The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Pasting Properties
2.3. Rheological Properties
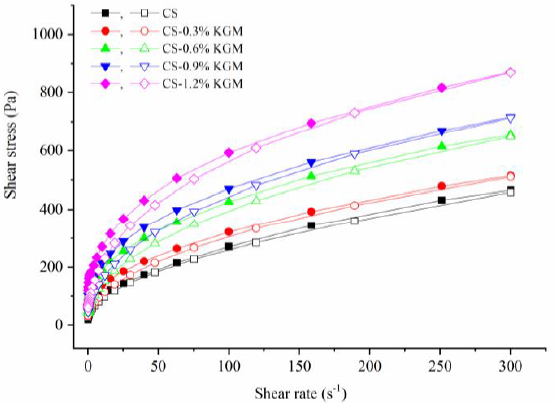
2.3.1. Flow Behavior
2.3.2. Frequency Sweep
2.3.3. Oscillation Time Sweep
2.4. Texture Analysis
2.5. Polarized Light Microscopy
2.6. Scanning Electron Microscopy
2.7. Differential Scanning Calorimetry (DSC)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pasting Properties
3.2. Rheological Properties
3.2.1. Flow Behaviors
3.2.2. Viscoelasticity
3.2.3. Time-Dependent Rheological Properties
3.3. Textural Properties
3.4. Morphological Structure of Starch Granules
3.5. Microstructure of Gelatinized Dispersions
3.6. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Varela, M.S.; Navarro, A.S.; Yamul, D.K. Effect of hydrocolloids on the properties of wheat/potato starch mixtures. Starch Stärke 2016, 68, 753–761. [Google Scholar] [CrossRef]
- Wang, X.; Reddy, C.K.; Xu, B. A systematic comparative study on morphological, crystallinity, pasting, thermal and functional characteristics of starches resources utilized in China. Food Chem. 2018, 259, 81–88. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Baranowska, H.M.; Szwengiel, A.; Le Thanh-Blicharz, J. Molecular Structure vs. Func-Tional Properties Of Waxy And Normal Corn Starch. In Proceedings of the 12th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 19–21 October 2016; Rapkova, R., Copikova, J., Sarka, E., Eds.; Czech Chemical Society: Praha, Czech Republic, 2016; p. 5. [Google Scholar]
- Matia-Merino, L.; Prieto, M.; Roman, L.; Gomez, M. The impact of basil seed gum on native and pregelatinized corn flour and starch gel properties. Food Hydrocoll. 2019, 89, 122–130. [Google Scholar] [CrossRef]
- Piyachomkwan, K.; Chotineeranat, S.; Kijkhunasatian, C.; Tonwitowat, R.; Prammanee, S.; Oates, C.G.; Sriroth, K. Edible canna (Canna edulis) as a complementary starch source to cassava for the starch industry. Ind. Crop Prod. 2002, 16, 11–21. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Puncha-Arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N.J.F.C. Quality assessment of noodles made from blends of rice flour and canna starch. Food Chem. 2015, 179, 85–93. [Google Scholar] [CrossRef]
- Hung, P.V.; Morita, N. Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydr. Polym. 2005, 61, 314–321. [Google Scholar] [CrossRef]
- Santoso, B.; Pratama, F.; Hamzah, B.; Pambayun, R. Physical and Chemical Characteristics of Canna edulis Kerr and Dioscorea hispida Dennst Modified Starch with Cross Linking Method. Agritech 2015, 35, 273–279. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A review: Interaction of starch/non-starch hydrocolloid blending and the recent food applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- Fang, F.; Luo, X.; BeMiller, J.N.; Schaffter, S.; Hayes, A.M.R.; Woodbury, T.J.; Hamaker, B.R.; Campanella, O.H. Neutral hydrocolloids promote shear-induced elasticity and gel strength of gelatinized waxy potato starch. Food Hydrocoll. 2020, 107, 105923. [Google Scholar] [CrossRef]
- Fang, F. Shear-induced synergistic effects of konjac glucomannan and waxy potato starch on viscosity and gel strength. Food Hydrocoll. 2021, 114, 106540. [Google Scholar] [CrossRef]
- Zheng, M.; Lin, Y.; Wu, H.; Zeng, S.; Zheng, B.; Zhang, Y.; Zeng, H. Water migration depicts the effect of hydrocolloids on the structural and textural properties of lotus seed starch. Food Chem. 2020, 315, 126240. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-Y.; Kwon, J.-Y.; Choi, J.; Kim, Y.-C.; Shin, M. Pasting properties of non-waxy rice starch-hydrocolloid mixtures. Starch Starke 2006, 58, 223–230. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Yoshimura, M.; Takaya, T.; Nishinari, K. Rheological studies on mixtures of corn starch and konjac glucomannan. Carbohydr. Polym. 1998, 35, 71–79. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Ma, S.; Wang, H. Physicochemical characterization of rice, potato, and pea starches, each with different crystalline pattern, when incorporated with Konjac glucomannan. Food Hydrocoll. 2020, 101, 105499. [Google Scholar] [CrossRef]
- Noda, T.; Tsuda, S.; Mori, M.; Takigawa, S.; Matsuura-Endo, C.; Salto, K.; Mangalika, W.H.A.; Hanaoka, A.; Suzuki, Y.; Yamauchi, H. The effect of harvest dates on the starch properties of various potato cultivars. Food Chem. 2004, 86, 119–125. [Google Scholar] [CrossRef]
- Chantaro, P.; Pongsawatmanit, R.; Nishinari, K. Effect of heating–cooling on rheological properties of tapioca starch paste with and without xanthan gum. Food Hydrocoll. 2013, 31, 183–194. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, B. Rheological properties of rice starch-xanthan gum mixtures. J. Food Eng. 2006, 75, 120–128. [Google Scholar] [CrossRef]
- Liu, K.; Hao, Y.; Chen, Y.; Gao, Q. Effects of dry heat treatment on the structure and physicochemical properties of waxy potato starch. Int. J. Biol. Macromol. 2019, 132, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Cui, B.; Yuan, C.; Zou, Y.; Liu, W.; Pan, Y. Effects of konjac glucomannan on the rheological, microstructure and digestibility properties of debranched corn starch. Food Hydrocoll. 2020, 100, 105342. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; You, X.; Fang, F.; Li, B. Impacts of guar and xanthan gums on pasting and gel properties of high-amylose corn starches. Int. J. Biol. Macromol. 2020, 146, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.H.; BeMiller, J.N. Effects of food gums on viscosities of starch suspensions during pasting. Carbohydr. Polym. 2002, 50, 7–18. [Google Scholar] [CrossRef]
- Liu, J.; Xu, B. A comparative study on texture, gelatinisation, retrogradation and potential food application of binary gels made from selected starches and edible gums. Food Chem. 2019, 296, 100–108. [Google Scholar] [CrossRef]
- Nagar, M.; Sharanagat, V.S.; Kumar, Y.; Singh, L.; Mani, S. Influence of xanthan and agar-agar on thermo-functional, morphological, Cileck for updates pasting and rheological properties of elephant foot yam (Amorphophallus paeoniifolius) starch. Int. J. Biol. Macromol. 2019, 136, 831–838. [Google Scholar] [CrossRef]
- Gani, A.; Ashwar, B.A.; Akhter, G.; Shah, A.; Wani, I.A.; Masoodi, F.A. Physico-chemical, structural, pasting and thermal properties of starches of fourteen Himalayan rice cultivars. Int. J. Biol. Macromol. 2017, 95, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Funami, T.; Kataoka, Y.; Omoto, T.; Goto, Y.; Asai, I.; Nishinari, K. Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch. Food Hydrocoll. 2005, 19, 1–13. [Google Scholar] [CrossRef]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, rheological, thermal and gel textural properties of pearl millet starch as modified by guar gum and its acid hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M.; Wang, F.; Wang, N. Effect of konjac glucomannan with different molecular weights on physicochemical properties of corn starch. Food Hydrocoll. 2019, 96, 663–670. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Cao, Y.; Liu, S.; Deng, D.; Zhang, J.; Huang, G. Effects of xanthan and konjac gums on pasting, rheology, microstructure, crystallinity and in vitro digestibility of mung bean resistant starch. Food Chem. 2021, 339, 128001. [Google Scholar] [CrossRef]
- Wang, R.; Wan, J.; Liu, C.; Xia, X.; Ding, Y. Pasting, thermal, and rheological properties of rice starch partially replaced by inulin with different degrees of polymerization. Food Hydrocoll. 2019, 92, 228–232. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Achayuthakan, P.; Suphantharika, M. Pasting and rheological properties of waxy corn starch as affected by guar gum and xanthan gum. Carbohydr. Polym. 2008, 71, 9–17. [Google Scholar] [CrossRef]
- Nagano, T.; Tamaki, E.; Funami, T. Influence of guar gum on granule morphologies and rheological properties of maize starch. Carbohydr. Polym. 2008, 72, 95–101. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.C.; Aman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Choi, H.M.; Yoo, B. Steady and dynamic shear rheology of sweet potato starch-xanthan gum mixtures. Food Chem. 2009, 116, 638–643. [Google Scholar] [CrossRef]
- Huang, M.; Kennedy, J.F.; Li, B.; Xu, X.; Xie, B.J. Characters of rice starch gel modified by gellan, carrageenan, and glucomannan: A texture profile analysis study. Carbohydr. Polym. 2007, 69, 411–418. [Google Scholar] [CrossRef]
- Le Thanh-Blicharz, J.; Lewandowicz, J. Functionality of Native Starches in Food Systems: Cluster Analysis Grouping of Rheological Properties in Different Product Matrices. Foods 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-L.; Zhang, Q.; Shen, Q.; Hu, X.-S.; Wu, J.-H. Effect of high hydrostatic pressure on modified non-crystalline granular starch of starches with different granular type and amylase content. Lebensm. Wiss. Technol. 2012, 47, 450–458. [Google Scholar] [CrossRef]
- Blaszczak, W.; Bidzinska, E.; Dyrek, K.; Fornal, J.; Michalec, M.; Wenda, E. Effect of phosphorylation and pretreatment with high hydrostatic pressure on radical processes in maize starches with different amylose contents. Carbohydr. Polym. 2011, 85, 86–96. [Google Scholar] [CrossRef]
- Błaszczak, W.; Lewandowicz, G. Light Microscopy as a Tool to Evaluate the Functionality of Starch in Food. Foods 2020, 9, 670. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Observations on the impact of amylopectin and amylose structure on the swelling of starch granules. Food Hydrocoll. 2020, 103, 105663. [Google Scholar] [CrossRef]
- Kou, X.; Luo, D.; Li, Y.; Xu, B.; Zhang, K.; Li, P.; Li, X.; Han, S.; Liu, J. Effect of inulin with different degree of polymerisation on textural and rheological properties of wheat starch. Int. J. Food Sci. Technol. 2018, 53, 2576–2585. [Google Scholar] [CrossRef]




| KGM Addition (%) | PV (mPa·s) | BV (mPa·s) | FV (mPa·s) | SV (mPa·s) | PT (°C) |
|---|---|---|---|---|---|
| 0 | 2453 ± 128 e | 1097 ± 80 e | 1824 ± 272 c | 437 ± 17 b | 68 ± 1.5 a |
| 0.3 | 3935 ± 98 d | 2449 ± 176 d | 2070 ± 112 bc | 458 ± 24 b | 68 ± 1.5 a |
| 0.6 | 4580 ± 104 c | 2836 ± 225 c | 2181 ± 114 b | 468 ± 24 b | 67 ± 0.6 a |
| 0.9 | 5361 ± 336 b | 3522 ± 229 b | 2390 ± 196 ab | 551 ± 18 a | 63 ± 2.2 b |
| 1.2 | 6170 ± 181 a | 4030 ± 193 a | 2598 ± 179 a | 584 ± 7 a | 56 ± 1.6 c |
| KGM Addition (%) | Up Curve | Down Curve | ||||
|---|---|---|---|---|---|---|
| K (Pa·sn) | n | R2 | K (Pa·sn) | n | R2 | |
| 0 | 43.645 ± 2.162 e | 0.408 ± 0.013 a | 0.9964 b | 31.701 ± 2.232 e | 0.464 ± 0.020 a | 0.9947 e |
| 0.3 | 69.655 ± 3.314 d | 0.342 ± 0.004 b | 0.9938 e | 39.943 ± 3.043 d | 0.445 ± 0.004 b | 0.9956 d |
| 0.6 | 96.813 ± 5.021 c | 0.329 ± 0.001 c | 0.9949 d | 54.279 ± 4.821 c | 0.433 ± 0.003 c | 0.9967 c |
| 0.9 | 109.143 ± 7.364 b | 0.324 ± 0.001 d | 0.9956 c | 62.678 ± 5.326 b | 0.426 ± 0.001 d | 0.9969 b |
| 1.2 | 143.335 ± 9.634 a | 0.311 ± 0.001 e | 0.9997 a | 90.093 ± 5.867 a | 0.398 ± 0.001 e | 0.9973 a |
| KGM Addition (%) | Hardness (N) | Cohesiveness | Chewiness (mJ) | Elasticity (mm) |
|---|---|---|---|---|
| 0 | 3.86 ± 0.2 a | 0.25 ± 0.02 c | 9.1 ± 0.2 e | 9.2 ± 0.3 e |
| 0.3 | 3.23 ± 0.2 b | 0.39 ± 0.06 b | 10.2 ± 0.1 d | 10.1 ± 0.2 d |
| 0.6 | 2.71 ± 0.2 c | 0.40 ± 0.00 b | 10.8 ± 0.3 c | 11.2 ± 0.3 c |
| 0.9 | 2.33 ± 0.1 d | 0.46 ± 0.05 a | 11.6 ± 0.2 b | 12.8 ± 0.3 b |
| 1.2 | 1.99 ± 0.1 e | 0.47 ± 0.06 a | 12.3 ± 0.3 a | 14.1 ± 0.1 a |
| KGM Addition (%) | To (°C) | Tp (°C) | Tc (°C) | ΔH (J·g−1) | Tc-To (°C) |
|---|---|---|---|---|---|
| 0 | 60.49 ± 0.21 a | 66.58 ± 0.01 c | 74.10 ± 0.01 e | 29.59 ± 0.01 a | 13.61 ± 1.05 e |
| 0.3 | 59.42 ± 0.15 a | 67.23 ± 0.02 b | 78.73 ± 0.01 c | 27.17 ± 0.01 b | 16.31 ± 1.02 d |
| 0.6 | 58.96 ± 0.92 a | 67.87 ± 0.01 b | 77.44 ± 0.00 d | 24.74 ± 0.02 c | 18.48 ± 0.87 c |
| 0.9 | 56.35 ± 0.82 b | 68.61 ± 0.02 a | 79.79 ± 0.01 b | 21.66 ± 0.07 d | 23.44 ± 0.76 b |
| 1.2 | 53.91 ± 0.62 c | 69.78 ± 0.01 a | 84.73 ± 0.01 a | 16.44 ± 0.08 e | 30.82 ± 0.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Q.; Fang, F.; Liu, J.; Wang, Z.; Chen, H.; Zhang, F. The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch. Foods 2021, 10, 422. https://doi.org/10.3390/foods10020422
Liu Y, Chen Q, Fang F, Liu J, Wang Z, Chen H, Zhang F. The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch. Foods. 2021; 10(2):422. https://doi.org/10.3390/foods10020422
Chicago/Turabian StyleLiu, Yuanqin, Qiaoli Chen, Fang Fang, Jiali Liu, Zhiying Wang, Hourong Chen, and Fusheng Zhang. 2021. "The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch" Foods 10, no. 2: 422. https://doi.org/10.3390/foods10020422
APA StyleLiu, Y., Chen, Q., Fang, F., Liu, J., Wang, Z., Chen, H., & Zhang, F. (2021). The Influence of Konjac Glucomannan on the Physicochemical and Rheological Properties and Microstructure of Canna Starch. Foods, 10(2), 422. https://doi.org/10.3390/foods10020422






