Dynamics of the Degradation of Acetyl-CoA Carboxylase Herbicides in Vegetables
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Carrot
3.2. Onion
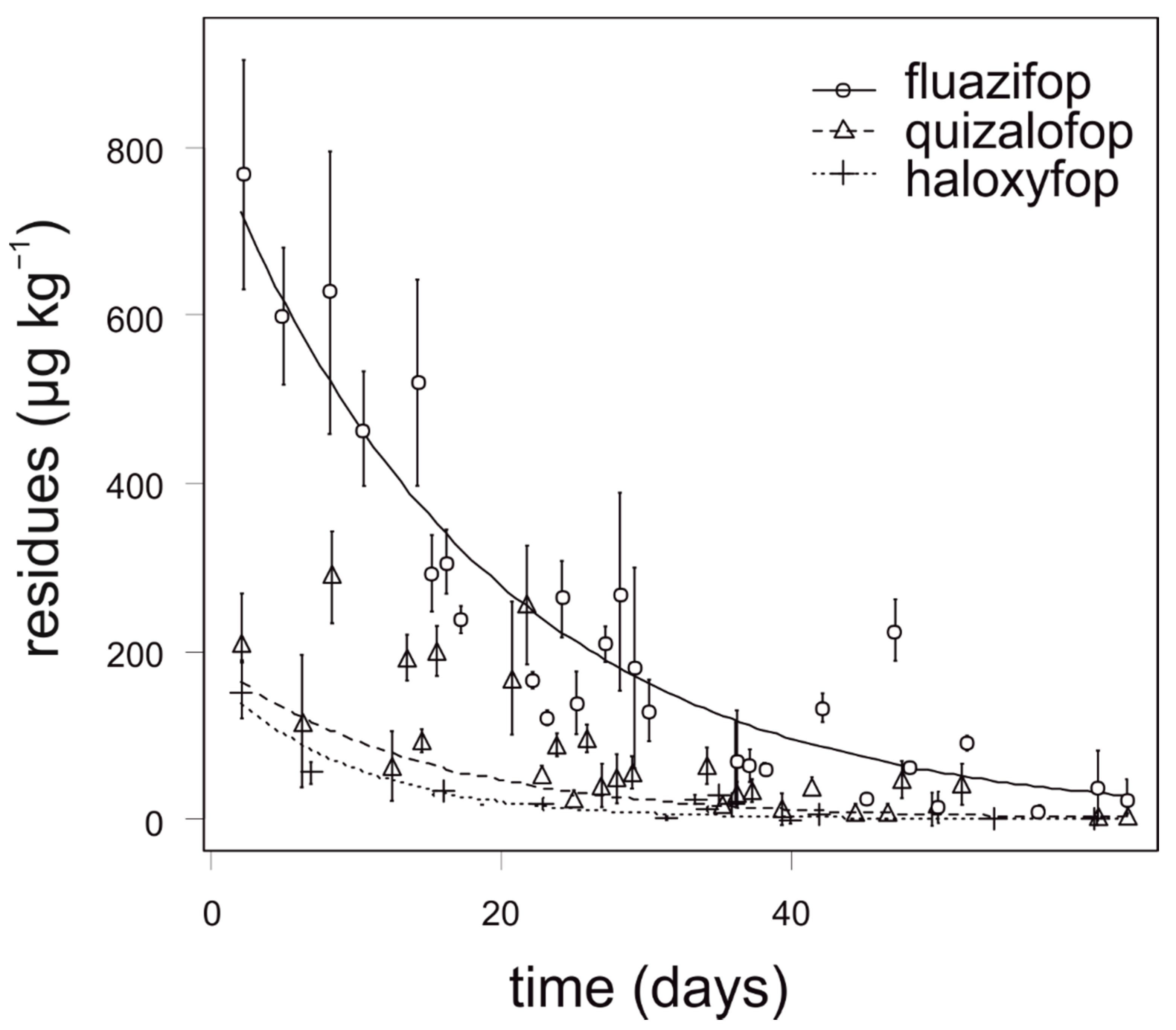
3.3. Lettuce
3.4. Cauliflower
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdulahi, A.E.; Modisa, O.; Molosiwa, O.; Mosarwe, L. Cynidon dactylon control in sunflower (Helianthus annuus) with postemergence graminicides in a semi-arid environment. Crop. Prot. 2001, 20, 411–414. [Google Scholar]
- Gelmini, G.A.; Novo, M.; Mattos, J.B.S. Poaceae control by fenoxaprop-p-ethyl + clethodim in lettuce crop. Eccossistema 2000, 25, 160–163. [Google Scholar]
- Vidrine, P.R.; Reynolds, D.B.; Blouin, D.C. Grasland control in soybean (Glycine max) with graminicides applied alone and with mixtures. Weed Technol. 1995, 9, 68–72. [Google Scholar] [CrossRef]
- Kukorelli, G.; Reisinger, P.; Pinke, G. ACCase inhibitor herbicide–selectivity, weed resistance and fitness cost: A review. Int. J. Pest. Manag. 2013, 59, 165–173. [Google Scholar] [CrossRef]
- Tichy, L.; Jursik, M.; Kolarova, M.; Hejnak, V.; Andr, J.; Martinkova, J. Sensitivity of sunflower cultivar PR63E82 to tribenuron and propaquizafop in different weather conditions. Plant. Soil Enviton. 2018, 64, 479–483. [Google Scholar]
- Deer, J.F.; Monaco, T.J.; Sheets, T.J. Uptake and translocation of fluazifop by three annual grass. Weed Sci. 1985, 33, 612–617. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Z.G.; Liang, S.; Lu, Z.B. Residue behavior and risk assessment of rimsulfuron and quizalofop-P-ethyl in potato under field condition. B Environ. Contam. Tox. 2020, 105, 602–606. [Google Scholar] [CrossRef]
- Li, Z.Y.; Li, Q.L.; Cheng, F.N.; Zhang, W.S.; Wang, W.X.; Li, J.Y. Enantioselectivity in degradation and transformation of quizalofop-ethyl in soils. Chirality 2012, 24, 552–557. [Google Scholar] [CrossRef]
- Kapsi, M.; Tsoutsi, C.; Paschalidou, A.; Albanis, T. Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (NW Greece). Sci. Total Environ. 2019, 650, 2188–2198. [Google Scholar] [CrossRef]
- Correia, N.M.; Carbonari, C.A.; Velini, E.D. Detection of herbicide in water bodies of the Samambaia River sub-basin in the Federal District and eastern Goias. J. Environ. Sci. Health B 2020, 55, 574–582. [Google Scholar] [CrossRef]
- Mantzos, N.; Karakitsou, A.; Nikolaki, S.; Leneti, E.; Konstantinou, I. Dissipitation and transport of quizalofop-p-ethyl herbicide in sunflower cultivation under field conditions. Environ. Sci. Pollut. Res. 2016, 23, 3481–3490. [Google Scholar] [CrossRef]
- Saha, A.; Bhaduri, D.; Pipariya, A.; Jain, N.K. Influence of imazethapyr and quizalofop-p-ethyl application on microbial biomass and enzymatic activity in peanut grown soil. Environ. Sci. Pollut. Res. 2016, 23, 23758–23771. [Google Scholar] [CrossRef]
- Horská, T.; Kocourek, F.; Stará, J.; Holý, K.; Mráz, P.; Krátký, F.; Kocourek, V.; Hajšlová, H. Evaluation of pesticide residue Dynamics in lettuce, onion, leak, carrot and parsley. Foods 2020, 9, 680. [Google Scholar] [CrossRef]
- Jacobsen, R.E.; Fantke, P.; Trapp, S. Analysing half-lives for pesticide dissipitation in plants. SAR QSAR Environ. Res. 2015, 26, 325–342. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Regulation (EC) no 396/2005 of the European Parliament and of the Council of 23 February 2005 a maximum residues levels of pesticides in or on foods and feed of plant and animal origin and amending Council Directive 91/414/EEC. This act has been changed. Latest consolidated version: 14/12/2019. Off. J. Eur Union 2005, 70, 1–16. [Google Scholar]
- Kocourek, F.; Stará, J.; Holý, K.; Horská, T.; Kocourek, V.; Kováčová, J.; Kohoutková, J.; Suchanová, M.; Hajšlová, J. Evaluation of pesticide residue dynamics in Chinese cabbage, head cabbage and cauliflower. Food Addit. Contam Part A 2017, 34, 980–989. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2006/125/EC of 5 December 2006 on processed cereal-based foods and baby foods for infants and young children. Off. J. Eur. Union 2006, 339, 16–35. [Google Scholar]
- Panseri, S.; Nobile, M.; Arioli, F.; Biolatti, C.; Pavlovic, R.; Chieasa, L.M. Occurrence of perchlorate, chlorate and polar herbicides in different baby food commodities. Food Chem. 2020, 330, 127205. [Google Scholar] [CrossRef]
- MacLoughlin, T.M.; Peluso, M.L.; Etchegoyen, M.A.; Alonso, L.L.; de Cstro, M.C.; Percudani, M.C.; Marino, D.J.G. Pesticide residues in fruits and vegetables of the argentine domestic market: Occurrence snd quality. Food Control 2018, 93, 129–138. [Google Scholar] [CrossRef]
- Ramadan, M.F.A.; Abdel-Hamid, M.M.A.; Altorgoman, M.M.F.; AlGaramah, H.A.; Alawi, M.A.; Shati, A.A.; Shweeta, H.A.; Awwad, N.S. Evaluation of Pesticide residue in vegetables from the Asir region, Saudi Arabia. Molecules 2020, 25, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakirci, G.T.; Acay, D.B.Y.; Bakirci, F.; Otles, S. Pesticide residues in fruits and vegetables from the Aegean region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- Elgueta, S.; Velenzuela, M.; Fuetes, M.; Meza, P.; Manzur, J.P.; Liu, S.; Zhao, G.Q.; Correa, A. Pesticide residues and health risk assessment in tomatoes and lettuces from Farms of metropolitan region Chile. Molecules 2000, 25, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skovgaard, M.; Encinas, S.R.; Jensen, O.C.; Andersen, J.H.; Condarco, G.; Jors, E. Pesticide residues in commercial lettuce, onion, and potato samples from Bolivia–A threat to public health? Environ. Health Insights 2017, 11, 1–8. [Google Scholar]
- Santarelli, G.A.; Migliorati, G.; Pomilio, F.; Marfoglia, C.; Centorame, P.; D’Agostino, A.; D’Aurelio, R.; Scarpone, R.; Battistelli, N.; Di Simome, F.; et al. Assessment of pesticide residues and microbial contamination in raw leafy green vegetables marked in Italy. Food Control 2018, 85, 350–358. [Google Scholar] [CrossRef]
- Chiarello, M.; Moura, S. Determination of pesticides in organic carrots by high-performance liquid chromatography/high-resolution mass spectrometry. Anal. Lett. 2018, 51, 2561–2574. [Google Scholar] [CrossRef]
- Singh, B.; Bhullar, M.S.; Walia, U.S.; Randhawa, S.K.; Phutela, R.P. Weed control in carrot (Daucas carota): Bio-efficacy and residues of pre-emergence herbicides. Indian J. Ecol. 2010, 37, 145–148. [Google Scholar]
- Saritha, J.D.; Ramprakash, T.; Rao, P.C. Persistence of metribuzin in tomato growing soils and tomato fruits. Nat. Env. Poll. Tech. 2017, 16, 505–508. [Google Scholar]
- Khan, S.U.; Belanger, A.; Hogue, E.J.; Hamilton, H.A.; Mathur, S.P. Residues of paraquat and linuron in an organic soil and their uptake by onions, lettuce and carrots. Can. J. Soil Sci. 1976, 56, 407–412. [Google Scholar] [CrossRef]
- Sondhia, S. Harvest time residues of pendimethalin in tomato, cauliflower, and radish under field conditions. Toxicol. Environ. Chem. 2013, 95, 254–259. [Google Scholar] [CrossRef]
- Esturk, O.; Yakar, Y.; Ayhan, Z. Pesticide residue analysis in parsley, lettuce and spinach by LC-MS/MS. J. Food Sci. Technol. 2014, 51, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Jursik, M.; Kovacova, J.; Kocarek, M.; Hamouzova, K.; Soukup, J. Effect of a non-woven fabric covering on the residual activity of pendimethalin in lettuce and soil. Pest. Manag. Sci. 2017, 73, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Jursik, M.; Kocarek, M.; Suchanova, M.; Kolarova, M.; Suk, J. Effect of irrigation and adjuvant on residual activity of pendimethalin and metazachlor in kohlrabi. Plant. Soil Environ. 2019, 65, 387–394. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. General Requirements for the Competence of Testing and Calibration Laboratories; ISO/IEC 17025:2005 (Revised by ISO/IEC 17025: 2017); BSI-ISO: London, UK, 2017. [Google Scholar]
- EN 12393-2 Standard (Foods of plant origin-Multiresidue methods for the determination of pesticide residues by GC or LC-MS/MS-Part 2: Methods for extraction and clean-up) and EN 12393-2 (Foods of plant origin-Multiresidue methods for the determination of pesticide residues by GC or LC-MS/MS-Part 3: Determination and confirmatory tests).
- European Commission. Guidance Document on Analytical Quality Control. And Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; SANTE/12682/2019; DG SANTE: Bruxelles, Belgium, 2019. [Google Scholar]
- Central Institute for Supervising and Testing in Agriculture. Available online: http://eagri.cz/public/web/en/ukzuz/portal (accessed on 22 December 2020).
- Doohan, D.J.; Monaco, T.J.; Sheets, T.J.; Leidy, R.B. Residues and efficacy of fluazifop-buthyl in strawberries. Weed Res. 1986, 26, 89–98. [Google Scholar] [CrossRef]
- Sufang, F.; Fengzu, Z.; Kailin, D.; Chuanshan, Y.; Shaowen, L.; Pengyue, Z.; Canping, P. Spinach or amaranth contains highest residues of metalaxyl, fluazifop-P-butyl, chlorpyrifos, and lambda-cyhalothrin on six leaf vegetables upon open field application. J. Agric. Food Chem. 2013, 61, 2039–2044. [Google Scholar]
- Balinova, A.M.; Lalova, M.P. Translocation, metabolism and residues of fluazifop-butyl in soybean plants. Weed Res. 1992, 32, 143–147. [Google Scholar] [CrossRef]
- Sondhia, S. Fluazifop-p-butyl residues in soybean crop and soil. Pestic. Res. J. 2007, 19, 248–250. [Google Scholar]
- Poonia, T.C.; Mathukia, R.K.; Karwasara, P.K. Residues of pendimethalin, oxyfluorfen, quizalofop-ethyl and imazethapyr in groundnut and their persistence in soil. J. Crop. Weed 2017, 13, 194–202. [Google Scholar]
- Zou, R.; Wu, D.; Wu, L.; Li, Y.; Zhang, Z.; Liu, H. Residual dynamics of quizalofop-P-ethyl in blueberry fruit and soil. J. Jilin Agric. Uni. 2009, 31, 652–655. [Google Scholar]
- Sahoo, S.K.; Mandal, K.; Singh, G.; Kumar, R.; Chahil, G.S.; Battu, R.S.; Singh, B. Residual behavior of quizalofop ethyl on onion (Allium cepa L.). Environ. Monit. Assess. 2013, 185, 1711–1718. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ghost, M.; Banerjee, H.; Bhattacharyya, A.; Roy, A. Pesticide behavior of haloxyfop–a new herbicide in/on onion. J. Crop. Weed 2005, 1, 41–44. [Google Scholar]
- Duhang, A.; Sing, S. Terminal residues of propaquizafop in cotton seed, lint and soil. J. Cotton Res. Dev. 2018, 32, 133–138. [Google Scholar]
- Suk, J.; Jursik, M.; Suchanova, M.; Schusterova, D.; Hamouzova, K. Dynamics of herbicide degradation in cauliflower. Plant. Soil Environ. 2018, 64, 551–556. [Google Scholar] [CrossRef] [Green Version]



| Vegetable | Crop Density (Plant m−2) | Inter-Row Spacing (m) | Date of Planting/Sowing | ||||
|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | |||
| carrot | 90 | 0.5 | 25.3 | 20.4 | 31.3 | 21.4 | 28.4 |
| onion | 70 | 0.3 | 29.3 | 15.4 | 12.3 | 30.3 | - |
| lettuce | 10 | 0.3 | 28.3 | 9.4 | 27.3 | - | - |
| cauliflower | 4 | 0.5 | 9.5 | 6.5 | 5.5 | - | - |
| Active Ingredient (ai) | Trade Name | Concentration of ai (g L−1) | Application Rate (g ha−1 ai) | Manufacturer |
|---|---|---|---|---|
| cycloxydim | Stratos Ultra | 100 | 200 | BASF |
| fluazifop | Fusilade Forte | 150 | 300 | Syngenta |
| haloxyfop | Gallant Super | 104 | 104 | Corteva |
| propaquizafop | Agil | 100 | 150 | ADAMA |
| quizalofop | Targa Super | 100 | 250 | Chemtura |
| Vegetable | Growth Stage | Date of Herbicide Application | ||||
|---|---|---|---|---|---|---|
| 2012 | 2013 | 2014 | 2015 | 2016 | ||
| carrot | 5 TL 1 | 27.6 | 24.6 | 16.6 | 17.6 | 16.6 |
| 7 TL | 9.7 | 7.7 | 23.6 | 30.6 | 28.6 | |
| 9 TL | - | 15.7 | 7.7 | - | - | |
| onion | 6 L 2 | 11.6 | 7.7 | 11.6 | 17.6. | - |
| 9 L | 27.6 | 22.7 | 7.7 | 13.7. | - | |
| lettuce | 4 WAP 3 | 15.5 | 13.5 | 23.5 | - | - |
| 6 WAP | 25.5 | 29.5 | 3.6 | - | - | |
| cauliflower | 6 WAP | 19.6 | 24.6 | 23.6 | - | - |
| 8 WAP | 27.6 | 7.7 | 7.7 | - | - | |
| Analyte | Quantification | Cone | Collision | Confirmation | Cone | Collision |
|---|---|---|---|---|---|---|
| Transition (m/z) | (V) | (V) | Transition (m/z) | (V) | (V) | |
| Cycloxydim | 326.3 > 280.2 | 30 | 13 | 326.3 > 180.4 | 30 | 25 |
| Fluazifop | 328.2 > 282.1 | 35 | 20 | 328.2 > 91.2 | 35 | 30 |
| Haloxyfop | 362 > 315.8 | 27 | 18 | 362 > 91 | 27 | 30 |
| Propaquizafop | 444.2 > 100.04 | 30 | 20 | 444.2 > 56.2 | 30 | 15 |
| Quizalofop | 344.46 > 298.83 | 54 | 18 | 346.46 > 300.83 | 54 | 18 |
| Herbicide | Carrot | Onion | Lettuce | Cauliflower | ||||
|---|---|---|---|---|---|---|---|---|
| LOQ 1 | MRL 2 | LOQ | MRL | LOQ | MRL | LOQ | MRL | |
| µg kg−1 | ||||||||
| cycloxydim | 2 | 5000 | - | 3000 | 2 | 1500 | 2 | 5000 |
| fluazifop | 2 | 400 | 1 | 300 | 1 | 20 | 1 | 10 |
| haloxyfop | 2 | 90 | 2 | 200 | - | 10 | - | 10 |
| propaquizafop | 2 | 200 | 2 | 40 | 2 | 400 | 2 | 200 |
| quizalofop | 2 | 200 | 1 | 40 | 1 | 400 | 1 | 200 |
| Vegetable | Active Ingredient | Parameter | |||||
|---|---|---|---|---|---|---|---|
| a 1 | SE 2 | b 1 | SE | F-Test 3 | p-Value | ||
| carrot | fluazifop | 804.09 | 121.91 | 18.82 | 3.39 | 0.55 | 0.91 |
| quizalofop | 78.56 | 13.19 | 32.89 | 7.85 | 1.67 | 0.16 | |
| haloxyfop | 153.15 | 19.57 | 17.66 | 2.43 | 0.94 | 0.64 | |
| onion | fluazifop | 458.81 | 67.95 | 10.30 | 1.18 | 2.28 | 0.12 |
| quizalofop | 108.76 | 25.03 | 9.24 | 1.73 | 0.40 | 0.90 | |
| haloxyfop | 81.98 | 12.08 | 18.57 | 2.94 | 7.57 | 0.13 | |
| lettuce | fluazifop | 1194.64 | 617.99 | 9.45 | 3.77 | 8.97 | 0.30 |
| quizalofop | 262.89 | 120.05 | 29.10 | 21.72 | 3.91 | 0.14 | |
| cauliflower | fluazifop | 5905.47 | 800.93 | 12.4 | 1.31 | 7.77 | 0.12 |
| quizalofop | 107.99 | 33.25 | 26.77 | 10.17 | 4.95 | 0.18 | |
| Vegetable | Active Ingredient | MRL 1 (µg kg−1) | Model (µg kg−1) | PHI 2 (Days) | APHIBF 3 (Days) | APHI25 4 (Days) | APHI50 4 (Days) |
|---|---|---|---|---|---|---|---|
| carrot | fluazifop | 400 | 13.14 | 49 | 110 | 52 | 35 |
| quizalofop | 200 | −0.81 5 | 45 | 55 | 25 | 12 | |
| haloxyfop | 90 | 9.38 | 56 | 64 | 45 | 29 | |
| onion | fluazifop | 300 | 4.38 | 28 | 53 | 25 | 15 |
| quizalofop | 40 | 9.24 | 42 | 29 | 29 | 21 | |
| haloxyfop | 200 | −9.29 5 | 28 | 47 | 15 | 2 | |
| lettuce | fluazifop | 20 | 38.64 | 42 | 60 | 69 | 60 |
| quizalofop | 400 | 5.22 | 30 | 71 | 31 | 19 | |
| cauliflower | fluazifop | 10 | 79.13 | 56 | 105 | 128 5 | 117 5 |
| quizalofop | 200 | 2.57 | 70 | 68 | 33 | 18 |
| Vegetable | Active Ingredient | MRL 10 1 (Days) | MRL 20 2 (Days) | MRL 50 3 (Days) | MRL 100 4 (Days) | MRL 200 5 (Days) | MRL 500 6 (Days) |
|---|---|---|---|---|---|---|---|
| carrot | fluazifop | 110 | 70 | 57 | 52 | 26 | 9 |
| quizalofop | 55 | 45 | 15 | 12 | x | x | |
| haloxyfop | 64 | 36 | 20 | 10 | x | x | |
| onion | fluazifop | 53 | 32 | 23 | 21 | 9 | x |
| quizalofop | 29 | 16 | 7 | 1 | x | x | |
| haloxyfop | 47 | 26 | 9 | 1.26 | x | x | |
| lettuce | fluazifop | 60 | 39 | 34 | 31 | 17 | 8 |
| quizalofop | 71 | 65 | 48 | 31 | 8 | x | |
| cauliflower | fluazifop | 105 | 83 | 75 | 67 | 42 | 31 |
| quizalofop | 68 | 45 | 21 | 18 | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jursík, M.; Hamouzová, K.; Hajšlová, J. Dynamics of the Degradation of Acetyl-CoA Carboxylase Herbicides in Vegetables. Foods 2021, 10, 405. https://doi.org/10.3390/foods10020405
Jursík M, Hamouzová K, Hajšlová J. Dynamics of the Degradation of Acetyl-CoA Carboxylase Herbicides in Vegetables. Foods. 2021; 10(2):405. https://doi.org/10.3390/foods10020405
Chicago/Turabian StyleJursík, Miroslav, Kateřina Hamouzová, and Jana Hajšlová. 2021. "Dynamics of the Degradation of Acetyl-CoA Carboxylase Herbicides in Vegetables" Foods 10, no. 2: 405. https://doi.org/10.3390/foods10020405