Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising
Abstract
:1. Introduction
2. Materials and Methods
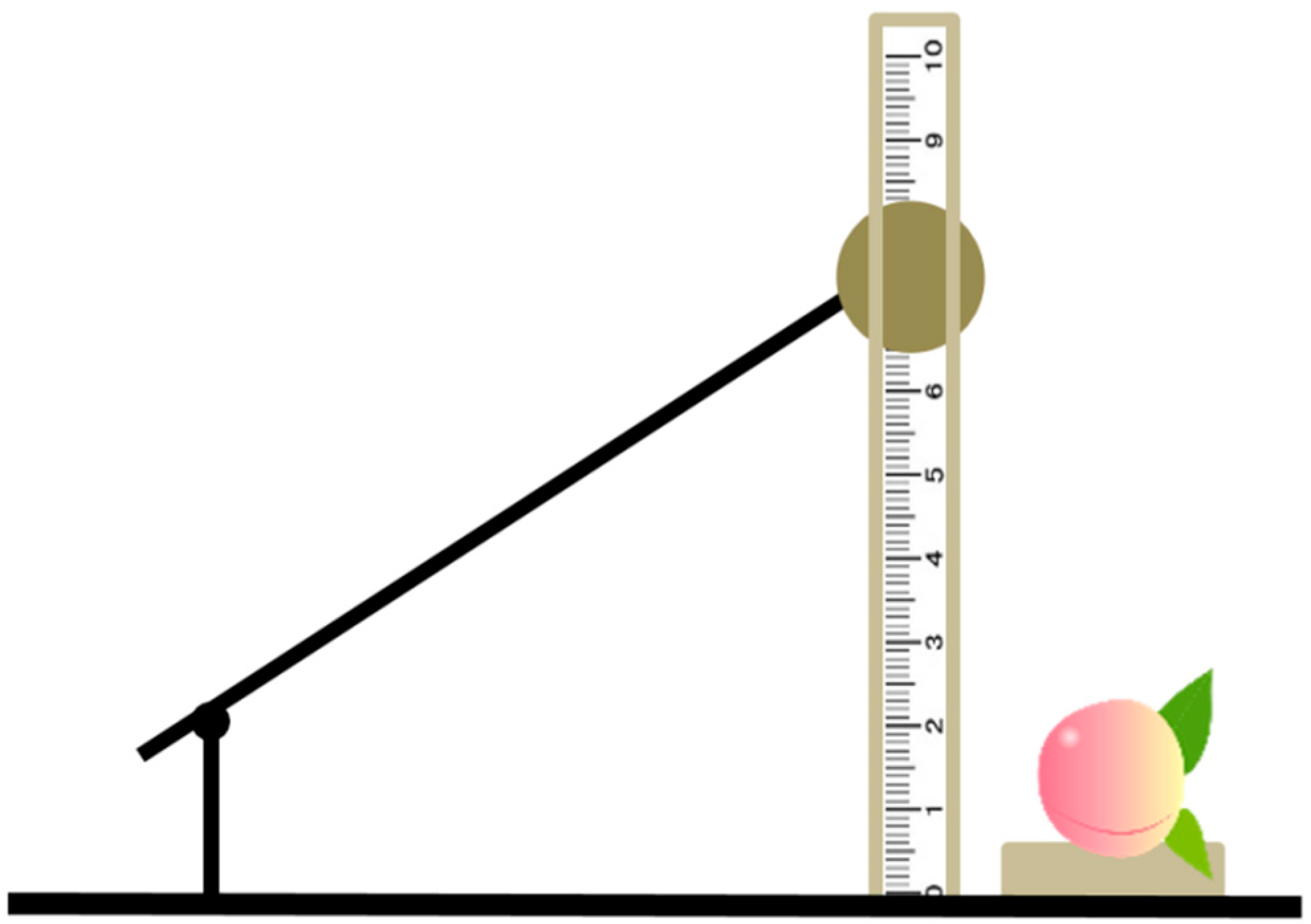
2.1. Sample Collection
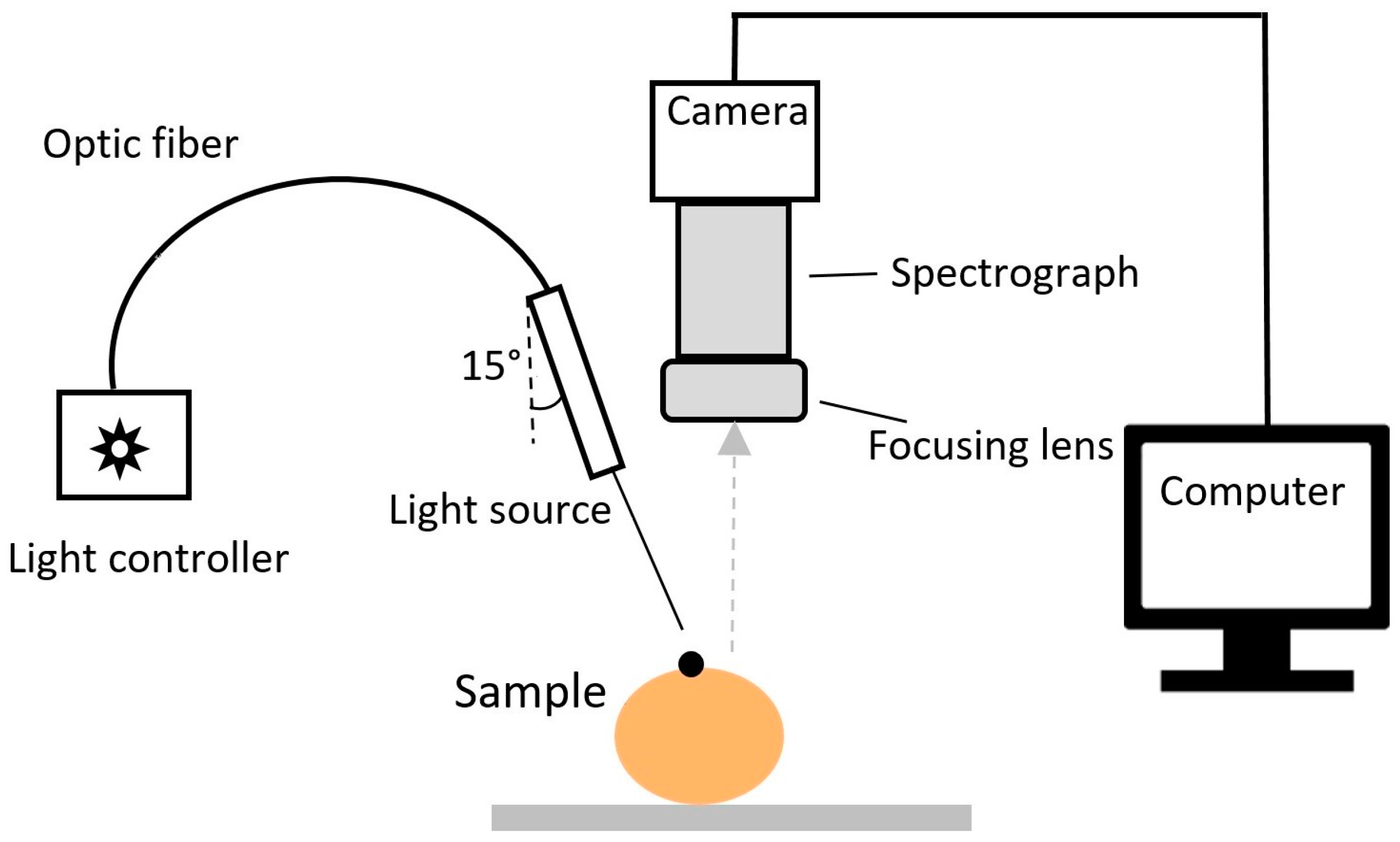
2.2. Image Acquisition and Pre-Processing
2.3. Color Measurement
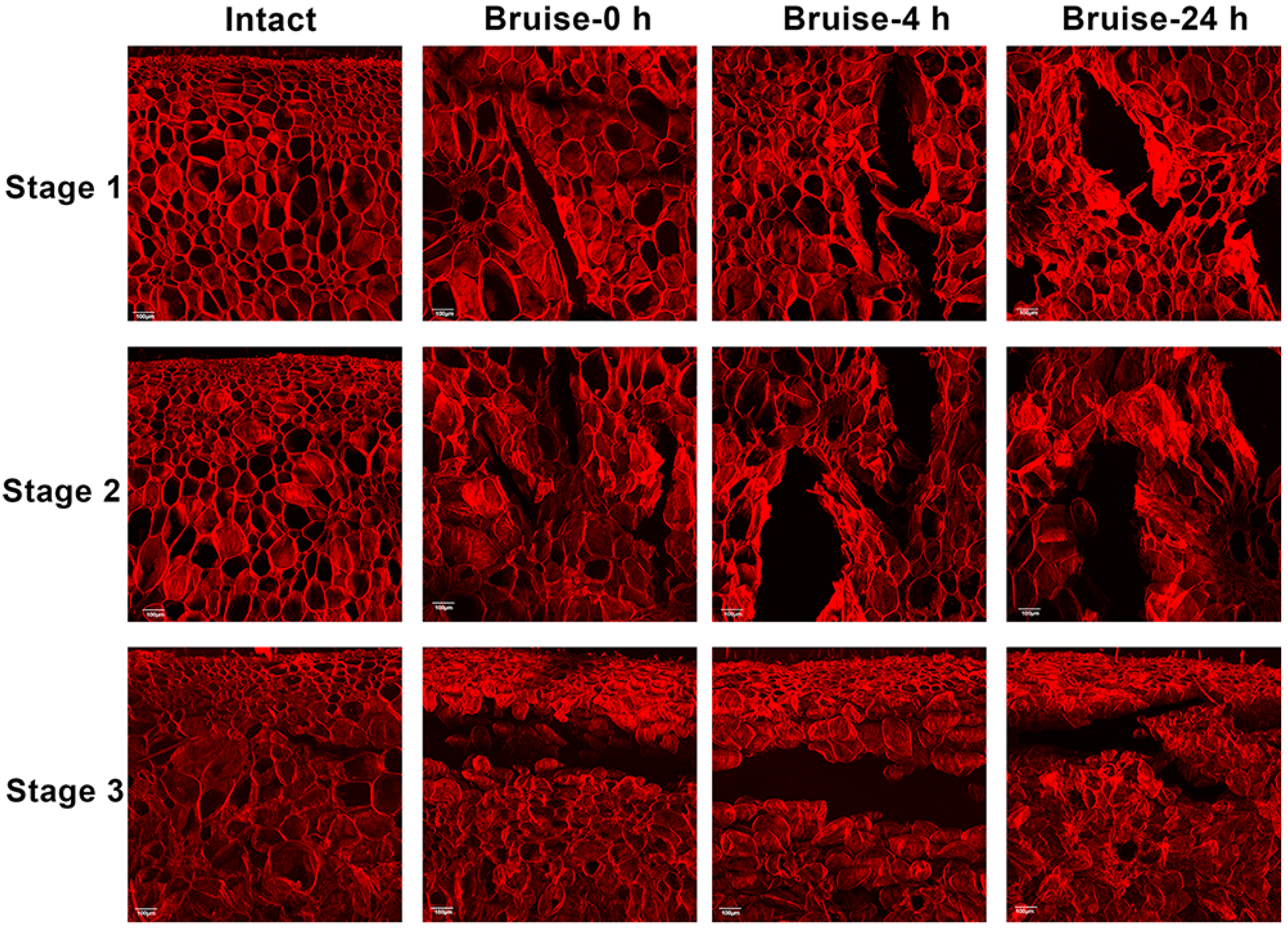
2.4. Microstructural Analysis
2.5. Data Processing and Analysis
3. Results and Discussion
3.1. Quality Parameter of Bruised Peaches
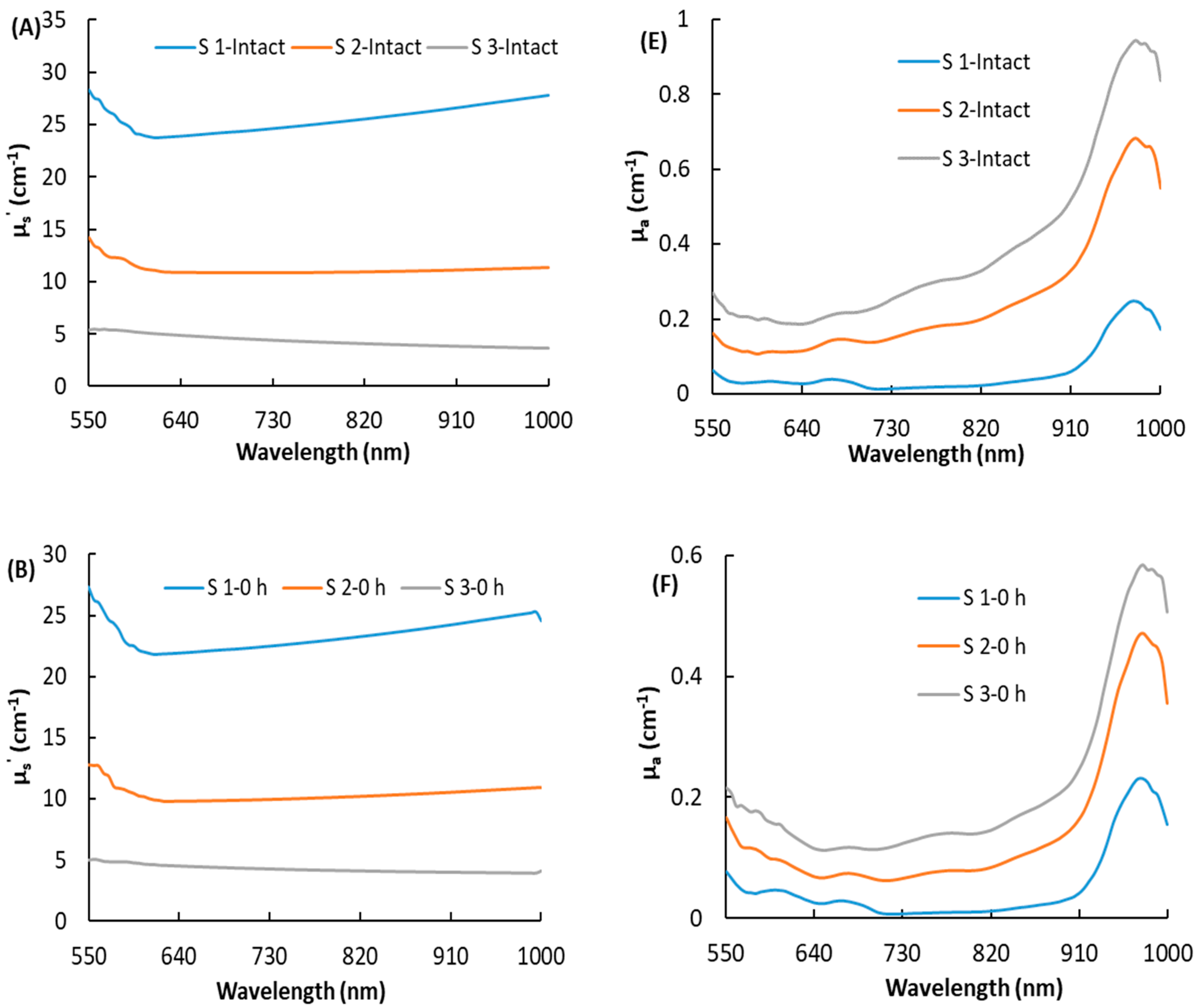
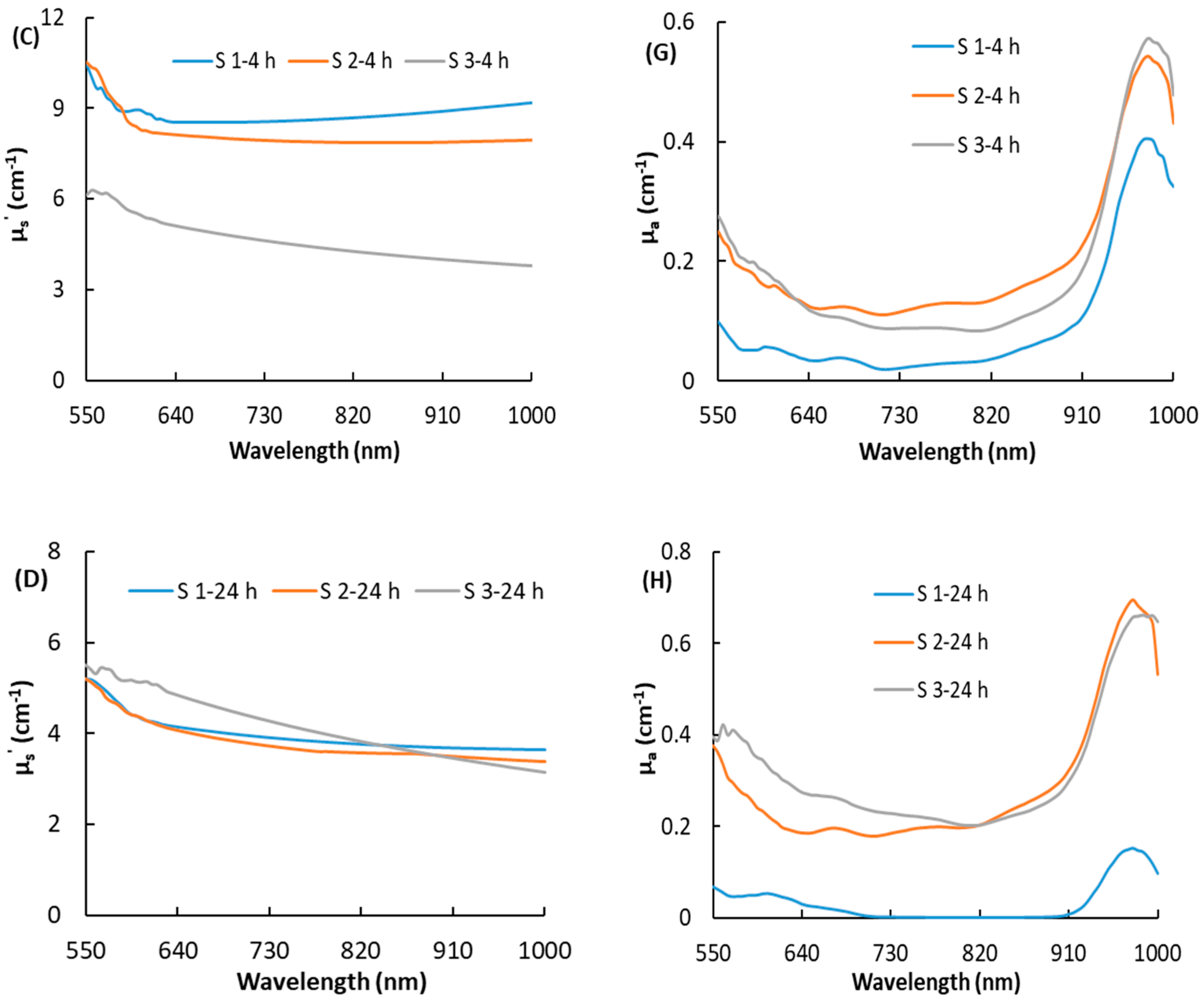
3.2. Optical Properties of Bruised Peaches
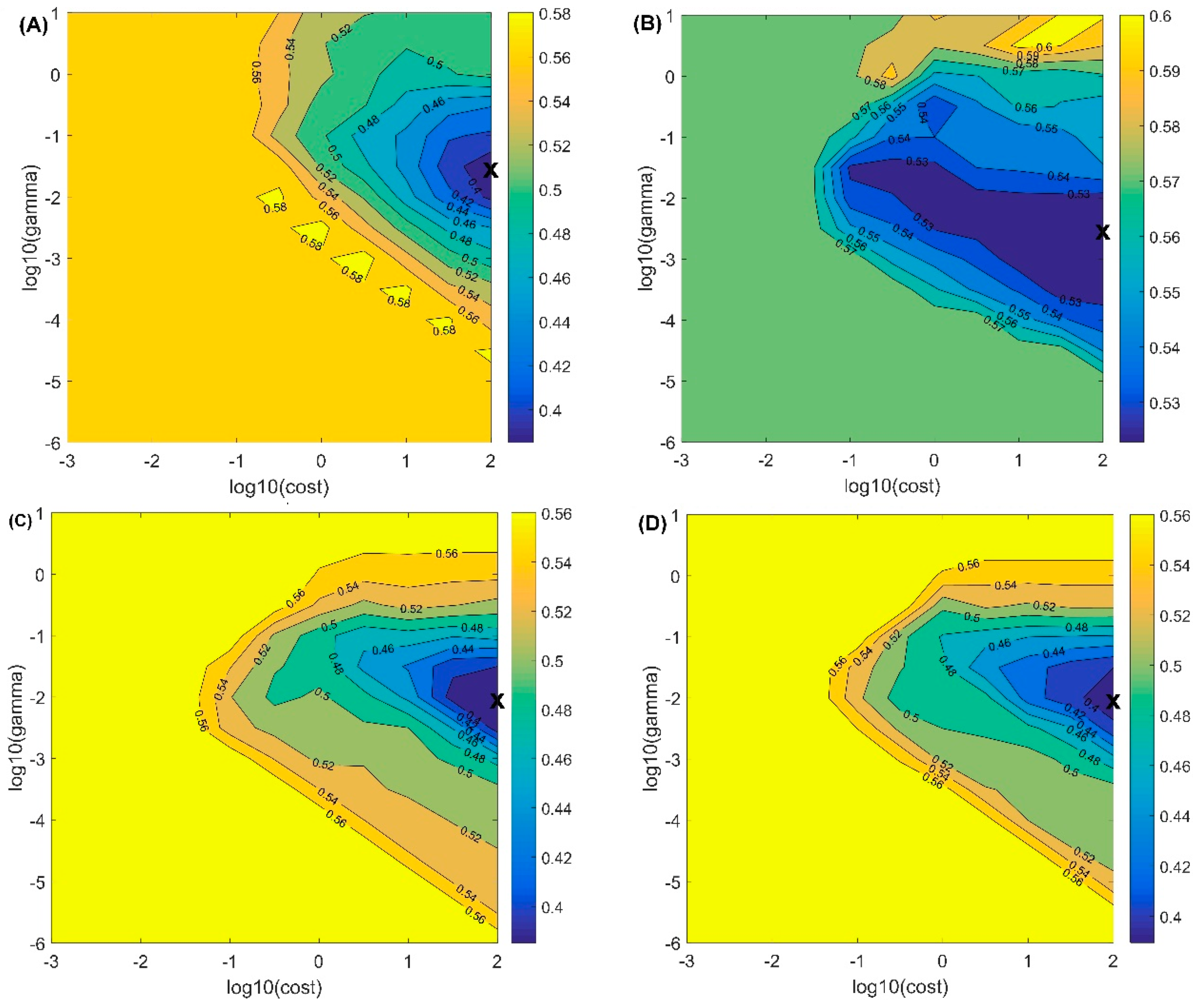
3.3. Classification of Bruised Peaches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Y.; Li, Y.; Pan, L.; Abbas, A.; Jiang, Y.; Wang, X. Authentication of the Geographic Origin of Yangshan Region Peaches Based on Hyperspectral Imaging. Postharvest Biol. Technol. 2021, 171, 111320. [Google Scholar] [CrossRef]
- Ahmadi, E.; Ghassemzadeh, H.R.; Sadeghi, M.; Moghaddam, M.; Neshat, S.Z. The Effect of Impact and Fruit Properties on the Bruising of Peach. J. Food Eng. 2010, 97, 110–117. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Harvest and Postharvest Factors Affecting Bruise Damage of Fresh Fruits. Hortic. Plant J. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise Damage Measurement and Analysis of Fresh Horticultural Produce—A Review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Leszczuk, A.; Chylińska, M.; Zięba, E.; Skrzypek, T.; Szczuka, E.; Zdunek, A. Structural Network of Arabinogalactan Proteins (Agps) and Pectins in Apple Fruit During Ripening and Senescence Processes. Plant Sci. 2018, 275, 36–48. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Pan, L.; Wang, X.; Tu, K. Assessment of the Optical Properties of Peaches with Fungal Infection Using Spatially-Resolved Diffuse Reflectance Technique and Their Relationships with Tissue Structural and Biochemical Properties. Food Chem. 2020, 321, 126704. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Sun, L.; Zhang, Q.; Tan, W.; Ye, D.; Zhang, D.; Liu, Y. Pixel Based Bruise Region Extraction of Apple Using Vis-Nir Hyperspectral Imaging. Comput. Electron. Agric. 2018, 146, 12–21. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, Y.; Li, C.; Yang, F. Fully Convolutional Networks for Blueberry Bruising and Calyx Segmentation Using Hyperspectral Transmittance Imaging. Biosyst. Eng. 2020, 192, 159–175. [Google Scholar] [CrossRef]
- Kuzy, J.; Jiang, Y.; Li, C. Blueberry Bruise Detection by Pulsed Thermographic Imaging. Postharvest Biol. Technol. 2018, 136, 166–177. [Google Scholar] [CrossRef]
- Mazhar, M.; Joyce, D.; Hofman, P.; Vu, N. Factors Contributing to Increased Bruise Expression in Avocado (Persea Americana M.) Cv. ‘Hass’ Fruit. Postharvest Biol. Technol. 2018, 143, 58–67. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Huang, W. Detection of Early Bruises on Peaches (Amygdalus Persica L.) Using Hyperspectral Imaging Coupled with Improved Watershed Segmentation Algorithm. Postharvest Biol. Technol. 2018, 135, 104–113. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xiao, H.; Gu, X.; Pan, L.; Tu, K. Hyperspectral Imaging Detection of Decayed Honey Peaches Based on Their Chlorophyll Content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, W.; Huang, X.; Du, R.; Zhu, X. Effect of Pressing and Impacting Bruises on Optical Properties of Kiwifruit Flesh. Postharvest Biol. Technol. 2021, 172, 111385. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Mendoza, F.A.; Ariana, D.P. Assessing Multiple Quality Attributes of Peaches Using Optical Absorption and Scattering Properties. Trans. ASABE 2012, 55, 647–657. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R.; Mendoza, F.; Beaudry, R.M. Relationship of the Optical Absorption and Scattering Properties with Mechanical and Structural Properties of Apple Tissue. Postharvest Biol. Technol. 2013, 85, 30–38. [Google Scholar] [CrossRef]
- Lu, R.; Cen, H.; Huang, M.; Ariana, D.P. Spectral Absorption and Scattering Properties of Normal and Bruised Apple Tissue. Trans. ASABE 2010, 53, 263–269. [Google Scholar] [CrossRef]
- He, X.; Fu, X.; Li, T.; Rao, X. Spatial Frequency Domain Imaging for Detecting Bruises of Pears. J. Food Meas. Charact. 2018, 12, 1266–1273. [Google Scholar] [CrossRef]
- Berardinelli, A.; Donati, V.; Giunchi, A.; Guarnieri, A.; Ragni, L. Damage to Pears Caused by Simulated Transport. J. Food Eng. 2005, 66, 219–226. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Wang, X. Evaluation of Fungal Infection in Peaches Based on Optical and Microstructural Properties. Postharvest Biol. Technol. 2020, 165, 111181. [Google Scholar] [CrossRef]
- Zhu, Q.; Guan, J.; Huang, M.; Lu, R.; Mendoza, F. Predicting Bruise Susceptibility of ‘Golden Delicious’ Apples Using Hyperspectral Scattering Technique. Postharvest Biol. Technol. 2016, 114, 86–94. [Google Scholar] [CrossRef]
- Cen, H.; Lu, R. Optimization of the Hyperspectral Imaging-Based Spatially-Resolved System for Measuring the Optical Properties of Biological Materials. Opt. Express 2010, 18, 17412–17432. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Bruise Damage Susceptibility of Pomegranates (Punica Granatum, L.) And Impact on Fruit Physiological Response During Short Term Storage. Sci. Hortic. 2019, 246, 664–674. [Google Scholar] [CrossRef]
- Ruiz-Altisent, M.; Moreda, G.P. Fruits, Mechanical Properties and Bruise Susceptibility; Springer: Amsteldam, The Netherlands, 2011. [Google Scholar]
- Kamruzzaman, M.; Elmasry, G.; Sun, D.W.; Allen, P. Application of Nir Hyperspectral Imaging for Discrimination of Lamb Muscles. J. Food Eng. 2011, 104, 332–340. [Google Scholar] [CrossRef]
- Khan, A.; Luqman, S.; Masood, N.; Singh, D.K.; Saeed, S.T.; Samad, A. Eclipta Yellow Vein Virus Enhances Chlorophyll Destruction, Singlet Oxygen Production and Alters Endogenous Redox Status in Andrographis Paniculata. Plant. Physiol. Biochem. 2016, 104, 165–173. [Google Scholar] [CrossRef]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest Factors Influencing Bruise Damage of Fresh Fruits–a Review. Sci. Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Jiménez, R.; Rallo, P.; Suárez, M.P.; Morales-Sillero, A.M.; Casanova, L.; Rapoport, H.F. Cultivar Susceptibility and Anatomical Evaluation of Table Olive Fruit Bruising. Acta Hortic. 2011, 924, 419–424. [Google Scholar] [CrossRef]
| Maturity | Intact | Detection Time | ||
|---|---|---|---|---|
| 0 h | 4 h | 24 h | ||
| S1 | Group 1 | Group 4 | Group 5 | Group 6 |
| S2 | Group 2 | Group 7 | Group 8 | Group 9 |
| S3 | Group 3 | Group 10 | Group 11 | Group 12 |
| Groups | Peel Color (L* Value) | Pulp Color (L* Value) | Bruises Diameter (mm) | |
|---|---|---|---|---|
| Intact | 1 | 49.38 ± 7.10 f | / | / |
| 2 | 46.75 ± 9.28 e,f | / | / | |
| 3 | 45.53 ± 10.02 c,d,e | / | / | |
| S1-bruise | 4 | 47.63 ± 5.05 e,f | / | / |
| 5 | 47.10 ± 7.17 e,f | / | / | |
| 6 | 45.95 ± 4.71 d,e | 67.26 ± 5.50 a | 10.5 ± 5.42 a | |
| S2-bruise | 7 | 46.51 ± 6.17 e,f | / | / |
| 8 | 44.61 ± 6.90 c,d,e | / | / | |
| 9 | 43.04 ± 7.96 c,d | 56.14 ± 11.62 b | 13.96 ± 4.94 b | |
| S3-bruise | 10 | 42.88 ± 5.29 c | / | / |
| 11 | 37.89 ± 7.67 b | / | / | |
| 12 | 35.13 ± 8.59 a | 47.11 ± 6.98 c | 20.37 ± 4.88 c | |
| Groups | µa 675 nm | µa 970 nm | µs’ 675 nm | µs’ 970 nm | |
|---|---|---|---|---|---|
| Intact | 1 | 0.04 ± 0.04 a | 0.24 ± 0.26 a | 24.64 ± 8.80 e | 27.86 ± 10.14 g |
| 2 | 0.09 ± 0.10 b,c | 0.34 ± 0.36 b | 15.79 ± 7.61 d | 14.74 ± 10.15 d | |
| 3 | 0.20 ± 0.07 e | 0.73 ± 0.20 e | 4.96 ± 0.72 a | 3.85 ± 0.67 b | |
| S1-bruise | 4 | 0.03 ± 0.03 a | 0.23 ± 0.21 a | 22.85 ± 9.52 e | 25.23 ± 9.83 f |
| 5 | 0.09 ± 0.10 b | 0.54 ± 0.27 c | 8.65 ± 6.42 b | 8.73 ± 6.65 e | |
| 6 | 0.11 ± 0.11 c,d | 0.58 ± 0.31 c | 4.01 ± 0.98 a | 3.88 ± 0.99 b | |
| S2-bruise | 7 | 0.07 ± 0.04 b | 0.34 ± 0.25 b | 13.90 ± 7.83 c | 16.90 ± 7.83 e |
| 8 | 0.11 ± 0.11 b,c,d | 0.53 ± 0.25 c | 7.05 ± 6.21 b | 7.05 ± 6.21 c | |
| 9 | 0.14 ± 0.08 d | 0.59 ± 0.20 c | 3.84 ± 0.87 a | 3.74 ± 0.87 b | |
| S3-bruise | 10 | 0.21 ± 0.12 e | 0.75 ± 0.07 e | 4.88 ± 2.65 a | 4.41 ± 0.65 b |
| 11 | 0.22 ± 0.12 e | 0.74 ± 0.27 e | 4.60 ± 2.13 a | 3.97 ± 1.94 b | |
| 12 | 0.27 ± 0.17 f | 0.68 ± 0.27 e | 4.33 ± 1.00 a | 3.22 ± 0.91 a | |
| Optical Parameter | Actual Class | Model Parameters | Training Set (%) | Testing Set | ||
|---|---|---|---|---|---|---|
| Predicted Intact Class | Predicted Bruise Class | Accuracy (%) | ||||
| (A) μa | Intact | Cost: 100 Gamma: 0.0316 Number of SVs: 245 | 90.00 | 76 | 24 | 76.00 |
| Bruise | 93.50 | 36 | 264 | 88.00 | ||
| Overall | 92.63 | 112 | 288 | 85.00 | ||
| (B) μs′ | Intact | Cost: 100 Gamma: 0.0316 Number of SVs: 353 | 22.50 | 24 | 76 | 24.00 |
| Bruise | 96.17 | 19 | 281 | 93.67 | ||
| Overall | 77.75 | 43 | 357 | 76.25 | ||
| (C) μa × μs′ | Intact | Cost: 100 Gamma: 0.01 Number of SVs: 251 | 83.50 | 62 | 38 | 62.00 |
| Bruise | 95.00 | 23 | 277 | 92.33 | ||
| Overall | 92.13 | 85 | 315 | 84.75 | ||
| (D) μeff | Intact | Cost: 100 Gamma: 0.01 Number of SVs: 243 | 85.00 | 63 | 37 | 63.00 |
| Bruise | 94.00 | 25 | 275 | 91.67 | ||
| Overall | 91.75 | 88 | 312 | 84.50 | ||
| Discrimination Result (%) | |||||
|---|---|---|---|---|---|
| Bruised Sample | Intact Sample | ||||
| Maturity | Detection Time | S1-Intact | S2-Intact | S3-Intact | Overall |
| S1 | 0 h-Bruise | 57.35 | 82.35 | 95.58 | 78.43 |
| 4 h-Bruise | 86.71 | 79.41 | 94.12 | 86.75 | |
| 24 h-Bruise | 94.12 | 95.59 | 94.12 | 94.61 | |
| Overall | 79.39 | 85.78 | 94.61 | / | |
| S2 | 0 h-Bruise | 79.41 | 91.18 | 95.58 | 88.72 |
| 4 h-Bruise | 98.53 | 94.12 | 94.12 | 95.59 | |
| 24 h-Bruise | 100 | 95.59 | 94.12 | 96.57 | |
| Overall | 92.65 | 93.63 | 94.61 | / | |
| S3 | 0 h-Bruise | 98.53 | 82.35 | 83.82 | 88.23 |
| 4 h-Bruise | 100 | 79.41 | 86.77 | 88.72 | |
| 24 h-Bruise | 100 | 95.59 | 94.12 | 96.57 | |
| Overall | 99.51 | 85.78 | 88.24 | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Huang, Y.; Pan, L.; Wang, X. Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising. Foods 2021, 10, 388. https://doi.org/10.3390/foods10020388
Sun Y, Huang Y, Pan L, Wang X. Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising. Foods. 2021; 10(2):388. https://doi.org/10.3390/foods10020388
Chicago/Turabian StyleSun, Ye, Yuping Huang, Leiqing Pan, and Xiaochan Wang. 2021. "Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising" Foods 10, no. 2: 388. https://doi.org/10.3390/foods10020388
APA StyleSun, Y., Huang, Y., Pan, L., & Wang, X. (2021). Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising. Foods, 10(2), 388. https://doi.org/10.3390/foods10020388








