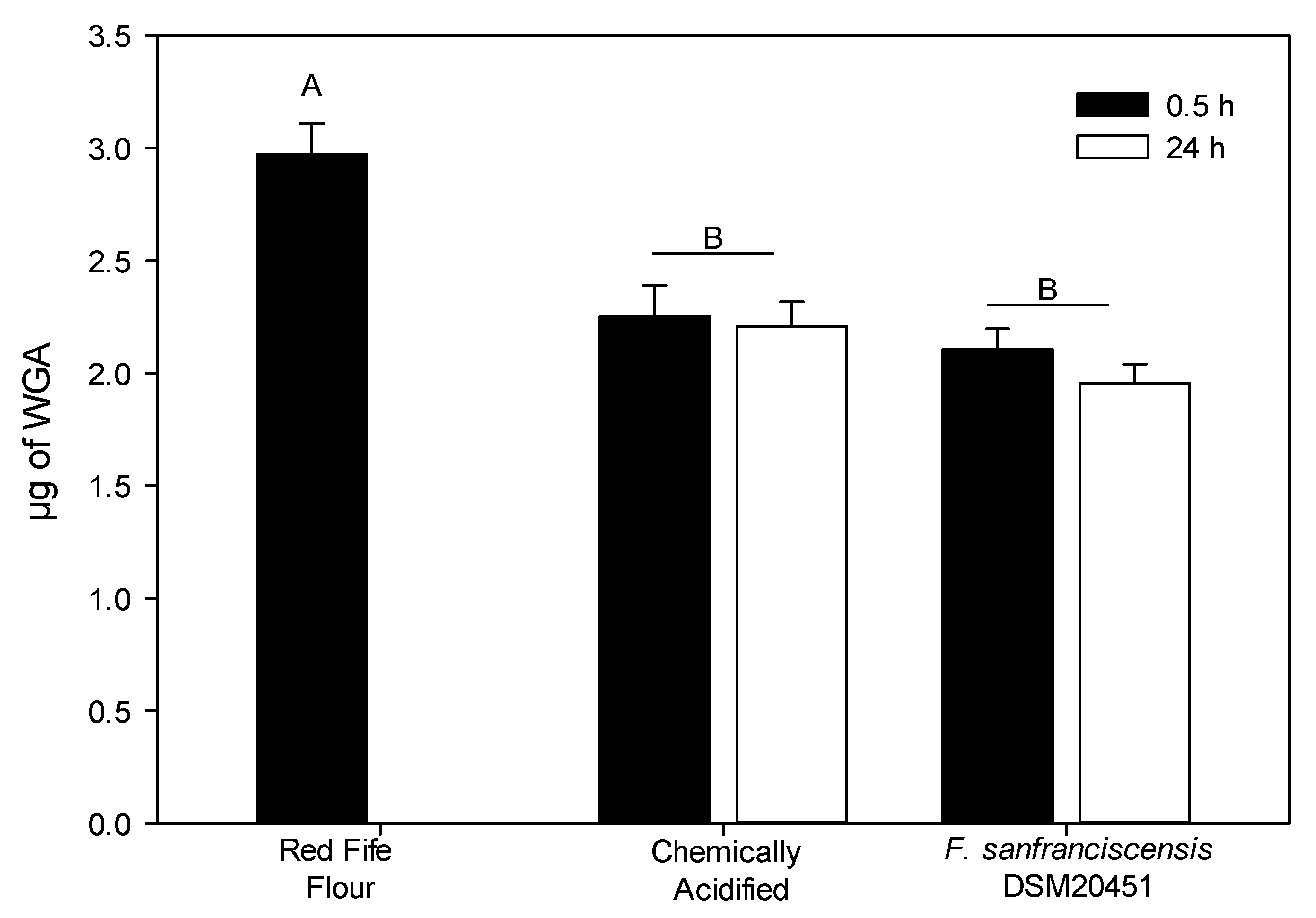
| pH | Cell Counts | |||||
|---|---|---|---|---|---|---|
| Strain | 0.5 h | 24 h | 24 h + Protease | 0.5 h | 24 h | 24 h + Protease |
| Commercial Whole Wheat Flour | ||||||
| Lt. sakei TMW1.22 | 5.78 ± 0.12 | 4.00 ± 0.03 | 3.99 ± 0.03 | 1.0 ± 0.1 × 108 | 1.1 ± 0.2 × 109 | 1.3 ± 0.2 × 109 |
| F. sanfranciscensis DSM20451 | 5.85 ± 0.1 | 4.14 ± 0.02 | 4.22 ± 0.01 | 7.3 ± 0.7 × 107 | 1.2 ± 0.8 × 109 | 1.2 ± 0.8 × 109 |
| F. sanfranciscensis DSM20451 ΔgshR | 5.75 ± 0.05 | 3.99 ± 0.17 | 4.06 ± 0.19 | 1.7 ± 0.8 × 107 | 1.8 ± 1.3 × 109 | 2.1 ± 1.6 × 109 |
| Chem. acidified | 4.04 ± 0.02 | 4.04 ± 0.03 | 4.09 ± 0.01 | n.d. * | n.d.* | n.d. * |
| Red Fife Wheat Flour | ||||||
| F. sanfranciscensis DSM20451 | 6.01 ± 0.01 | 3.84 ± 0.01 | n.d. * | 9.5 ± 5.3 × 107 | 1.1 ± 0.7 × 109 | n.d. * |
| Chem. acidified | 4.07 ± 0.11 | 3.93 ± 0.03 | n.d. * | n.d. * | n.d. * | n.d. * |
| (WGA) (μg WGA/g Flour) | |
|---|---|
| Glutathione | 3.03 ± 0.03 |
| Hydrogen Peroxide | 3.05 ± 0.61 |
| Water | 2.97 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar, L.E.R.; Gänzle, M.G. Degradation of Wheat Germ Agglutinin during Sourdough Fermentation. Foods 2021, 10, 340. https://doi.org/10.3390/foods10020340
Tovar LER, Gänzle MG. Degradation of Wheat Germ Agglutinin during Sourdough Fermentation. Foods. 2021; 10(2):340. https://doi.org/10.3390/foods10020340
Chicago/Turabian StyleTovar, Luis E. Rojas, and Michael G. Gänzle. 2021. "Degradation of Wheat Germ Agglutinin during Sourdough Fermentation" Foods 10, no. 2: 340. https://doi.org/10.3390/foods10020340
APA StyleTovar, L. E. R., & Gänzle, M. G. (2021). Degradation of Wheat Germ Agglutinin during Sourdough Fermentation. Foods, 10(2), 340. https://doi.org/10.3390/foods10020340







