Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking
Abstract
1. Introduction
2. Materials and Methods
2.1. Dissolved Oxygen Measurement
2.2. Model Wine
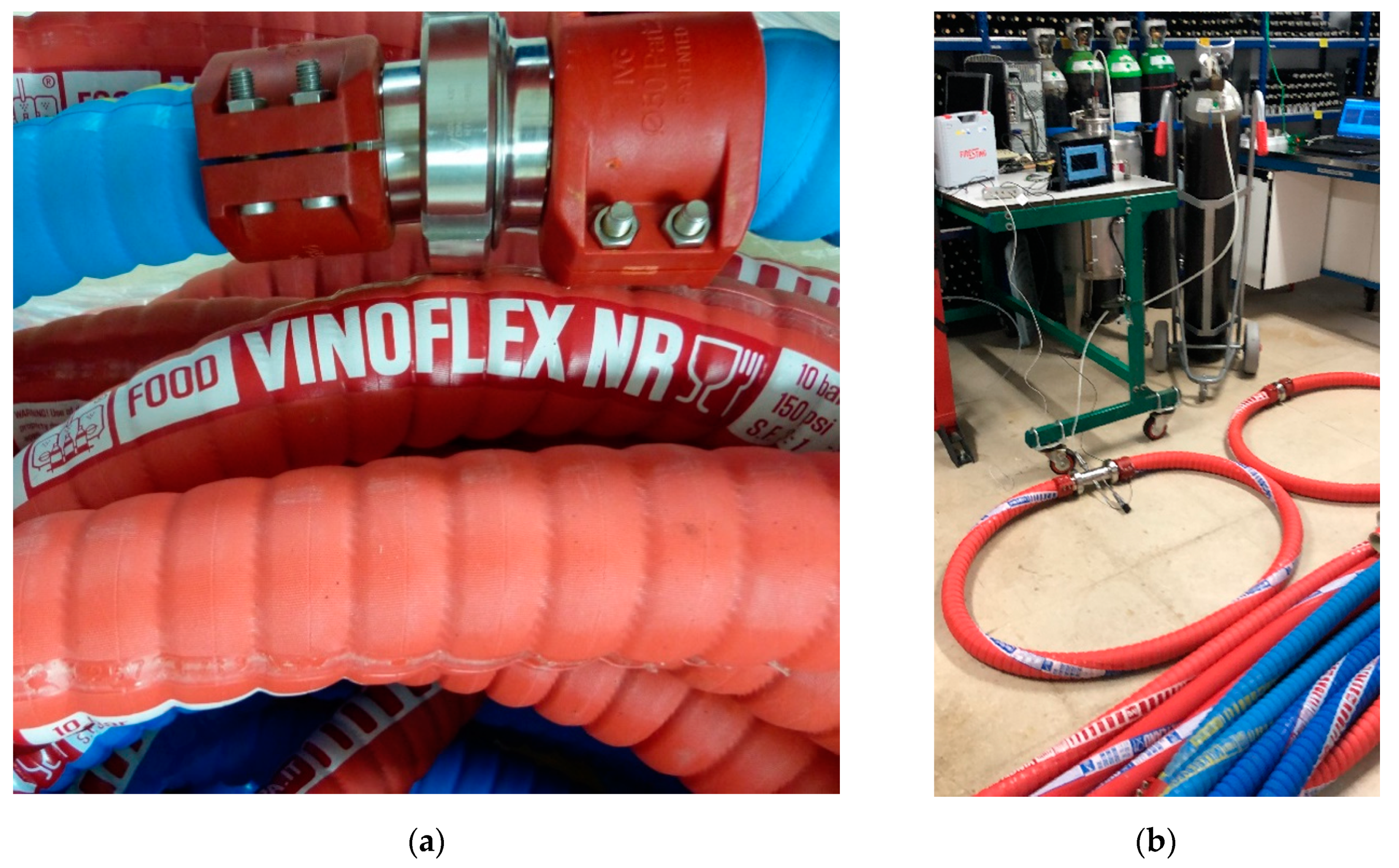
2.3. Hoses
2.3.1. Butyl Rubber
2.3.2. Nitrile Butadiene Rubber
2.3.3. Ethylene Propylene Diene Monomer Rubber
2.3.4. Ultra-High-Molecular-Weight Polyethylene
2.3.5. Natural Rubber
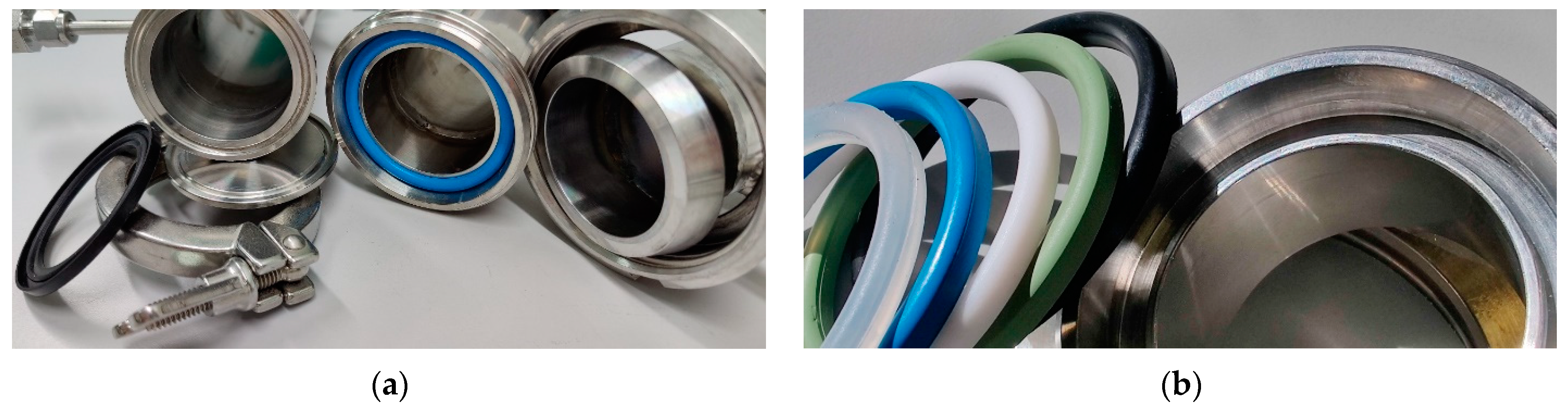
2.4. Connections
2.5. Inert Gases
2.5.1. Nitrogen (N2)
2.5.2. Carbon Dioxide (CO2)
2.5.3. Argon (Ar)
2.5.4. Mixture of Nitrogen and Carbon Dioxide (N2 + CO2)
2.5.5. Mixture of Argon and Carbon Dioxide (Ar + CO2)
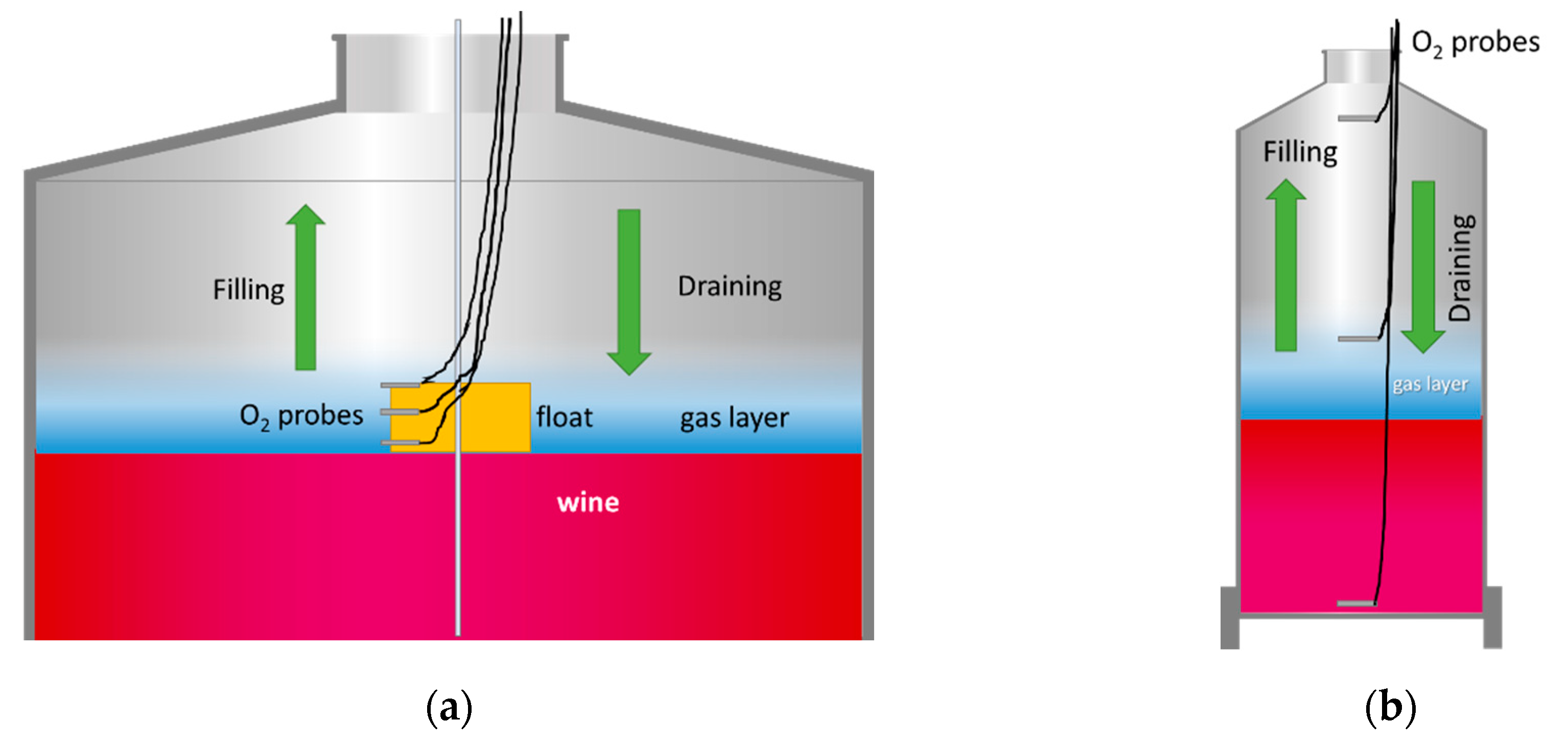
2.6. Measurement Protocols
2.6.1. Oxygen Permeability Measurement of Hoses and Connectors
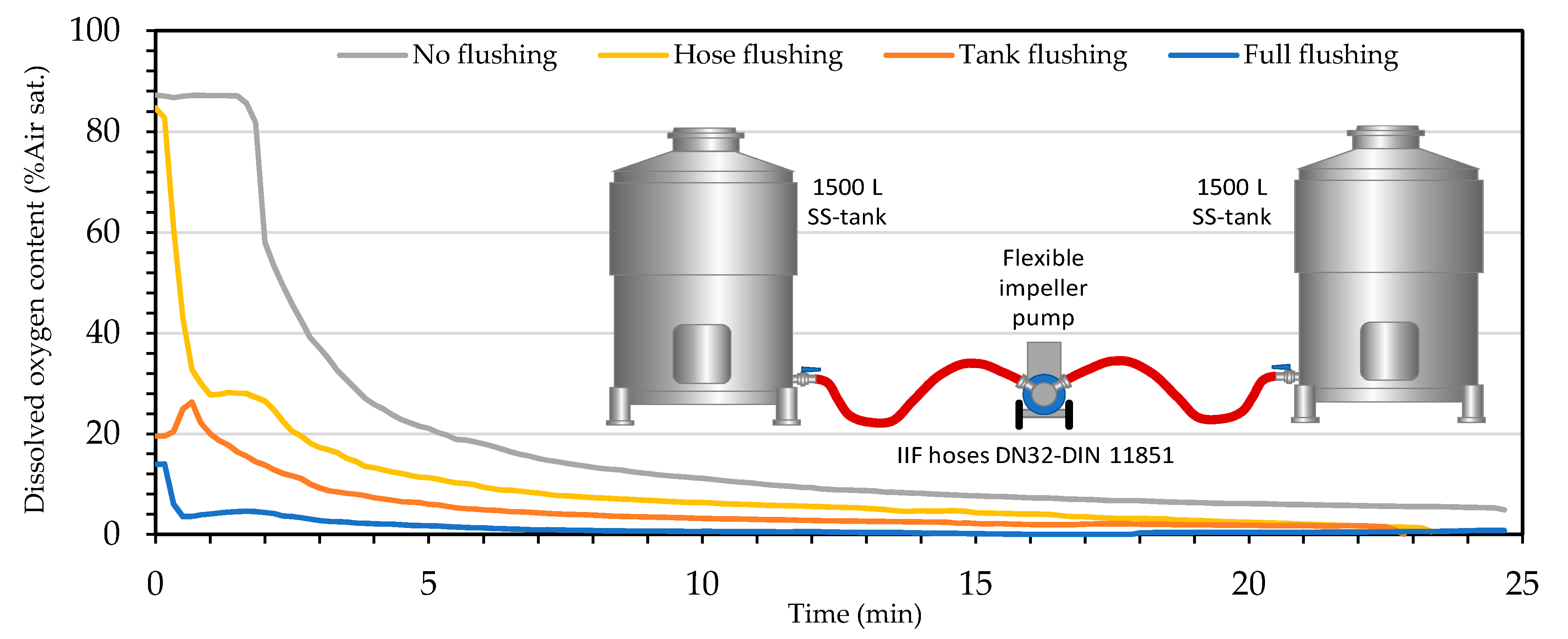
2.6.2. Hose Flushing with Inert Gases
2.6.3. Tank Flushing with Inert Gases
- Gas Phase Protocol
- Liquid Phase Protocol
2.7. Statistical Analysis
3. Results
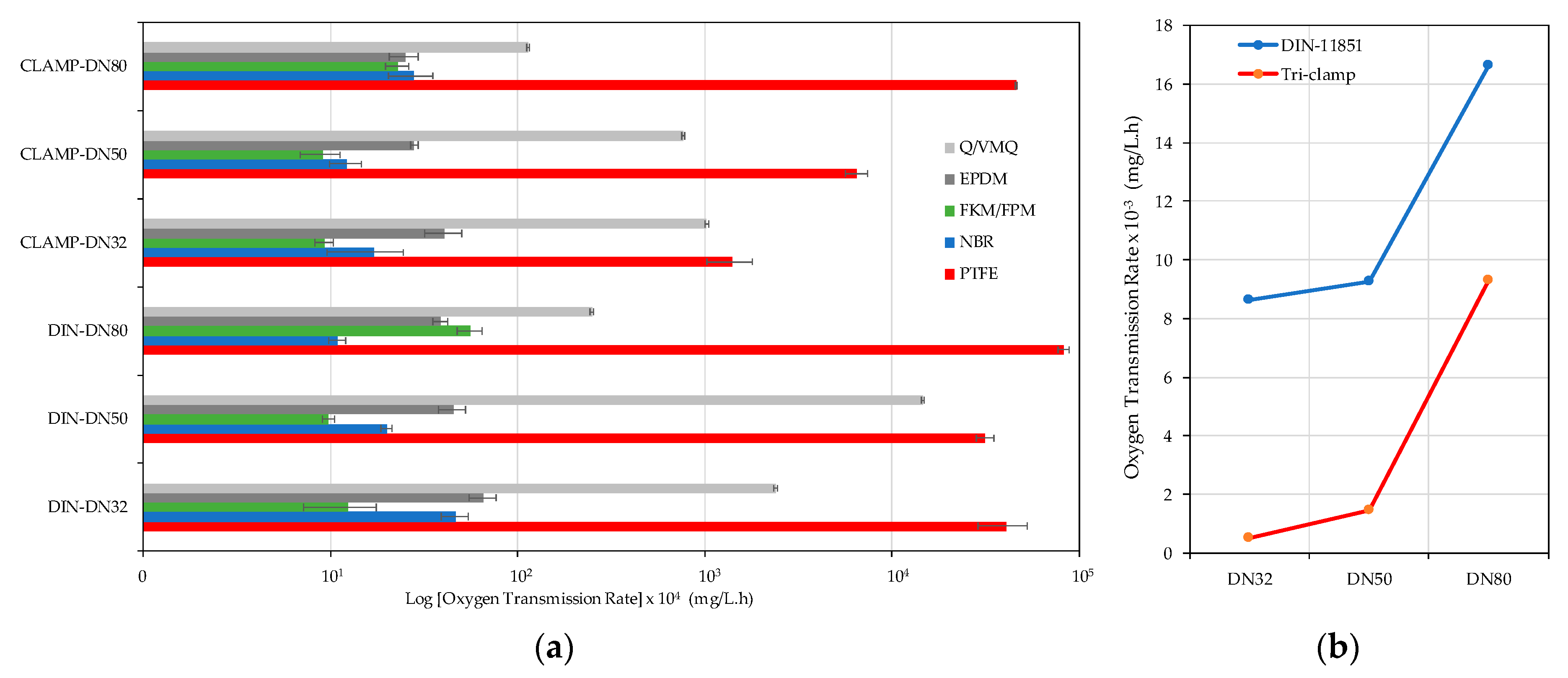
3.1. Connections
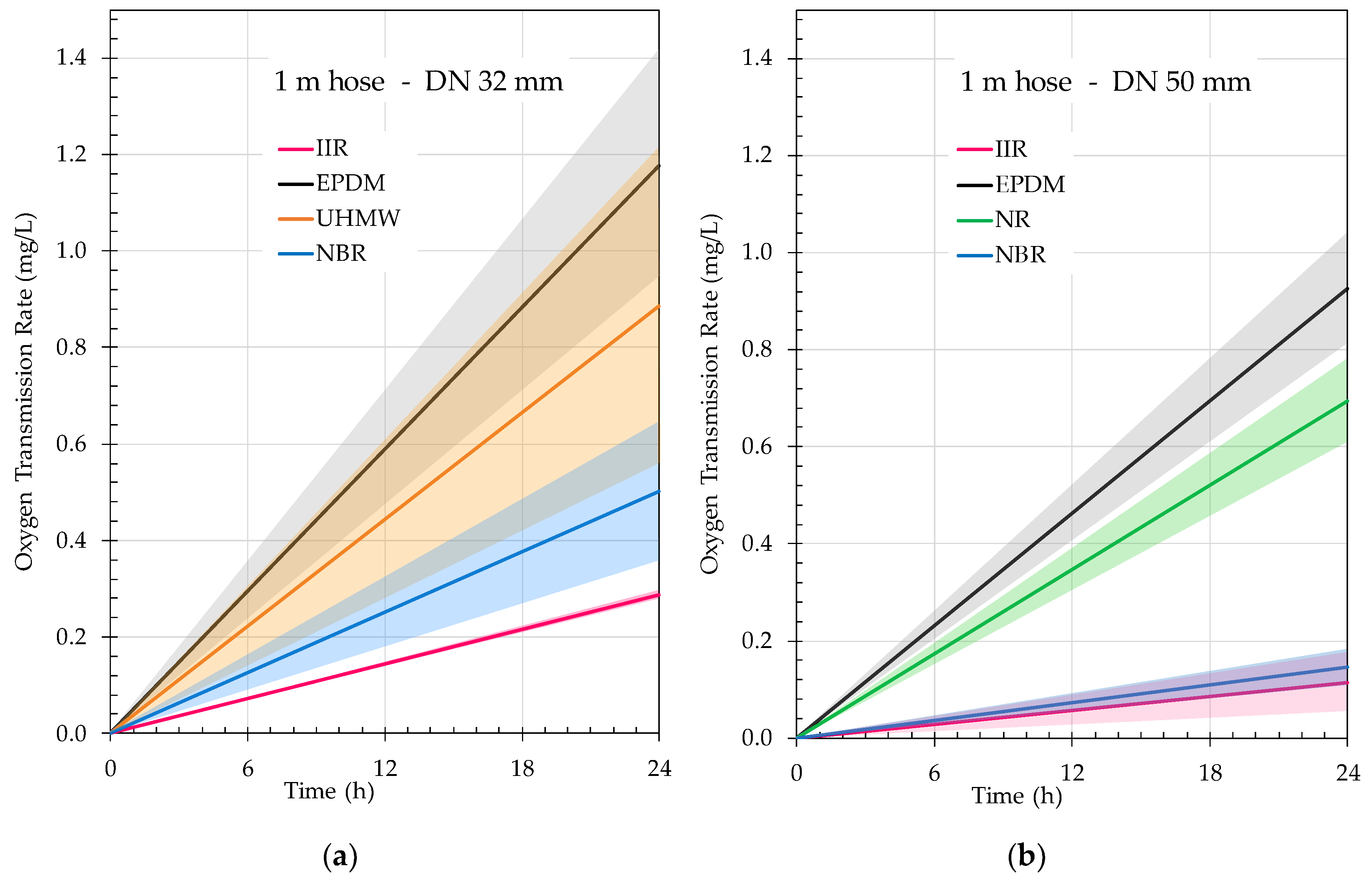
3.2. Hose Permeation
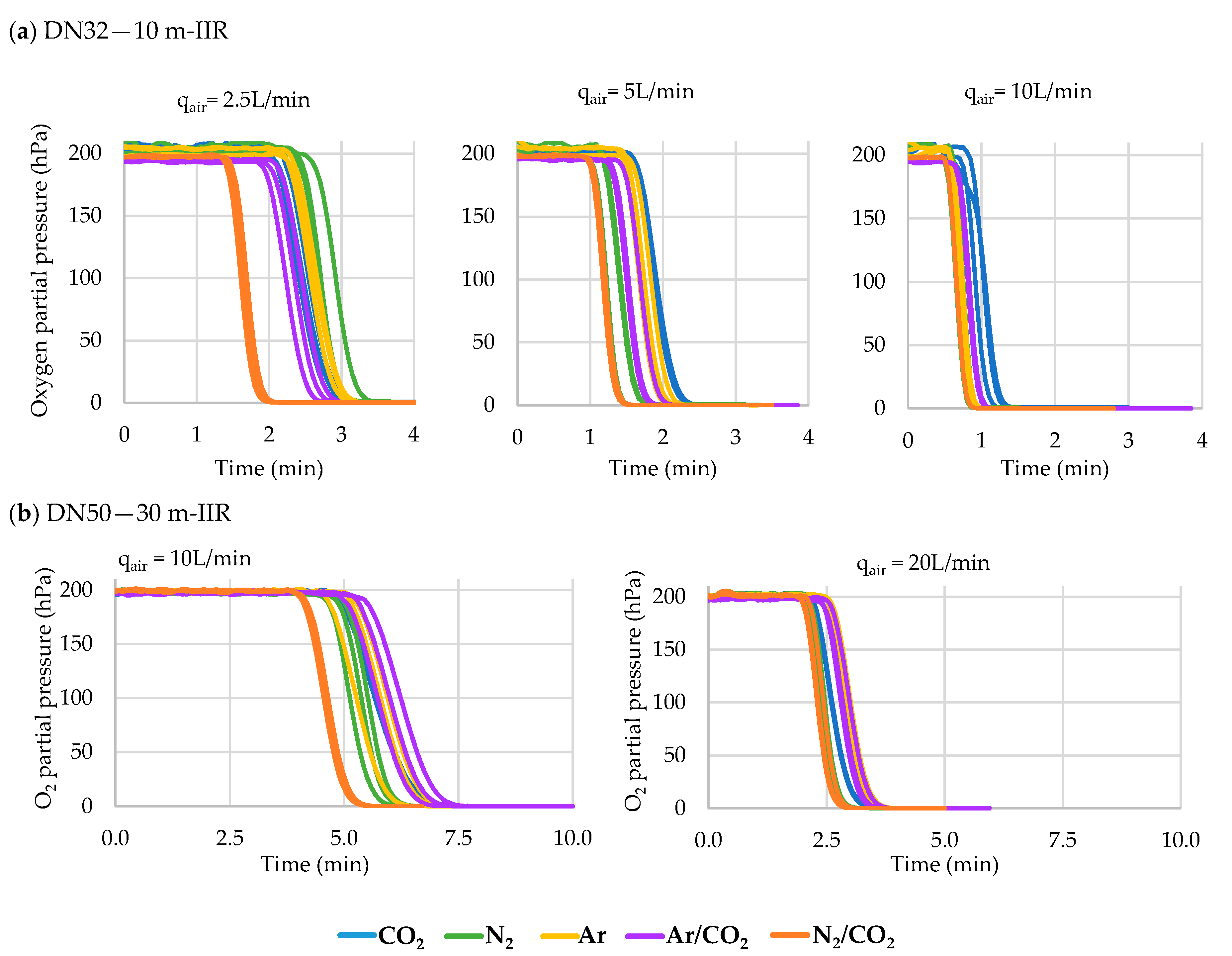
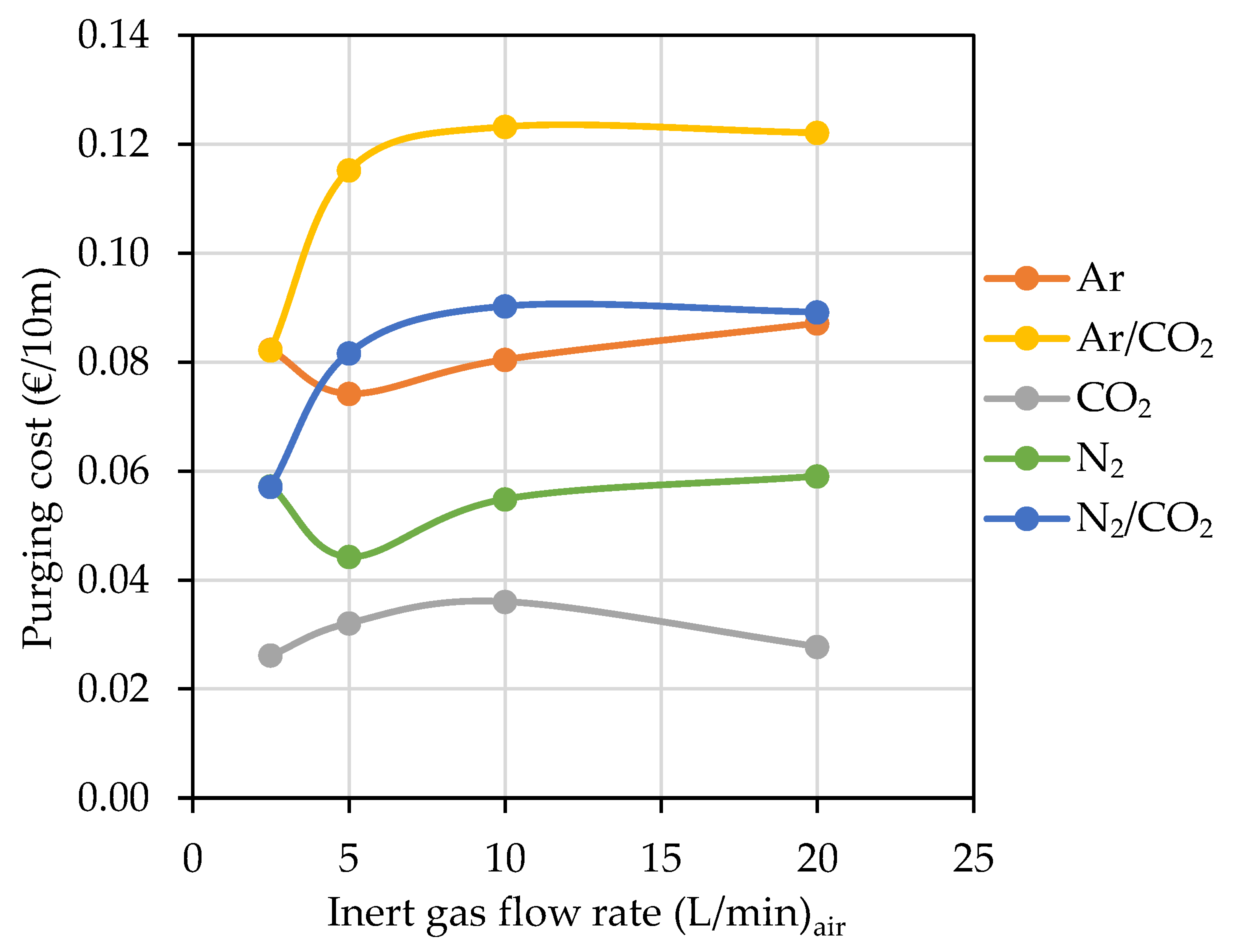
3.3. Hose Purging
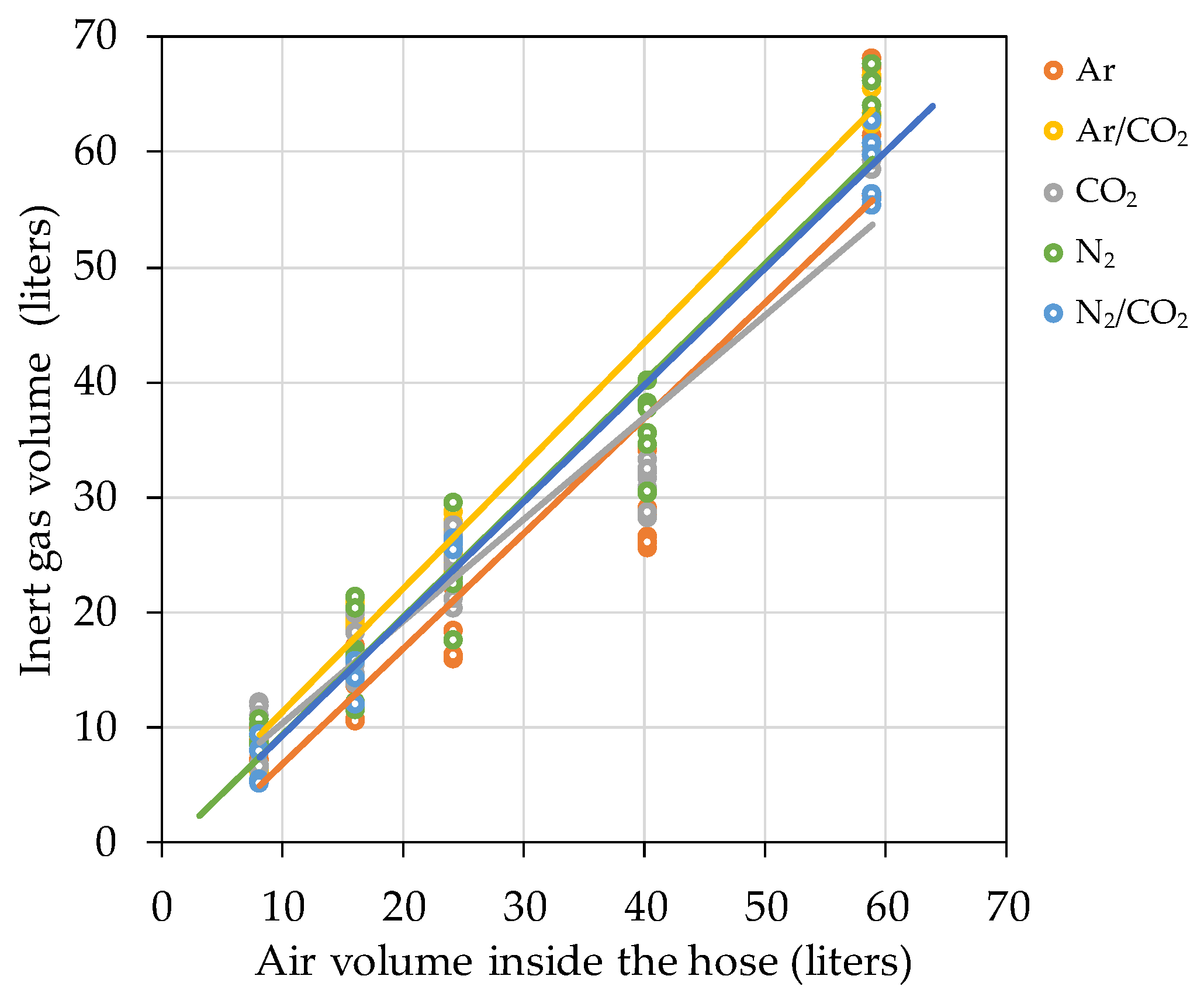
The Effect of Hose Length
3.4. Racking Tank to Tank
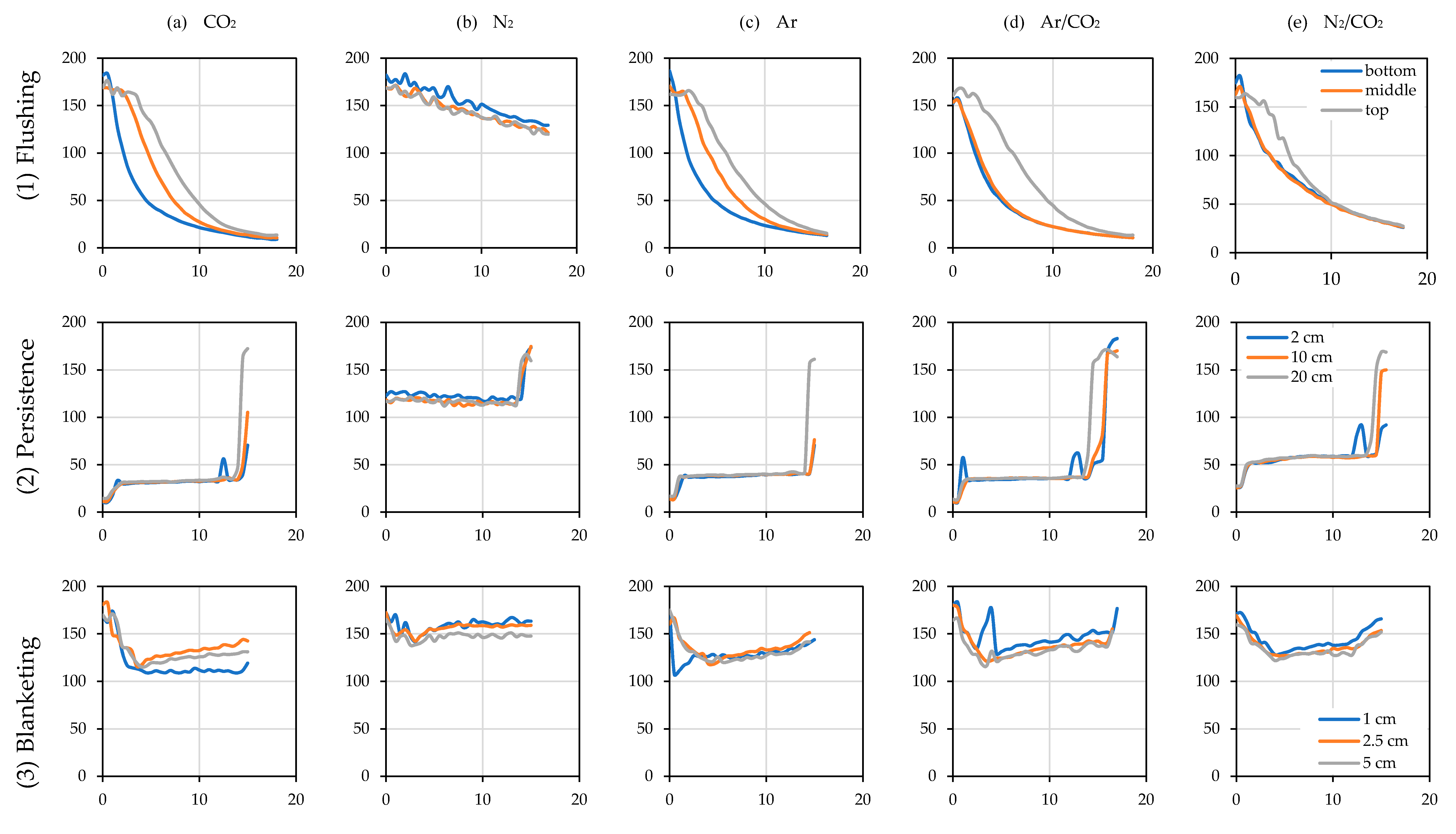
3.4.1. Tank Purging and Blanketing
3.4.2. Efficiency and Intensity in the Use of Inert Gases
3.4.3. Impact of Wine Transfer at Different Stages of Inert Gas Uses
4. Discussion
4.1. Connections
4.2. Hose Permeation
4.3. Hose Purging
4.4. Wine Racking and the Use of Inert Gases
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurie, V.F.; Clark, A.C. Wine oxidation. In Oxidation in Foods and Beverages and Antioxidant Applications. Volume 2: Management in Different Industry Sectors; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 445–475. ISBN 9781845699831. [Google Scholar]
- Moutounet, M.; Mazauric, J.-P. L’oxygène dissous dans les vins: Les gaz en oenologie. Rev. Française Oenol. 2001, 186, 12–15. [Google Scholar]
- Boulton, R.B. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996; ISBN 9780412064111. [Google Scholar]
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- Castellari, M.; Simonato, B.; Tornielli, G.B.; Spinelli, P.; Ferrarini, R. Effects of different enological treatments on dissolved oxygen in wines. Ital. J. Food Sci. 2004, 16, 387–396. [Google Scholar]
- Calderón, J.F.; del Alamo-Sanza, M.; Nevares, I.; Laurie, F. The influence of selected winemaking equipment and operations on the concentration of dissolved oxygen in wines. Cienc. Investig. Agrar. 2014, 41, 273–280. [Google Scholar] [CrossRef]
- Vidal, J.C.; Boulet, J.C.; Moutounet, M. Les apports d’oxygène au cours des traitements des vins. Bilan des observations sur site. 2ème partie. Rev. Française Oenol. 2003, 32–34, 36–38. [Google Scholar]
- Vidal, J.C.; Boulet, J.C.; Moutounet, M. Les apports d’oxygène au cours des traitements des vins. Bilan des observations sur site. 3ème partie. Rev. Française Oenol. 2004, 205, 25–33. [Google Scholar]
- Vidal, J.C.; Dufourcq, T.; Boulet, J.C.; Moutounet, M. Les apports d’oxygène au cours des traitements des vins. Bilan des observations sur site. 1ère partie. Rev. Française Oenol. 2001, 190, 24–31. [Google Scholar]
- Aziz, M.B.; M’Barek, H.N.; Douieb, H.; Hajjaj, H. Influence of Enological Treatments on Dissolved Oxygen Content of Moroccan Red Wine. Int. J. Adv. Res. 2016, 4, 156–160. [Google Scholar]
- Vidal, J.C.; Moutounet, M. La Maîtrise des apports d’oxygène au conditionnement. Rev. Française Oenol. 2009, 229, 19–29. [Google Scholar]
- Catarino, A.; Alves, S.; Mira, H. Influence of Technological Operations in the Dissolved Oxygen Content of Wines. J. Chem. Chem. Eng. 2014, 8, 390–394. [Google Scholar] [CrossRef]
- Ma, C.; Shero, E.; Verma, N.; Gilbert, S.; Shadman, F. Permeation of Moisture and Oxygen Through Polymeric O-Rings. J. IEST 1995, 38, 43–46. [Google Scholar] [CrossRef]
- Laurenson, L.; Dennis, N.T.M. Permeability of common elastomers for gases over a range of temperatures. J. Vac. Sci. Technol. A Vac. Surf. Films 1985, 3, 1707–1710. [Google Scholar] [CrossRef]
- Peacock, R.N. Practical Selection of Elastomer Materials for Vacuum Seals. J. Vac. Sci. Technol. 1979, 17, 330–336. [Google Scholar] [CrossRef]
- Sturm, P.; Leuenberger, M.; Sirignano, C.; Neubert, R.E.M.; Meijer, H.A.J.; Langenfelds, R.; Brand, W.A.; Tohjima, Y. Permeation of atmospheric gases through polymer O-rings used in flasks for air sampling. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Holland, L.; Steckelmacher, W.; Yarwood, J. Vacuum Manual; Holland, L., Steckelmacher, W., Yarwood, J., Eds.; E & FN Spon Ltd.: London, UK, 1974; ISBN 9789401181228. [Google Scholar]
- Zhang, H.; Cloud, A. The Permeability Characteristics of Silicone Rubber. In Proceedings of the Global Advances in Materials and Process Engineering: SAMPE Fall Technical Conference, Dallas, TX, USA, 6–9 November 2006; Holland, L., Steckelmacher, W., Yarwood, J., Eds.; Society for the Advancement of Material and Process Engineering: Dallas, TX, USA, 2006. [Google Scholar]
- Massey, L.K. Permeability Properties of Plastics and Elastomers—A Guide to Packaging and Barrier Materials; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Maolin, C.; Changchun, W. Principles of and tips for nitrogen displacement in gas pipeline commissioning. Nat. Gas Ind. B 2015, 2, 263–269. [Google Scholar]
- Johnson, J.E.; Svedeman, S.J.; Kuhl, C.A.; Gregor, J.G.; Lambeth, A.K. Pipeline Purging Principles and Practice. In Proceedings of the Volume 2: Design, Construction, and Operation Innovations; Compression and Pump Technology; SCADA, Automation, and Measurement; System Simulation; Geotechnical and Environmental, Calgary, AB, Canada, 9–13 June 1996; American Society of Mechanical Engineers: New York, NY, USA, 1996; Volume 2, pp. 765–775. [Google Scholar]
- Lonvaud-Funel, A.; Matsumoto, M. Le coefficient de solubilité du gaz carbonique dans les vins. VITIS J. Grapevine Res. 1979, 18, 137–147. [Google Scholar]
- McKetta, J. Encyclopedia of Chemical Processing and Design; Marcel Dekker: New York, NY, USA; Basel, Switzerland; Hong Kong, China, 1996; Volume 56, ISBN 9780824724962. [Google Scholar]
- Hall, S. Rules of Thumb for Chemical Engineers, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128110386. [Google Scholar]
- Girardon, P. Gases in Enology. In Gases in Agro-Food Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 433–449. ISBN 9780128124659. [Google Scholar]
- Goode, J. Oxygen & Wine. Research gets specific about oxygen, closures, bottling and aging. Wines Vines 2009, 90, 26–32. [Google Scholar]
- Dimkou, E.; Ugliano, M.; Dieval, J.B.; Vidal, S.; Aagaard, O.; Rauhut, D.; Jung, R. Impact of Headspace Oxygen and Closure on Sulfur Dioxide, Color, and Hydrogen Sulfide Levels in a Riesling Wine. Am. J. Enol. Vitic. 2011, 62, 261–269. [Google Scholar] [CrossRef]


| Fluid | EPDM | NBR | Q/VMQ | FKM/FPM | PTFE | NR | UHMW | IIR |
|---|---|---|---|---|---|---|---|---|
| Wines and yeast | 4 | 3 | 4 | 2–3 | 3–4 | 4 | 4 | 4 |
| Concentrated fruit juices | 1 | 1 | 1 | 3 | 3–4 | 4 | 4 | 4 |
| Brewery | 3–4 | 3 | 1–2 | 2–3 | 3–4 | 4 | 4 | 4 |
| Alcohols | 2–3 | 1–3 | 3–4 | 3–4 | 3–4 | 4 | 4 | 4 |
| Ozone and Atmospheric Conditions | 4 | 1–2 | 4 | 3–4 | 3–4 | 4 | 3 | 4 |
| Connection Type | Diameter (mm) | Materials | |||||
|---|---|---|---|---|---|---|---|
| NBR | FKM/FPM | Q/VMQ | EPDM | PTFE | p Level | ||
| DIN 11851 | DN32 | 3.50 ± 0.57 c | 0.93 ± 0.39 a | 179.08 ± 4.13 b | 4.94 ± 0.83 a | 3046.83 ± 893.96 b | 0.0102 * |
| DN50 | 1.50 ± 0.10 b | 0.73 ± 0.06 a | 1093.15 ± 21.02 c | 3.40 ± 0.56 a | 2366.80 ± 251.10 a | 0.0001 *** | |
| DN80 | 0.82 ± 0.08 a | 4.20 ± 0.66 b | 18.68 ± 0.48 a | 2.90 ± 0.26 a | 6194.63 ± 400.80 c | 0.0000 *** | |
| Tri-clamp | DN32 | 1.28 ± 0.56 a | 0.70 ± 0.08 a | 76.70 ± 1.64 c | 3.06 ± 0.68 a | 105.25 ± 28.26 a | 0.005 ** |
| DN50 | 0.92 ± 0.18 b | 0.68 ± 0.16 b | 57.40 ± 1.02 b | 2.10 ± 0.10 a | 487.97 ± 67.26 b | 0.0003 *** | |
| DN80 | 2.08 ± 0.55 c | 1.72 ± 0.24 c | 8.53 ± 0.14 a | 1.88 ± 0.33 a | 3459 ± 41.21 c | 0.0000 *** | |
| Diameter (mm) | Material | OTR Measured (hPa/h) | OTR Hose (1,2) (hPa/h) | OTR Hose (1,2) (hPa/h·m) |
|---|---|---|---|---|
| DN32 | IIR | 0.0291 ± 0.0003 a | 0.0268 ± 0.00001 a | 0.0089 ± 0.00001 a |
| EPDM | 0.1125 ± 0.0098 b | 0.1102 ± 0.0095 b | 0.0367 ± 0.0095 a | |
| UHMW | 0.0852 ± 0.0136 b | 0.0829 ± 0.0133 b | 0.0276 ± 0.0133 a | |
| NBR | 0.0493 ± 0.0060 a | 0.0470 ± 0.0057 a | 0.0157 ± 0.0057 a | |
| p level | 0.0093 ** | 0.0084 ** | 0.2508 | |
| DN50 | IIR | 0.0200 ± 0.0041 a | 0.0107 ± 0.0015 a | 0.0036 ± 0.0025 a |
| EPDM | 0.0958 ± 0.0090 b | 0.0865 ± 0.0074 c | 0.0288 ± 0.0074 c | |
| NR | 0.0742 ± 0.0064 b | 0.0650 ± 0.0048 b | 0.0217 ± 0.0048 bc | |
| NBR | 0.0230 ± 0.0018 a | 0.0137 ± 0.0002 a | 0.0046 ± 0.0002 ab | |
| p level | 0.0019 ** | 0.0007 *** | 0.0405 * |
| Diameter | Material | Flow Rate | CO2 | N2 | Ar | Ar/CO2 | N2/CO2 | p Level |
|---|---|---|---|---|---|---|---|---|
| DN32 10 m | IIR | 10 | 1.433 ± 0.076 a | 1.000 ± 0.050 a | 1.050 ± 0.000 a | 1.200 ± 0.000 a | 0.950 ± 0.000 a | 0.0000 *** |
| 5 | 2.533 ± 0.029 b | 1.830 ± 0.156 b | 2.300 ± 0.100 b | 2.067 ± 0.115 b | 1.617 ± 0.029 b | 0.0000 *** | ||
| 2.5 | 3.233 ± 0.076 c | 3.450 ± 0.132 c | 3.333 ± 0.058 c | 3.000 ± 0.150 c | 2.150 ± 0.050 c | 0.0000 *** | ||
| NBR | 10 | 1.283 ± 0.029 a | 1.117 ± 0.058 a | 0.767 ± 0.664 a | 1.200 ± 0.000 a | 1.000 ± 0.000 a | 0.0000 *** | |
| 5 | 2.083 ± 0.029 b | 1.810 ± 0.053 b | 2.517 ± 0.029 b | 2.217 ± 0.076 b | 1.700 ± 0.000 b | 0.0000 *** | ||
| 2.5 | 3.150 ± 0.087 c | 2.383 ± 0.029 c | 4.017 ± 0.176 c | 3.067 ± 0.029 c | 1.883 ± 0.029 c | 0.3146 | ||
| UHMW | 10 | 1.317 ± 0.029 a | 1.067 ± 0.029 a | 1.250 ± 0.000 a | 1.050 ± 0.000 a | 0.950 ± 0.000 a | 0.0000 *** | |
| 5 | 2.053 ± 0.046 b | 1.667 ± 0.029 b | 2.083 ± 0.029 b | 2.067 ± 0.029 b | 1.633 ± 0.029 b | 0.0000 *** | ||
| 2.5 | 3.300 ± 0.050 c | 2.467 ± 0.029 c | 3.100 ± 0.087 c | 2.983 ± 0.029 c | 2.200 ± 0.000 c | 0.0000 *** | ||
| EPDM | 10 | 1.417 ± 0.029 a | 1.133 ± 0.029 a | 1.167 ± 0.029 a | 1.150 ± 0.000 a | 1.050 ± 0.000 a | 0.0000 *** | |
| 5 | 2.313 ± 0.129 b | 1.890 ± 0.052 b | 2.367 ± 0.029 b | 2.400 ± 0.050 b | 1.717 ± 0.029 b | 0.0000 *** | ||
| 2.5 | 3.317 ± 0.076 c | 3.167 ± 0.275 c | 3.167 ± 0.029 c | 3.183 ± 0.076 c | 2.217 ± 0.029 c | 0.0000 *** | ||
| DN50 30 m | IIR | 20 | 3.633 ± 0.029 a | 3.150 ± 0.087 a | 3.950 ± 0.050 a | 3.800 ± 0.132 a | 3.117 ± 0.076 a | 0.5211 |
| 10 | 4.917 ± 4.258 b | 6.483 ± 0.176 b | 7.067 ± 0.416 b | 7.450 ± 0.300 b | 5.700 ± 0.050 b | 0.0000 *** | ||
| NBR | 20 | 3.683 ± 0.275 a | 3.117 ± 0.116 a | 3.700 ± 0.132 a | 3.650 ± 0.132 a | 3.000 ± 0.265 a | 0.0535 | |
| 10 | 6.883 ± 1.358 b | 6.050 ± 0.218 b | 7.567 ± 0.202 b | 7.717 ± 0.633 b | 6.367 ± 0.104 b | 0.0023 ** | ||
| UHMW | 20 | 3.867 ± 0.076 a | 2.650 ± 0.000 a | 4.017 ± 0.076 a | 3.917 ± 0.104 a | 3.150 ± 0.050 a | 0.0000*** | |
| 10 | 8.217 ± 0.189 b | 6.083 ± 0.058 b | 7.867 ± 0.058 b | 8.033 ± 0.176 b | 6.783 ± 0.104 b | 0.0000 *** | ||
| EPDM | 20 | 3.450 ± 0.132 a | 2.917 ± 0.116 a | 3.550 ± 0.050 a | 3.767 ± 0.144 a | 3.267 ± 0.362 a | 0.0031 ** | |
| 10 | 6.983 ± 0.765 b | 5.833 ± 0.318 b | 7.550 ± 0.087 b | 7.750 ± 0.278 b | 6.650 ± 0.520 b | 0.0032 ** | ||
| NR | 20 | 3.883 ± 0.340 a | 3.117 ± 0.231 a | 3.750 ± 0.218 a | 3.633 ± 0.104 a | 3.367 ± 0.116 a | 0.0001 *** | |
| 10 | 8.300 ± 0.755 b | 5.783 ± 0.116 b | 7.600 ± 0.328 b | 7.283 ± 0.202 b | 6.650 ± 0.132 b | 0.0105 ** |
| Flow Rate (L/min) | Inert Gas | Lengths (m) | |||
|---|---|---|---|---|---|
| 10 m | 20 m | 30 m | 50 m | ||
| 5 | CO2 | 3.23 ± 0.08 b,c | 3.73 ± 0.12 b | 6.18 ± 0.37 c | 7.32 ± 0.55 a |
| N2 | 3.45 ± 0.13 c | 2.33 ± 0.08 a | 3.45 ± 0.00 a | 5.97 ± 0.03 a | |
| Ar | 3.33 ± 0.06 b,c | 2.47 ± 0.03 a | 3.78 ± 0.03 a | 6.12 ± 0.13 a | |
| Ar/CO2 | 3.00 ± 0.15 b | 4.35 ± 0.09 b | 6.20 ± 0.25 c | ||
| N2/CO2 | 2.15 ± 0.05 a | 2.45 ± 0.00 a | 5.30 ± 0.09 b | ||
| p level | 0.0015 ** | 0.0000 *** | 0.0005 *** | 0.1077 | |
| 10 | CO2 | 2.53 ± 0.03 b | 2.32 ± 0.12 b | 3.25 ± 0.13 c | 3.88 ± 0.08 a |
| N2 | 1.83 ± 0.15 ab | 1.62 ± 0.03 a | 2.22 ± 0.03 a | 3.80 ± 0.13 a | |
| Ar | 2.30 ± 0.10 c | 1.62 ± 0.03 a | 2.15 ± 0.00 a | 3.43 ± 0.10 a | |
| Ar/CO2 | 2.07 ± 0.12 bc | 2.47 ± 0.03 b | 2.82 ± 0.03 b | ||
| N2/CO2 | 1.62 ± 0.03 a | 1.45 ± 0.00 a | 2.62 ± 0.03 b | ||
| p level | 0.0069 ** | 0.0002 *** | 0.0003 *** | 0.1061 | |
| 20 | CO2 | 1.43 ± 0.08 c | 0.88 ± 0.03 ab | 1.32 ± 0.08 a | 2.03 ± 0.03 b |
| N2 | 1.00 ± 0.05 a | 1.02 ± 0.03 c | 1.45 ± 0.00 a | 1.72 ± 0.03 a | |
| Ar | 1.05 ± 0.00 ab | 0.97 ± 0.03 bc | 1.33 ± 0.06 a | 1.98 ± 0.03 b | |
| Ar/CO2 | 1.20 ± 0.00 b | 1.12 ± 0.03 d | 1.67 ± 0.03 b | ||
| N2/CO2 | 0.95 ± 0.00 a | 0.80 ± 0.00 a | 1.32 ± 0.03 a | ||
| p level | 0.0025 ** | 0.0024 ** | 0.0142 *** | 0.010 * | |
| Gas | Blanketing Full Tank | Flushing Empty Tank | Both Tanks Inertized | |||
|---|---|---|---|---|---|---|
| 1000 L | 1500 L | 1000 L | 1500 L | 1000 L | 1500 L | |
| CO2 | 11.67 | 12.32 | 10.33 | 9.32 | 11.08 | 13.84 |
| N2 | 12.00 | 8.39 | 5.30 | 8.38 | 4.84 | 11.73 |
| Ar | 11.58 | 5.64 | 6.26 | 6.74 | 5.34 | 11.06 |
| Ar/CO2 | 11.30 | 10.69 | 5.99 | 9.33 | 6.33 | 11.03 |
| N2/CO2 | 12.22 | 6.00 | 6.06 | 8.87 | 7.39 | 12.69 |
| No gas | 14.92 | 12.61 | 15.13 | 11.80 | 15.07 | |
| Gas Volume | 1500 L Tank (Ar) | 1000 L Tank (CO2) | ||||
|---|---|---|---|---|---|---|
| Initial pO2 | Final pO2 | ΔpO2 | Initial pO2 | Final pO2 | ΔpO2 | |
| Half tank | 1.431 | 5.345 | 3.914 | 0.736 | 5.206 | 4.470 |
| Third tank | 0.367 | 7.108 | 6.741 | 0.860 | 6.158 | 5.298 |
| Quarter tank | 1.322 | 8.528 | 7.206 | 1.462 | 8.419 | 6.957 |
| O2 Addition to Wine | No Inert Gases | Hoses Purged (N2) | Tank Flushed (CO2) | Full Flushing & Blanketing |
|---|---|---|---|---|
| hPa | 12.80 | 8.40 | 4.30 | 0.25 |
| % air sat. | 6.70 | 4.39 | 2.23 | 0.13 |
| % O2 | 1.39 | 0.92 | 0.47 | 0.03 |
| mg/L | 0.56 | 0.38 | 0.18 | 0.01 |
| Material | DIN 11851 | Tri-Clamp | ||||
|---|---|---|---|---|---|---|
| DN32 | DN50 | DN80 | DN32 | DN50 | DN80 | |
| PTFE | 3294 | 3227 | 1475 | 150 | 718 | 2011 |
| NBR | 4 | 2 | 0.2 | 2 | 1 | 1 |
| FKM/FPM | 1 | 1 | 1 | 1 | 1 | 1 |
| Q/VMQ | 194 | 1491 | 4 | 110 | 84 | 5 |
| EPDM | 5 | 5 | 1 | 4 | 3 | 1 |
| Gas | ||||||
| Gas Properties | CO2 | Ar | N2/CO2 | N2 | Ar/CO2 | |
| Density (g/L) | 1.840 | 1.669 | 1.300 | 1.169 | 1.410 | |
| Specific gravity * | 1.502 | 1.362 | 1.061 | 0.955 | 1.151 | |
| Gas cost €/L | 0.0040 | 0.0116 | 0.0109 | 0.0065 | 0.0130 | |
| Hose material | Inertization data | CO2 | Ar | N2/CO2 | N2 | Ar/CO2 |
| IIR | Iva/vg | 0.8857 | 1 | 1.0128 | 1.0225 | 1.0678 |
| Purge cost (10−3 €/L) | 3.54 | 11.59 | 11.00 | 6.67 | 13.87 | |
| R2 | 0.9314 | 0.8984 | 0.9852 | 0.9275 | 0.9899 | |
| NBR | Iva/vg | 0.8872 | 1.0501 | 1.0062 | 1.0534 | 1.1099 |
| Purge cost (10−3 €/L) | 3.54 | 12.17 | 10.92 | 6.87 | 14.42 | |
| R2 | 0.9833 | 0.9967 | 0.9889 | 0.9957 | 0.993 | |
| EPDM | Iva/vg | 0.9583 | 1.0496 | 1.1751 | 0.9433 | 1.0839 |
| Purge cost (10−3 €/L) | 3.83 | 12.16 | 12.76 | 6.16 | 14.08 | |
| R2 | 0.9912 | 0.9949 | 0.9969 | 0.9976 | 0.9975 | |
| UHMW | Iva/vg | 1.1035 | 1.1615 | 1.1107 | 0.9702 | 1.1594 |
| Purge cost (10−3 €/L) | 4.41 | 13.46 | 12.06 | 6.33 | 15.06 | |
| R2 | 0.9952 | 0.9972 | 0.9947 | 0.9857 | 0.9978 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevares, I.; Fernández-Díaz, A.; del Alamo-Sanza, M. Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking. Foods 2021, 10, 386. https://doi.org/10.3390/foods10020386
Nevares I, Fernández-Díaz A, del Alamo-Sanza M. Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking. Foods. 2021; 10(2):386. https://doi.org/10.3390/foods10020386
Chicago/Turabian StyleNevares, Ignacio, Ainara Fernández-Díaz, and Maria del Alamo-Sanza. 2021. "Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking" Foods 10, no. 2: 386. https://doi.org/10.3390/foods10020386
APA StyleNevares, I., Fernández-Díaz, A., & del Alamo-Sanza, M. (2021). Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking. Foods, 10(2), 386. https://doi.org/10.3390/foods10020386









