Establishment of an Indirect Competitive Enzyme-Linked Immunosorbent Method for the Detection of Heavy Metal Cadmium in Food Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals and Samples
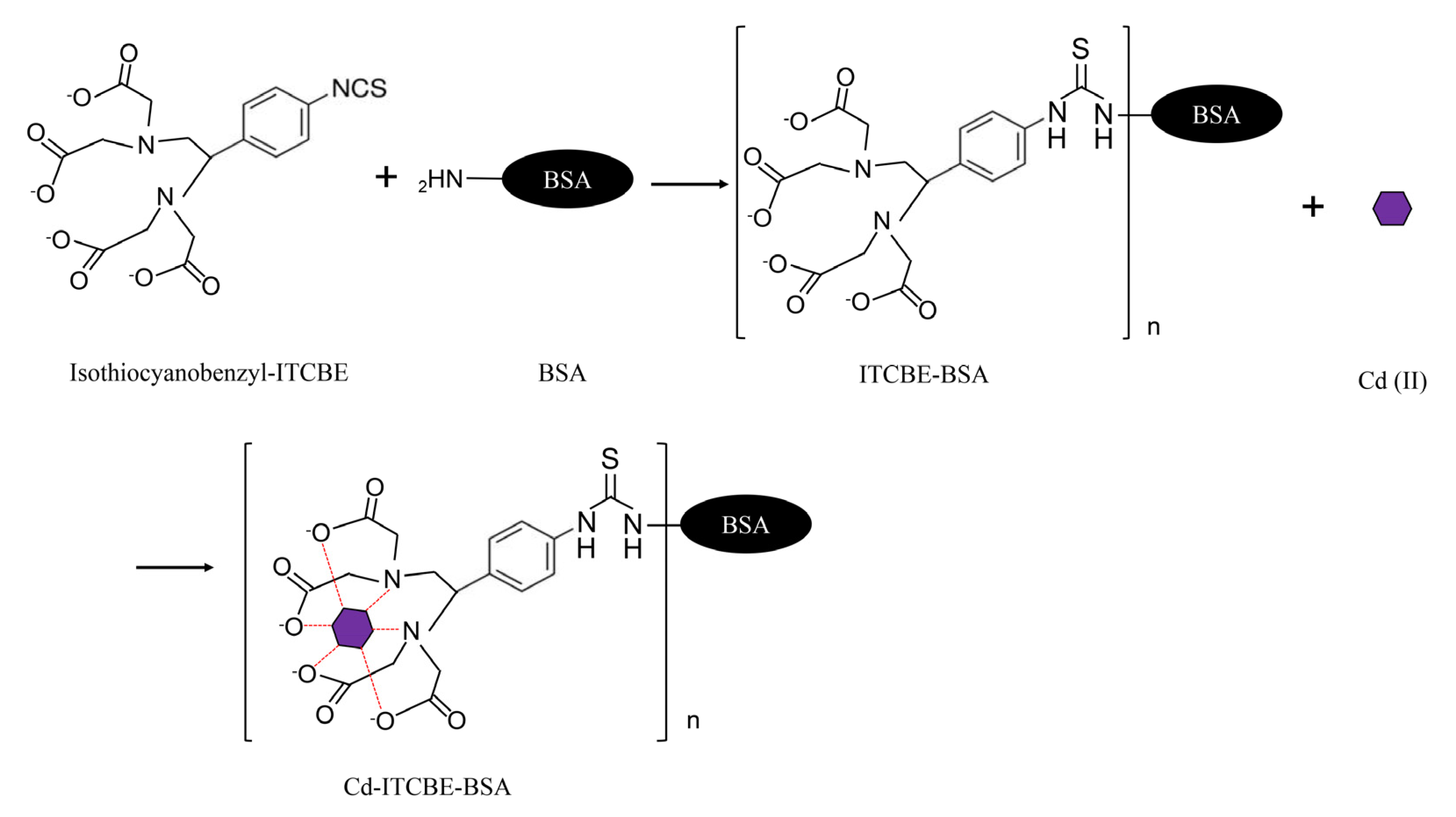
2.2. Preparation of Cd-ITCBE-BSA and Cd-ITCBE-OVA
2.3. Production of Monoclonal Antibody
2.4. Optimal Working Concentration of Anti-Cadmium Monoclonal Antibody and Coating Anti-Gen
2.5. Standard Curve and Sensitivity Analysis
2.6. Specificity Analysis of Ic-ELISA
2.7. Minimum Detection Limit and Minimum Limit of Quantification
2.8. Precision and Accuracy of Ic-ELISA Analysis
2.9. Comparison of Ic-ELISA with ICP-AES
3. Results and Discussion
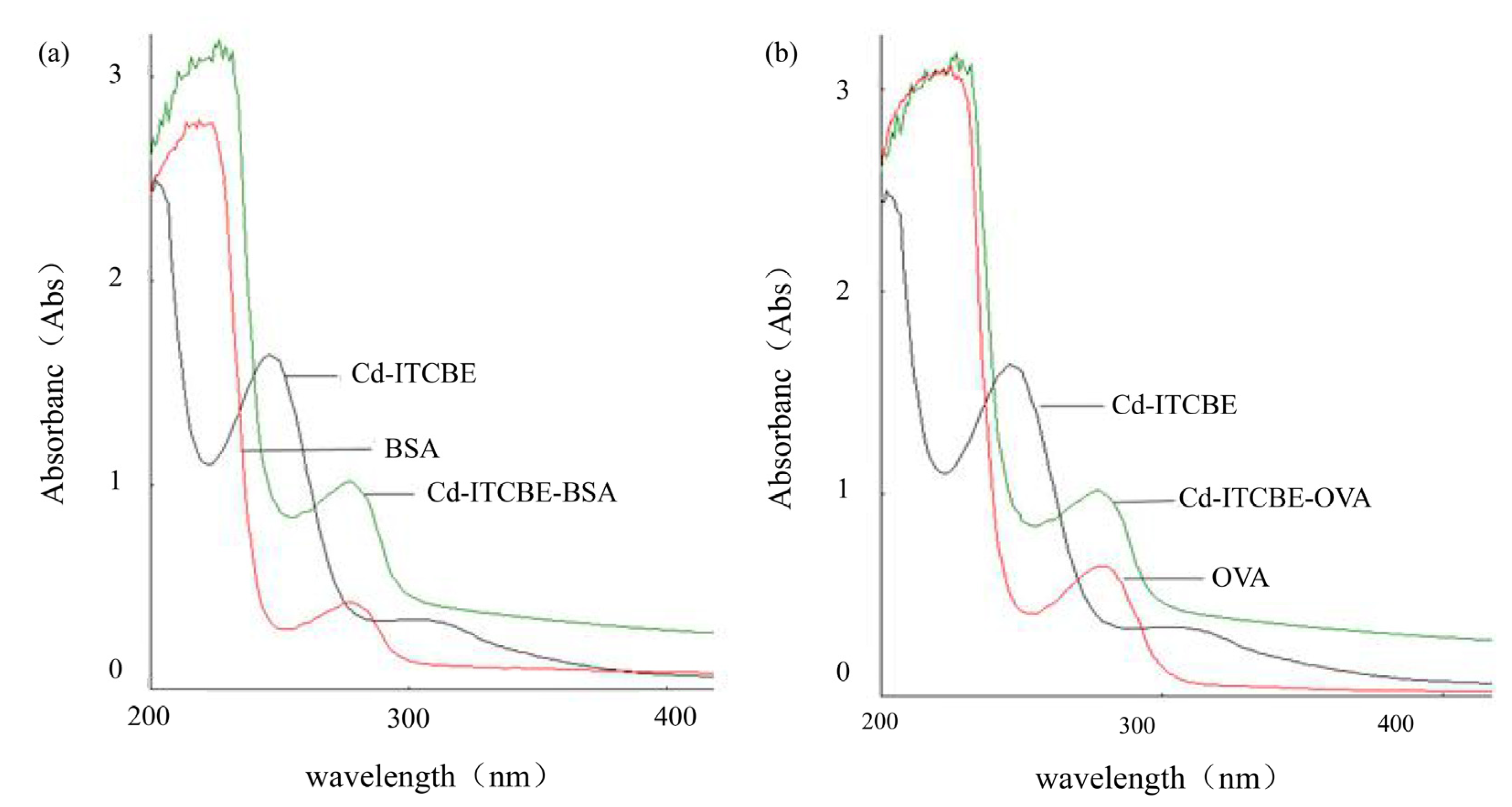
3.1. UV-Scanning Identification of Cd-ITCBE-BSA and Cd-ITCBE-OVA
3.2. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Identification of Cd-ITCBE-BSA and Cd-ITCBE-OVA
3.3. Identification of Anti-Cadmium Monoclonal Antibody
3.4. Determination of Optimal Working Concentration of Monoclonal Antibody and Coated Anti-Gen for Cd (II)
3.5. Standard Curve Linear Range and Detection Sensitivity
3.6. Specificity Analysis of Ic-ELISA
3.7. Analysis of Limits of Detection and Limits of Quantitation
3.8. Precision and Accuracy of Ic-ELISA Analysis
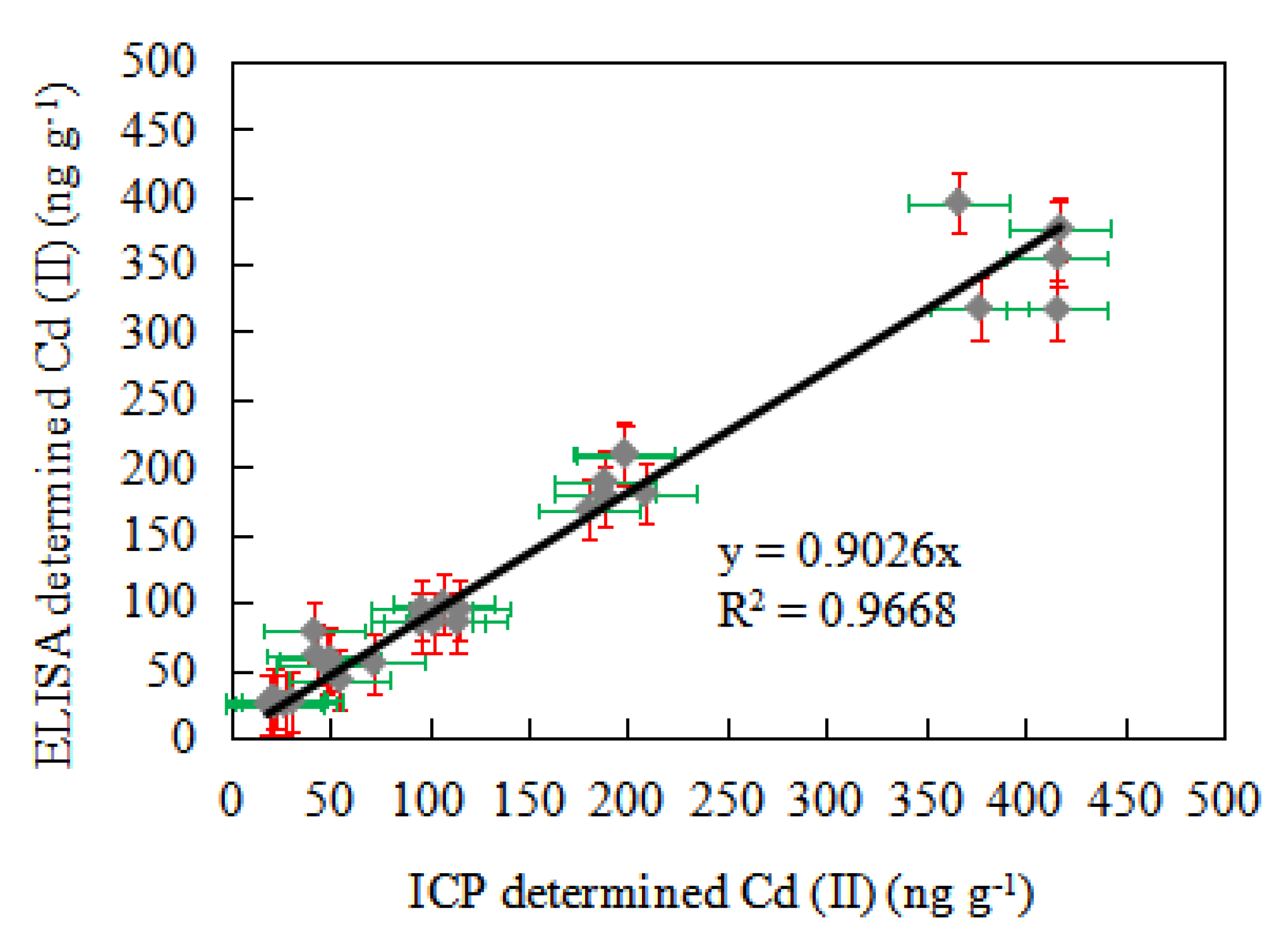
3.9. Comparison of Ic-ELISA and ICP-AES Detection in Spiked Sample
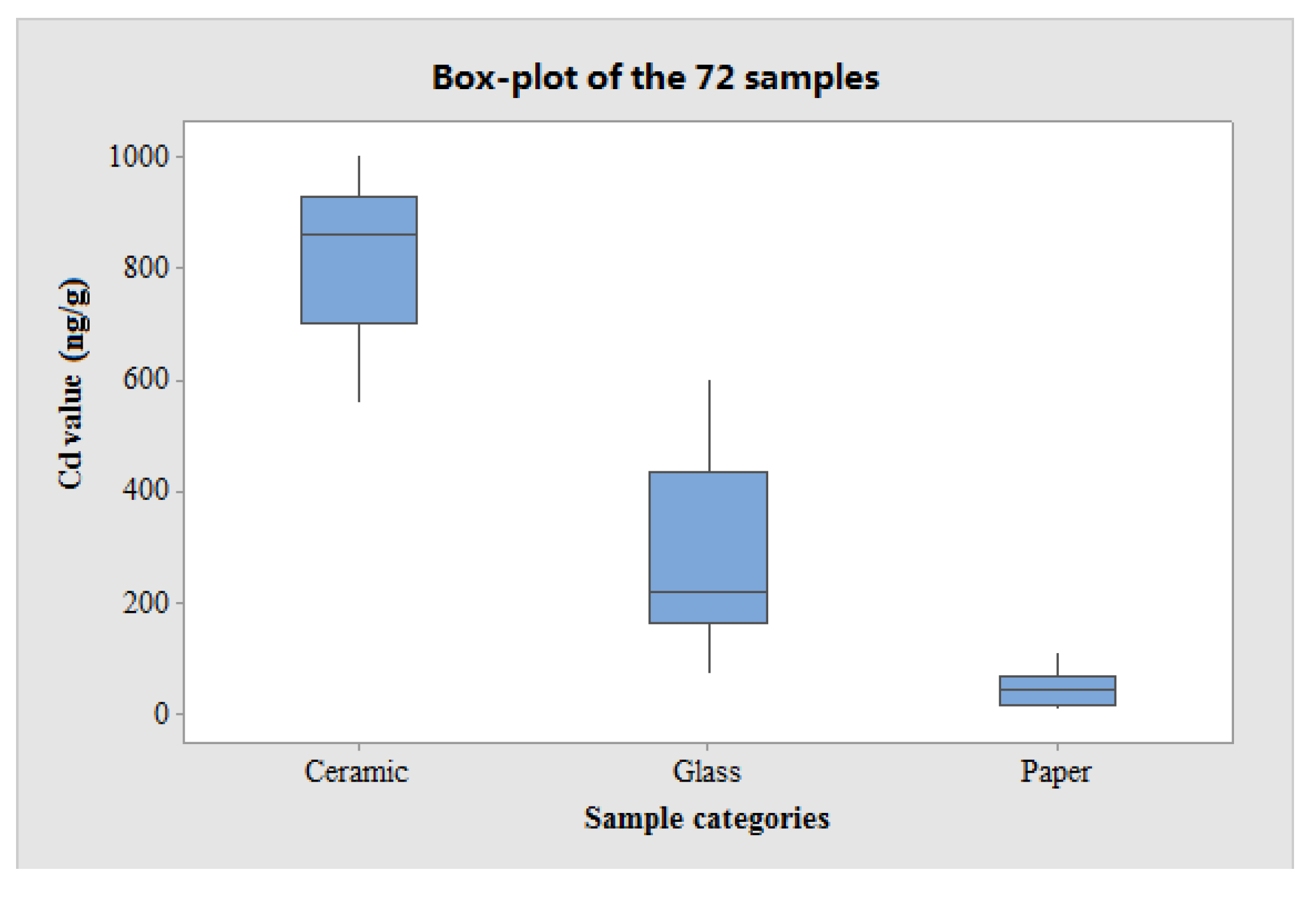
3.10. Statistical Analysis of the 72 Actual Samples
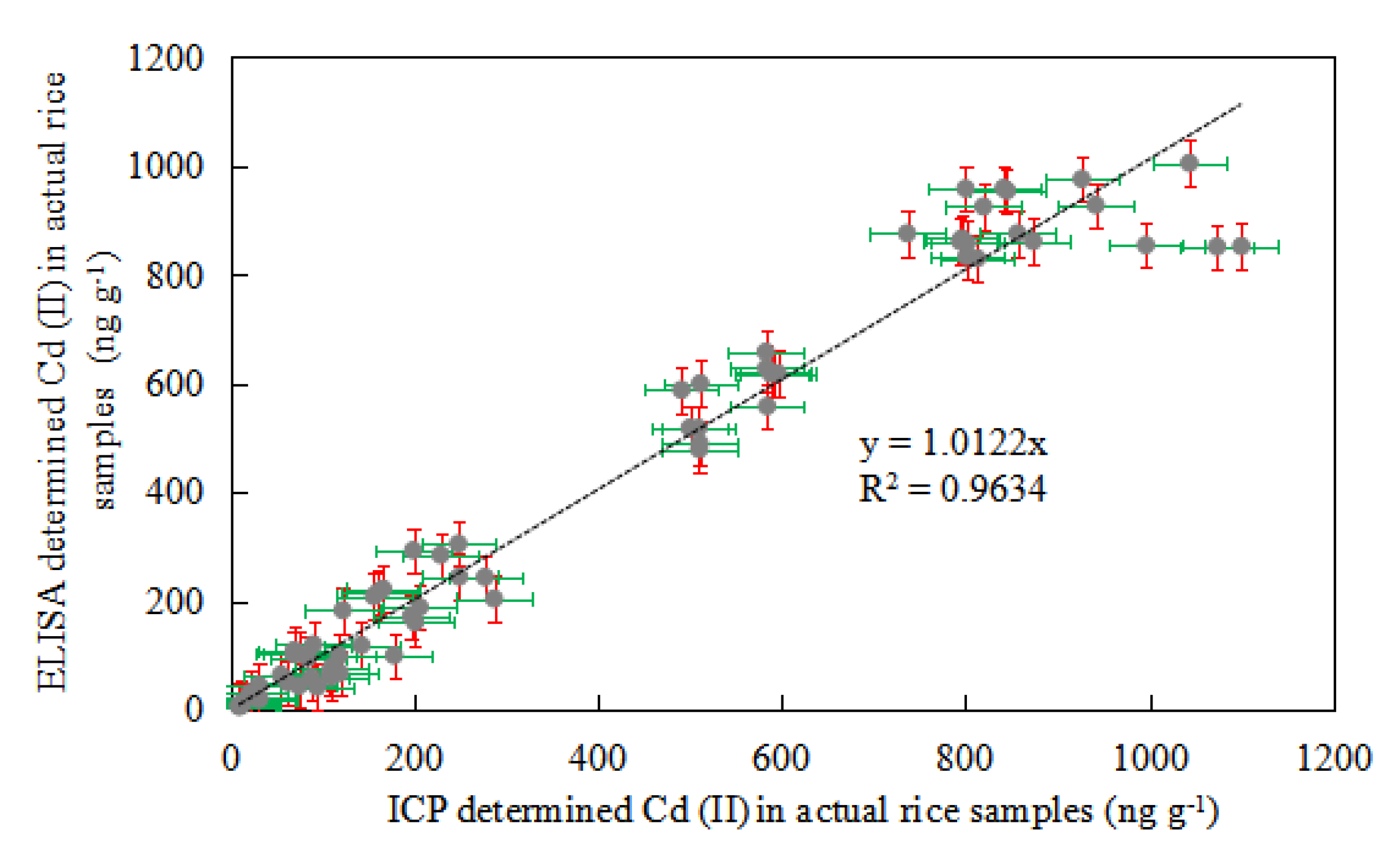
3.11. Comparison of Ic-ELISA and ICP-AES Detection in Actual Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borchers, A.; Teuber, S.S.; Keen, C.L.; Gershwin, M.E. Food Safety. Clin. Rev. Allergy Immunol. 2009, 39, 95–141. [Google Scholar] [CrossRef]
- Skrzydlewska, E.; Balcerzak, M.; Vanhaecke, F. Determination of chromium, cadmium and lead in food-packaging materials by axial inductively coupled plasma time-of-flight mass spectrometry. Anal. Chim. Acta 2002, 479, 191–202. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Chen, C.S.; Shun, S.; Huang, Y.C. Inductively coupled plasma orthogonal acceleration time-of-flight mass spectrometry (ICP-oa-TOF-MS) analysis of heavy metal content in Indocalamus tesselatus samples. Food Chem. 2013, 141, 2154–2157. [Google Scholar] [CrossRef]
- Santos1, É.J.D.; Oliveira1, F.M.P.; Herrmann, A.B.; Sturgeon, R.E. ICP OES Determination of Contaminant Elements Leached from Food Packaging Films. Biol. Appl. Sci. 2017, 60, e17160465. [Google Scholar] [CrossRef] [Green Version]
- Goodlaxson, B.; Curtzwiler, G.; Vorst, K. Evaluation of methods for determining heavy metal content in polyethylene terephthalate food packaging. J. Plast. Film. Sheet. 2018, 34, 119–139. [Google Scholar] [CrossRef]
- Ramos, K.; Gómez-Gómez, M.M.; Cámara, C.; Ramos, L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICP-MS. Talanta 2015, 151, 83–90. [Google Scholar] [CrossRef]
- Prunier, J.; Maurice, L.; Perez, E.; Gigault, J.; Wickmann, A.P.; Davranche, M.; Halle, A. Trace metals in polyethylene debris from the North Atlantic subtropical gyre. Environ. Pollut. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Watts, M.J. Chromium speciationin foodstuffs: A review. Food Chem. 2018, 250, 105–112. [Google Scholar] [CrossRef]
- Ogungbesan, A.; Neal-Kluever, A.; Rice, P. Exploring the use of current immunological assays for the developmental immunotoxicity assessment of food contact materials. Food Chem. Toxicol. 2019, 133, 110801. [Google Scholar] [CrossRef] [PubMed]
- Brunnbauer, L.; Mayr, M.; Larisegger, S.; Nelhiebel, M.; Pagnin, L.; Wiesinger, R.; Schreiner, M.; Limbeck, A. Combined LA‑ICP‑MS/LIBS: Powerful analytical tools for the investigation of polymer alteration after treatment under corrosive conditions. Sci. Rep. 2020, 10, 12513. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Dordevic, D.; Gambuś, F.; Szczurowska, K.; Zając, M. Heavy metal contamination, microbiological spoilage and biogenic amine content in sushi available on the Polish market. J. Sci. Food Agric. 2018, 98, 2809–2815. [Google Scholar] [CrossRef]
- Martinis, E.M.; Olsina, R.A.; Altamiranoa, J.C.; Wuillouda, R.G. On-line ionic liquid-based preconcentration system coupled to flame atomic absorption spectrometry for trace cadmium determination in plastic food packaging materials. Talanta 2009, 78, 857–862. [Google Scholar] [CrossRef]
- Jackson, L.S.; Knize, M.G.; Morgan, J.N. Effects of processing on heavy metal content of foods. Adv. Exp. Med. Biol. 1999, 459, 195–211. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Q.; Liu, J.; Liu, W.; Wang, T.; Zhang, Q.; Jiang, G. High levels of heavy metals in rice (sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 2008, 71, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Parry, S.J.; Benzing, R. The validation of a method for determining the migration of trace elements from food packaging materials into food. J. Radioanal. Nucl. Chem. 1997, 217, 147–150. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snedeker, S.M. Methylnaphthalene in Food Packaging and Cadmium in Food Packaging and Household Items: Overview of Exposure, Toxicology, Regulatory Aspects, and Research Needs[M]. In Toxicants in Food Packaging and Household Plastics Book Series. Molecular and Integrative Toxicology; Springer: London, UK, 2014; pp. 245–263. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Gritsenko, V.A.; Skalnaya, M.G.; Cherkasov, S.V.; Aaseth, J.; Skalny, A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018, 235, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Xie, H.; Song, H.; Xu, H.; Wu, Y. Bioconcentration factors and potential human health risks of heavy metals in cultivated Lentinus edodes in Chengdu, People’s Republic of China. J. Food Protect. 2015, 78, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Kawde, A.; Muraza, O.; Sanhoob, M.A.; Abdul, A.M.; Al-Betar, A.R. Modified lanthanum–zeolite for sensitive electrochemical detection of heavy metal ions. Arab. J. Sci. Eng. 2018, 44, 217–226. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakovae, O.P.; Skalnaya, M.G.; Aasethf, J.; Bjørklundh, G.; Gatiatulinai, E.R.; Popova, E.V.; Nemereshinai, O.N.; Huangk, P.T.; et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Wolk, A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: Systematic review and meta analysis of cohort studies. Int. J. Epidemiol. 2015, 45, 782–791. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Nordberg, G.; Ye, T.; Bo, M.; Wang, H.; Zhu, G.; Kong, Q.; Bernard, A. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ. Res. 2004, 96, 353–359. [Google Scholar] [CrossRef]
- GB 31604.24-2016. National Standard for Food Safety, Determination of Cadmium Migration in Food Contact Materials and Products. 2016, National Standard of the People’s Republic of China; National Health and Family Planning Commission: Beijing, China, 2016.
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V. Migration phenomenon in food packaging. Food-package interactions, mechanisms, types of migrants, testing and relative legislation-A review. Food. Bioproc. Tech. 2014, 7, 21–36. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Ullah, Z.; Asi, M.R.; Jinap, S.; Sultan, M.T.; Malik, N. Heavy metals in selected vegetables from markets of Faisalabad, Pakistan. J. Food Protect. 2018, 81, 806–809. [Google Scholar] [CrossRef]
- Lahimer, M.C.; Ayed, N.; Horriche, J.; Belgaied, S. Characterization of plastic packaging additives: Food contact, stability and toxicity. Arab. J. Chem. 2013, 10, S1938–S1954. [Google Scholar] [CrossRef] [Green Version]
- Bakircioglu, D.; Kurtulus, Y.B.; Ucar, G. Determination of some traces metal levels in cheese samples packaged in plastic and tin containers by ICP-OES after dry, wet and microwave digestion. Food Chem. Toxicol. 2011, 49, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Adriana, G.; Nabanita, S.; Takeshi, K.; Petr, S. Hydrothermal effect and mechanical stress properties of carboxymethylcellulose based hydrogel food packaging. Carbohydr. Polym. 2015, 117, 559–568. [Google Scholar] [CrossRef]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The challenge of identifying non-intentionally added substances from food packaging materials: A review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Perring, L.; Alonso, M.I.; Andrey, D.; Bourqui, B.; Zbinden, P. An evaluation of analytical techniques for determination of lead, cadmium, chromium, and mercury in food-packaging materials. Fresenius J. Anal. Chem. 2011, 370, 76–81. [Google Scholar] [CrossRef]
- Abe, K.; Nakamura, K.; Arao, T.; Sakurai, Y.; Nakano, A.; Suginuma, C.; Tawarada, K.; Sasaki, K. Immunochromatography for the rapid determination of cadmium concentrations in wheat grain and eggplant. J. Sci. Food Agric. 2011, 91, 1392–1397. [Google Scholar] [CrossRef]
- Abe, K.; Nakamura, K.; Naito, S. Interlaboratory study of immunochromatography for the rapid determination of cadmium concentrations in cereals and soybeans. J. AOAC Int. 2014, 97, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.D.; Diane, A.B. Development and Validation of a One-Step Immunoassay for Determination of Cadmium in Human Serum. Anal. Chem. 2002, 74, 52–58. [Google Scholar] [CrossRef]
- Arai, H.; Nakamura, K.; Yoshida, Y.; Tawarada, K. Development of quantitative immunochromatographic kit and visual screening immunoassay strip for cadmium. Anal. Bioanal. Chem. 2016, 408, 6063–6068. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Sobolev, A.M.; Tessier, M.D.; Hens, Z.; Goryacheva, I.Y.; Saeger, S.D. Fluorescently labelled multiplex lateral flow immunoassay based on cadmium-free quantum dots. Methods 2017, 116, 141–148. [Google Scholar] [CrossRef]
- Jia, C.; Yong, H.; Shulin, Z.; Xin, L.; Jianniao, T. Gold nanoparticles-based fluorescence resonance energy transfer for competitive immunoassay of biomolecules. Analyst 2012, 137, 5885. [Google Scholar] [CrossRef]
- Kiyataka, P.H.M.; Dantas, S.T.; Pallone, J.A.L. Method for analysis and study of migration of Lead, Cadmium, Mercury and Arsenic from polypropylene packaging into ice cream and simulant. Food Anal. Methods 2015, 8, 2331–2338. [Google Scholar] [CrossRef]
- Im, J.H.; Kim, H.R.; An, B.G.; Chang, Y.W.; Kang, M.J.; Lee, T.G.; Jin, G.S.; Park, J.G.; Pyun, J.C. In situ-synthesized cadmium sulfide nanowire photosensor with a parylene passivation layer for chemiluminescent immunoassays. Biosens. Bioelectron. 2017, 92, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, Y.; Liu, B.; Chen, H.; Chen, G.; Tang, D. Cadmium ion-doped magnetic poly(styrene-acrylic acid) nanospheres for sensitive electrochemical immunoassay. Biosens. Bioelectron. 2012, 35, 461–465. [Google Scholar] [CrossRef]
- López_Marzo, A.M.; Pons, J.; Blake, D.A.; Merkoçi, A. High sensitive gold-nanoparticle based lateral flow Immunodevice for Cd2+ detection in drinking waters. Biosens. Bioelectron. 2013, 47, 190–198. [Google Scholar] [CrossRef]
- Kong, T.; Li, X.B.; Liu, G.W.; Zhang, Z.G.; Yang, Z.J.; Wang, Z.; Tang, J.; Yang, J.; Sun, J. Preparation of Novel Monoclonal Antibodies Against Chelated Cadmium Ions. Biol. Trace Elem. Res. 2013, 152, 117–124. [Google Scholar] [CrossRef]
- Gong, Y.F.; Zhang, M.Z.; Wang, M.Z.; Chen, Z.L.; Xi, X. Development of immuno-based methods for detection of melamine. Arab. J. Sci. Eng. 2014, 39, 5315–5324. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cho, Y.A.; Lee, H.S.; Lee, Y.T.; Gee, S.J.; Hammock, B.D. Synthesis of haptens forimmunoassay of organophosphorus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity. Anal. Chim. Acta 2003, 475, 85–96. [Google Scholar] [CrossRef]
- Zhu, X.X.; Xu, L.; Lou, Y.; Yu, H.N.; Li, X.; Blake, D.A.; Liu, F.Q. Preparation of specific monoclonal antibodies (MAbs) against heavy metals: MAbs that recognize chelated cadmium ions. J. Agric. Food Chem. 2007, 55, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.L.; Wang, J.F.; Li, Z.Y.; Liang, S.Z.; Wang, X.N. Immunoassay for cadmium detection and quantification. Biomed. Environ. Sci. 2009, 22, 188–193. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Li, Z.; Liang, S.; Liu, J.; Wang, X. Development of Direct Competitive Enzyme-Linked Immunosorbent Assay for the Determination Cadmium Residue in Farm Produce. Appl. Biochem. Biotech. 2009, 159, 708–717. [Google Scholar] [CrossRef]
- Darwish, I.A.; Blake, D.A. One-step competitive immunoassay for cadmium ions: Development and validation for environmental water samples. Anal. Chem. 2001, 73, 1889–1895. [Google Scholar] [CrossRef]
- Asadollah, B.; Kamyar, P.; Mitra, A.D. Thiodiethanethiol modified silica coated magnetic nanoparticles for preconcentration and determination of ultratrace amounts of Mercury, Lead, and Cadmium in environmental and food samples. Anal. Lett. 2014, 47, 210–1223. [Google Scholar] [CrossRef]
- Wen, X.; Yang, Q.; Yan, Z.; Deng, Q. Determination of cadmium and copper in water and food samples by dispersive liquid–liquid microextraction combined with UV–vis spectrophotometry. Microchem. J. 2011, 97, 249–254. [Google Scholar] [CrossRef]


| Cd-ITCBE-BSA Dilution Times | Cd (II) Concentration (ng/mL) | Anti-Cadmium Monoclonal Antibody | |||
|---|---|---|---|---|---|
| 1:20,000 | 1:40,000 | 1:60,000 | 1:80,000 | ||
| 1:20,000 | 0 | 2.3 ± 0.1 | 1.6 | 1.1 ± 0.1 | 1.0 |
| 10 | 1.6 ± 0.1 | 0.8 | 0.6 | 0.4 | |
| Inhibition rate (%) | 31.0 | 46.8 | 43.8 | 60.2 | |
| 1:30,000 | 0 | 2.2 ± 0.1 | 1.5 | 0.9 | 0.9 ± 0.1 |
| 10 | 1.3 ± 0.1 | 0.7 | 0.5 ± 0.1 | 0.3 | |
| Inhibition rate (%) | 38.4 | 54.6 | 39.9 | 65.9 | |
| 1:40,000 | 0 | 2.0 ± 0.1 | 1.3 ± 0.1 | 0.8 | 0.7 |
| 10 | 0.9 ± 0.1 | 0.5 | 0.3 | 0.2 | |
| Inhibition rate (%) | 53.9 | 65.1 | 64.4 | 69.6 | |
| 1:50,000 | 0 | 1.8 ± 0.1 | 1.2 | 0.8 | 0.7 |
| 10 | 0.8 ± 0.1 | 0.4 | 0.3 | 0.2 | |
| Inhibition rate (%) | 56.0 | 67.0 | 66.0 | 73.0 | |
| Metal Ion | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|
| Cd (II) | 5.53 ± 0.76 | 100 |
| Al (III) | >810 | <0.1 |
| Ca (II) | >810 | <0.1 |
| Cu (II) | >810 | <0.1 |
| Fe (III) | >810 | <0.1 |
| Hg (II) | >810 | <0.1 |
| Mg (II) | >810 | <0.1 |
| Mn (II) | >810 | <0.1 |
| Pb (II) | >810 | <0.1 |
| Zn (II) | >810 | <0.1 |
| Cr (III) | >810 | <0.1 |
| Ni (II) | >810 | <0.1 |
| Detection Item | Concentration (ng g−1) |
|---|---|
| Mean (X) | 25.82 |
| Standard deviation (SD) | 1.57 |
| Limit of Detection (LOD) | 30.53 |
| Limit of Quantification (LOQ) | 35.24 |
| Concentration of Cd (II) (ng/mL) | 0 | 0.33 | 1 | 3 | 9 | 27 | 81 | |
|---|---|---|---|---|---|---|---|---|
| Intra-assay (n = 12) | CV (%) | 2.83 | 5.95 | 8.12 | 4.59 | 9.03 | 11.47 | 8.43 |
| Mean | 7.20% | |||||||
| Inter-assay (n = 12) | CV (%) | 4.25 | 6.00 | 7.10 | 7.66 | 9.23 | 6.92 | 5.99 |
| Mean | 6.74% | |||||||
| Spiked Concentration (ng/mL) | Average Measured Value ± SD (ng/mL) | Average Recovery ± SD (%) | CV (%) |
|---|---|---|---|
| 0 | 25.82 ± 1.57 | / | / |
| 100 | 92.34 ± 4.26 | 92.34 ± 4.26 | 4.61 |
| 200 | 178.07 ± 21.60 | 89.03 ± 10.80 | 11.68 |
| 400 | 383.24 ± 45.61 | 95.81 ± 11.40 | 9.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Chen, E.; Ma, B.; Xu, Y.; Hao, P.; Zhang, M.; Ye, Z.; Yu, X.; Li, C.; Ji, Q. Establishment of an Indirect Competitive Enzyme-Linked Immunosorbent Method for the Detection of Heavy Metal Cadmium in Food Packaging Materials. Foods 2021, 10, 413. https://doi.org/10.3390/foods10020413
Fu X, Chen E, Ma B, Xu Y, Hao P, Zhang M, Ye Z, Yu X, Li C, Ji Q. Establishment of an Indirect Competitive Enzyme-Linked Immunosorbent Method for the Detection of Heavy Metal Cadmium in Food Packaging Materials. Foods. 2021; 10(2):413. https://doi.org/10.3390/foods10020413
Chicago/Turabian StyleFu, Xianshu, Erjing Chen, Biao Ma, Ying Xu, Peiying Hao, Mingzhou Zhang, Zihong Ye, Xiaoping Yu, Chaofeng Li, and Qingge Ji. 2021. "Establishment of an Indirect Competitive Enzyme-Linked Immunosorbent Method for the Detection of Heavy Metal Cadmium in Food Packaging Materials" Foods 10, no. 2: 413. https://doi.org/10.3390/foods10020413







