Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Jeotgal Alternative
2.3. Glutamic Acid Content Analysis
2.4. Free Amino Acids Analysis
2.5. Volatile Compounds Analysis
2.6. Preparation of Kimchi by Adding Jeotgal Alternative
2.7. Physicochemical Analysis
2.8. Microbial Analysis
2.9. Statistical Analysis
3. Results and Discussion
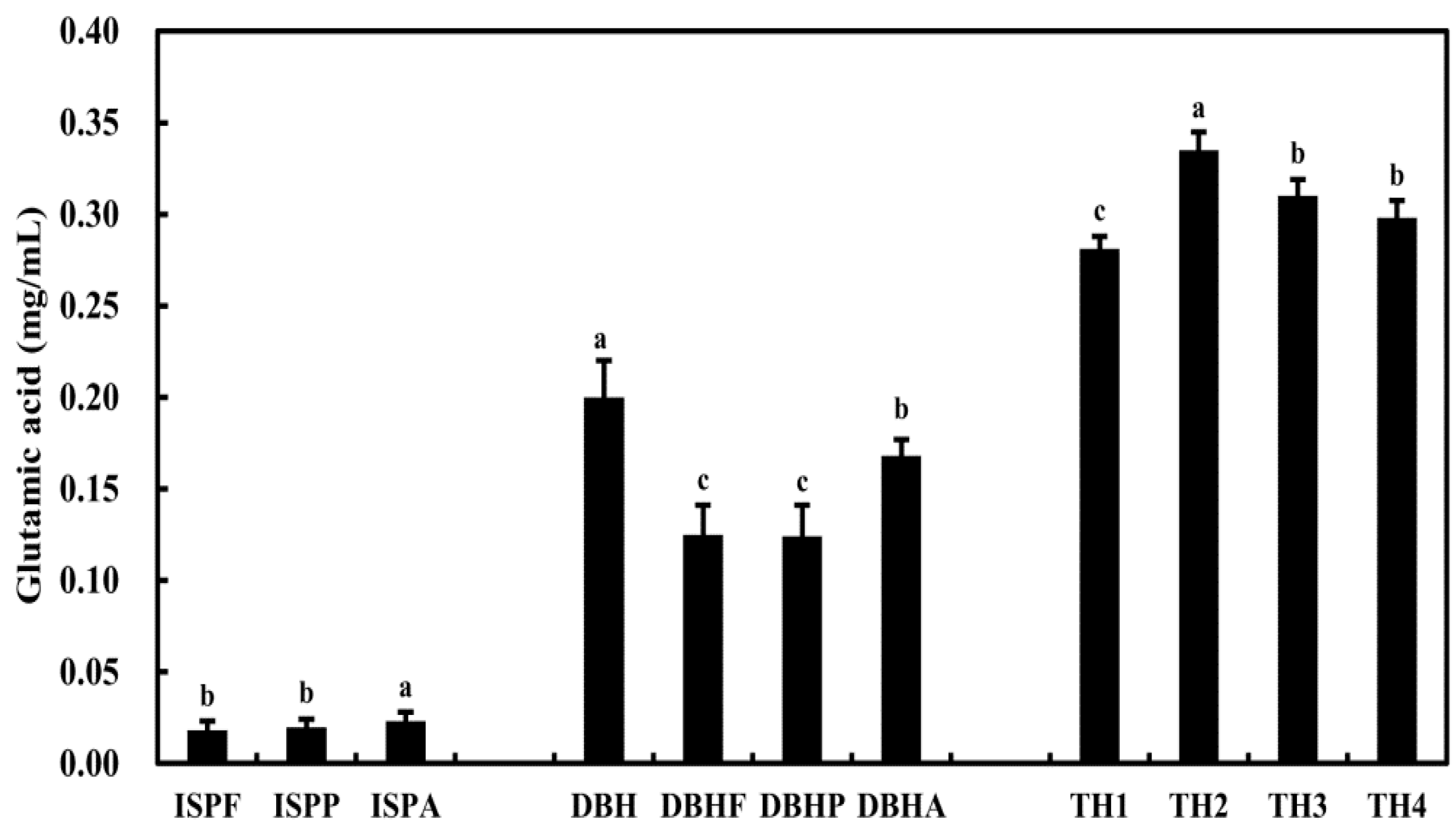
3.1. Glutamic Acid Content of Jeotgal Alternative
3.2. Free Amino Acid Composition of Selected Jeotgal Alternative
3.3. Volatile Compounds Composition of Jeotgal Alternatives
3.4. pH, Total Acidity, and Salinity of Kimchi Prepared with Jeotgal Alternatives
3.5. Microbial Profiles of Kimchi Prepared with Jeotgal Alternatives
3.6. Free Amino Acid Composition of Kimchi Added Jeotgal Alternatives
3.7. Volatile Compounds Composition of Prepared with Jeotgal Alternatives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, O.K.; Lee, S.J.; Chung, K.R.; Jang, D.J.; Yang, H.J.; Kwon, D.Y. Korean traditional fermented fish products: Jeotgal. J. Ethn. Foods 2016, 3, 107–116. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, K.H. Processing and Characterization of Salt-Fermented Fish (Jeotgal) Using Seafood By-Products in Korea. Seafood Processing By-Products; Springer: New York, NY, USA, 2014; pp. 63–99. [Google Scholar]
- Jung, M.Y.; Kim, T.W.; Lee, C.; Kim, J.Y.; Song, H.S.; Kim, Y.B.; Ahn, S.W.; Kim, J.S.; Roh, S.W.; Lee, S.H. Role of jeotgal, a Korean traditional fermented fish sauce, in microbial dynamics and metabolite profiles during kimchi fermentation. Food Chem. 2018, 265, 135–143. [Google Scholar] [CrossRef]
- Ninomiya, K. Natural occurrence. Food Rev. Int. 1988, 14, 177–211. [Google Scholar] [CrossRef]
- Fuke, S.; Konosu, S. Taste-active components in some foods: A review of Japanese research. Physiol. Behav. 1991, 49, 863–868. [Google Scholar] [CrossRef]
- Park, K.Y.; Kwon, D.Y.; Lee, K.W.; Park, S. Korean Functional Foods: Composition, Processing and Health Benefits. Jeotgal (Fermented Fish); CRC Press: Boca Raton, FL, USA, 2018; pp. 183–209. [Google Scholar]
- Zabat, M.A.; Sano, W.H.; Cabral, D.J.; Wurster, J.I.; Belenky, P. The impact of vegan production on the kimchi microbiome. Food Microbiol. 2018, 74, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Andayni, S.N.; Lioe, H.N.; Wijaya, C.H.; Ogawa, M. Umami fractions obtained from water-soluble extracts of red oncom and black oncom-Indonesian fermented soybean and peanut products. J. Food Sci. 2020, 85, 657–665. [Google Scholar] [CrossRef]
- Zhu, X.; Sun-Waterhouse, D.; Chen, J.; Cui, C.; Wang, W. Comparative study on the novel umami-active peptides of the whole soybeans and the defatted soybeans fermented soy sauce. J. Sci. Food Agric. 2020, 101, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Skurray, G.R.; Pucar, N. L-glutamic acid content of fresh and processed foods. Food Chem. 1988, 27, 177–180. [Google Scholar] [CrossRef]
- Al-Sayed, A.; Thomason, I. Meloidogyne incognita and tomato response to thiamine, ascorbic acid, L-arginine, and L-glutamic acid. J. Nematol. 1988, 20, 451–456. [Google Scholar]
- Augusto, S.; Gisela, F.; Silvana, B.B.; Estela, M.V. Free amino acid production during tomato fruit ripening: A focus on L-glutamate. Amino Acids 2010, 38, 1523–1532. [Google Scholar]
- Kim, J.H.; Jeon, M.Y.; Lee, C.H. Physicochemical and sensory characteristics of commercial, frozen, dry, and wet-aged Hanwoo sirloins. Asian-Australas. J. Anim. Sci. 2019, 32, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Miyazaki, Y.; Tsuyuki, R.; Kato, K.; Egusa, A.; Ogoshi, H.; Nishimura, T. Changes in taste compounds, breaking properties, and sensory attributes during dry aging of beef from Japanese black cattle. Meat Sci. 2016, 112, 46–51. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 15th ed.; Association of Official Analytical Chemist: Washington, DC, USA, 1975. [Google Scholar]
- Valerio, F.; Conte, A.; Biase, M.D.; Lattanzio, V.M.T.; Lonigro, S.L.; Padalino, L.; Pontonio, D.; Lavermicocca, P. Formulation of yeast-leavened bread with reduced salt content by using a Lactobacillus plantarum fermentation product. Food Chem. 2017, 15, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.K. Introduction to Aroma Compounds in Foods. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–30. [Google Scholar]
- Zhang, H.; Huang, D.; Pu, D.; Zhang, Y.; Chen, H.; Sun, B.; Ren, F. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis). Food Res. Int. 2020, 133, 109112. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K.; Faithong, N. Changes in volatile compounds, ATP-related compounds and antioxidative properties of Kapi, produced from Acetes vulgaris, during processing and fermentation. Food Biosci. 2017, 19, 49–56. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.R.; Liu, R.S.; He, L.; Li, H.M.; Tang, Y.L.; Liang, X.H.; Chen, T.; Tang, Y.J. Aroma improvement by repeated freeze-thaw treatment during Tuber melanosporum fermentation. Sci. Rep. 2015, 5, 17120. [Google Scholar] [CrossRef] [PubMed]
- Vercammen, A.; Vivijs, B.; Lurquin, I.; Michiels, C.W. Germination and inactivation of Bacillus coagulans and Alicyclobacillus acidoterrestris spores by high hydrostatic pressure treatment in buffer and tomato sauce. Int. J. Food Microbiol. 2012, 152, 162–167. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, S.J.; Choi, Y.J.; Lee, M.A.; Yun, Y.R.; Min, S.G.; Seo, H.Y.; Her, J.Y.; Park, S.H. Evaluation of onion juices quality following heat-treatment and their application as a sugar substitute in Kimchi. J. Food Sci. Technol. 2020, 57, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, H.W.; Yang, J.H.; Hong, S.W.; Park, S.H.; Lee, M.A. Changes in quality properties of Kimchi based on the nitrogen content of fermented anchovy sauce, myeolchi aekjeot, during fermentation. Food Sci. Biotechnol. 2018, 27, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Hawer, W.D.; Ha, J.H.; Seog, H.M.; Nam, Y.J.; Shin, D.W. Changes in the taste and flavour compounds of kimchi during fermentation. Korean J. Food Sci. Technol. 1988, 20, 511–517. [Google Scholar]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Fact. 2011, 10, S5. [Google Scholar] [CrossRef]
- Guo, Q.; Guo, L.; Wang, Z.; Zhuang, Y.; Gu, Z. Response surface optimization and identification of isothiocyanates produced from broccoli sprouts. Food Chem. 2013, 141, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, J.H.; Min, S.; Min, D.B. Changes of volatile compounds, lactic acid bacteria, pH, and headspace gases in Kimchi, a traditional Korean fermented vegetable product. J. Food Sci. 2003, 68, 849–854. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, H.; Cadwallader, K.R. Aroma-active compounds in kimchi during fermentation. J. Agric. Food Chem. 1998, 46, 1944–1953. [Google Scholar] [CrossRef]
- Hong, S.P.; Lee, E.J.; Kim, Y.H.; Ahn, D.U. Effect of fermentation temperature on the volatile composition of kimchi. J. Food Sci. 2016, 81, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.N.; Lee, M.A.; Lee, K.G. Determination of compositional quality and volatile flavor characteristics of radish-based Kimchi suitable for Chinese consumers and its correlation to consumer acceptability. Food Sci. Biotechnol. 2018, 27, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Keleş, D.; Taşkin, H.; Baktemur, G.; Kafkas, E.; Büyükalaca, S. Comparitive study on volatile aroma compounds of two different garlic types (Kastamonu and Chinese) using gas chromatography mass spectrometry (HS-GC/MS) technique. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 217–220. [Google Scholar] [CrossRef]
- Shahzadi, P.; Muhammad, A.; Mehmood, F.; Chaudhry, M. Synthesis of 3, 7-dimethyl-2, 6-octadienal acetals from citral extracted from lemon grass, Cymbopogon citrates L. J. Antivir. Antiretrovir. 2014, 6, 28–31. [Google Scholar] [CrossRef]
| Parameters | Conditions |
|---|---|
| HPLC system | Ultimate 3000 |
| Column | VDSpher 100 C18-E |
| Mobile phase | A: 40 mM sodium phosphate dibasic, pH 7.8 |
| B: water/acetonitrile/methanol (10:45:45 v/v%) | |
| Flow rate | 1.5 mL/min |
| Detector | FL Detector 1260 FLD |
| : Emission 450 nm, Excitation 340 nm (OPA) | |
| : Emission 305 nm, Excitation 266 nm (FMOC) | |
| UV Detector | |
| : 338 nm | |
| Oven Temperature 40 °C | |
| Parameters | Conditions |
|---|---|
| GC System with MS | Agilent 7890 with 5977BA |
| Separation column | DB-WAX (60 m × 0.25 mm × 0.25 µM) |
| Injection temperature | 250 °C |
| Carrier gas flow | 1 mL/min (He) |
| Oven temperature | 40 °C (3 min) → 150 °C (2 °C /min)(10 min) → 200 °C (4 °C/min)(10) |
| Ingredient | CON (g) | JA1 (g) | JA2 (g) |
|---|---|---|---|
| Brined Kimchi cabbage | 700 | 700 | 700 |
| Red pepper | 66 | 66 | 66 |
| Garlic | 36 | 36 | 36 |
| Ginger | 10.2 | 10.2 | 10.2 |
| Rice paste | 57.3 | 57.3 | 57.3 |
| Water | 98.1 | 98.1 | 98.1 |
| Sugar | 1.5 | 1.5 | 1.5 |
| Salt | 2.28 | 2.28 | 2.28 |
| Jeotgal | 28.62 | - | - |
| DBH | - | - | 14.31 |
| TH2 | - | 28.62 | 14.31 |
| Total | 1000 | 1000 | 1000 |
| Free Amino Acids | CON (mg/kg) | DBH (mg/kg) | ISPA (mg/kg) | TH2 (mg/kg) |
|---|---|---|---|---|
| Aspartic acid | 12,591.76 ± 65.40 a | 488.85 ± 31.64 c | 57.78 ± 3.00 d | 8425.89 ± 77.47 b |
| Glutamic acid | 21,032.41 ± 407.62 b | 7815.70 ± 332.44 c | 192.49 ± 9.41 d | 33,328.32 ± 212.79 a |
| Asparagine | 85.72 ± 1.90 c | 2152.63 ± 110.63 b | 110.74 ± 4.28 c | 4515.82 ± 21.08 a |
| Serine | 6548.43 ± 78.05 a | 5163.78 ± 306.21 a | 132.22 ± 6.92 c | 1169.69 ± 6.54 b |
| Glutamine | 117.89 ± 4.17 c | 18,764.66 ± 945.22 a | 37.89 ± 0.46 d | 6278.53 ± 8.15 b |
| Histidine | 6141.59 ± 250.82 a | 2128.26 ± 105.86 b | 122.26 ± 1.35 d | 773.99 ± 0.41 c |
| Glycine | 4841.74 ± 8.73 a | 4025.58 ± 464.57 a | 26.02 ± 1.87 c | 160.82 ± 3.76 b |
| Threonine | 7394.65 ± 48.64 a | 3983.79 ± 173.73 b | 31.62 ± 0.62 d | 1997.02 ± 3.13 c |
| Arginine | 6246.24 ± 22.23 a | 4173.57 ± 655.57 b | 212.44 ± 18.74 d | 1468.70 ± 43.62 c |
| Alanine | 9501.16 ± 35.15 b | 14,997.51 ± 655.57 a | 145.19 ± 5.38 d | 666.53 ± 35.05 c |
| Taurine | 2189.53 ± 38.50 | ND | ND | ND |
| GABA | ND | ND | ND | 7467.79 ± 139.30 |
| Tyrosine | 668.90 ± 64.66 b | 2976.79 ± 86.99 a | 126.07 ± 28.41 d | 474.99 ± 37.33 c |
| Valine | 7689.68 ± 26.09 | 4440.78 ± 121.56 | ND | 324.92 ± 9.16 |
| Methionine | 2744.33 ± 2.94 | 2543.58 ± 100.97 | ND | 193.65 ± 9.11 |
| Tryptophane | 1721.02 ± 13.16 | 845.61 ± 53.55 | ND | 469.91 ± 10.98 |
| Phenylalanine | 4437.97 ± 40.53 | 3226.94 ± 158.98 | ND | 1650.90 ± 22.61 |
| Isoleucine | 4919.20 ± 52.41 | 3221.46 ± 124.82 | ND | 563.90 ± 4.58 |
| Leucine | 6295.06 ± 47.16 a | 5884.54 ± 249.84 a | 128.94 ± 1.70 c | 782.21 ± 10.40 b |
| Lysine | 11,471.60 ± 40.38 a | 6181.05 ± 1019.20 b | 221.65 ± 7.68 d | 782.21 ± 10.40 c |
| Proline | 2565.24 ± 122.12 | 2629.67 ± 380.10 | ND | 192.56 ± 12.43 |
| Volatile Compounds 1 | CON (Peak Area) | DBH (Peak Area) | ISPA (Peak Area) | TH2 (Peak Area) |
|---|---|---|---|---|
| 2,4-Bis(1,1-dimethylethyl) phenol | 0.53 ± 0.19 NS | 2.68 ± 0.75 | 0.07 ± 0.00 | 0.46 ± 0.15 |
| 2,5-Dimethylpyrazine | 0.12 ± 0.04 b | 0.30 ± 0.10 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| 2-Furanmethanol | 0.14 ± 0.05 | ND | ND | 0.01 ± 0.00 |
| 2-Methylpentanoic acid | 0.11 ± 0.04 | ND | 0.00 ± 0.00 | ND |
| 2-Methylpropanoic acid | 0.14 ± 0.05 | 0.03 ± 0.01 | ND | ND |
| 3-Methylbutanoic acid | 1.79 ± 0.57 a | 0.06 ± 0.02 b | 0.00 ± 0.00 b | 0.01 ± 0.00 b |
| 4-Methylpentanoic acid | 0.10 ± 0.03 | 0.01 ± 0.00 | ND | ND |
| Acetic acid | 0.11 ± 0.04 b | 0.42 ± 0.14 a | 0.01 ± 0.00 c | 0.07 ± 0.01 b |
| Benzaldehyde | 0.17 ± 0.05 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.00 b |
| Butanoic acid | 0.37 ± 0.12 b | 0.59 ± 0.21 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| Phenol | 0.10 ± 0.03 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.00 b |
| Trimethylpyrazine | 0.15 ± 0.05 | 1.31 ± 0.47 | ND | 0.00 ± 0.00 |
| Week | Samples | pH | Total Acidity (%) | Salinity (%) |
|---|---|---|---|---|
| 0 | CON | 4.90 ± 0.00 a | 0.50 ± 0.01 c | 1.87 ± 0.04 a |
| JA1 | 4.54 ± 0.02 c | 0.79 ± 0.00 a | 1.13 ± 0.03 b | |
| JA2 | 4.94 ± 0.01 b | 0.69 ± 0.00 b | 1.03 ± 0.02 b | |
| 1 | CON | 4.35 ± 0.01 c | 0.73 ± 0.01 c | 1.85 ± 0.00 a |
| JA1 | 4.41 ± 0.01 b | 0.85 ± 0.01 b | 1.13 ± 0.01 b | |
| JA2 | 4.55 ± 0.02 a | 0.97 ± 0.01 a | 1.03 ± 0.02 b | |
| 2 | CON | 4.03 ± 0.01 c | 1.12 ± 0.02 b | 1.85 ± 0.01 a |
| JA1 | 4.23 ± 0.01 b | 1.15 ± 0.01 b | 1.12 ± 0.00 b | |
| JA2 | 4.31 ± 0.02 a | 1.36 ± 0.02 a | 1.02 ± 0.02 b |
| Week | Samples | Total Viable Bacteria (CFU/mL) | Lactic Acid Bacteria (CFU/mL) |
|---|---|---|---|
| 0 | CON | 6.93 ± 0.03 b | 6.87 ± 0.02 NS |
| JA1 | 7.02 ± 0.01 a | 6.88 ± 0.05 | |
| JA2 | 6.98 ± 0.03 b | 6.84 ± 0.02 | |
| 1 | CON | 8.19 ± 0.06 a | 8.00 ± 0.05 NS |
| JA1 | 7.93 ± 0.05 b | 7.80 ± 0.07 | |
| JA2 | 7.99 ± 0.04 b | 7.91 ± 0.01 | |
| 2 | CON | 8.49 ± 0.01 b | 8.50 ± 0.05 b |
| JA1 | 8.83 ± 0.05 a | 8.74 ± 0.05 a | |
| JA2 | 8.51 ± 0.12 b | 8.42 ± 0.04 b |
| Free Amino Acids | Week | CON (mg/kg) | JA1 (mg/kg) | JA2 (mg/kg) |
|---|---|---|---|---|
| Glutamic acid | 0 | 413.25 ± 2.19 c | 1246.23 ± 6.95 a | 686.59 ± 1.67 b |
| 1 | 428.60 ± 3.65 c | 1057.81 ± 1.38 a | 796.84 ± 6.81 b | |
| 2 | 466.21 ± 2.88 c | 1231.37 ± 10.95 a | 910.89 ± 18.50 b | |
| Glycine | 0 | 106.31 ± 0.83 a | 55.63 ± 0.45 b | 95.48 ± 0.03 a |
| 1 | 114.99 ± 1.65 b | 59.46 ± 0.21 c | 124.98 ± 1.82 a | |
| 2 | 125.06 ± 0.30 b | 63.12 ± 0.25 c | 145.24 ± 2.88 a | |
| Arginine | 0 | 429.60 ± 3.92 b | 467.05 ± 1.70 a | 446.23 ± 0.86 b |
| 1 | 443.33 ± 6.29 b | 462.16 ± 2.55 b | 514.60 ± 4.76 a | |
| 2 | 460.33 ± 4.11 b | 487.34 ± 9.22 b | 569.39 ± 12.09 a | |
| Alanine | 0 | 513.44 ± 3.09 b | 412.26 ± 1.32 c | 533.65 ± 2.09 a |
| 1 | 526.47 ± 7.12 b | 408.20 ± 1.89 c | 718.40 ± 7.18 a | |
| 2 | 572.12 ± 4.73 b | 428.67 ± 4.48 c | 783.50 ± 16.69 a | |
| Taurine | 0 | 46.13 ± 1.11 b | 26.50 ± 0.12 b | 293.33 ± 1.84 a |
| 1 | 44.96 ± 1.03 b | 23.96 ± 0.54 b | 313.50 ± 2.68 a | |
| 2 | 44.70 ± 0.66 b | 25.11 ± 0.18 b | 341.98 ± 5.40 a | |
| GABA | 0 | 243.81 ± 4.16 c | 477.81 ± 1.06 a | 342.11 ± 1.88 b |
| 1 | 230.97 ± 3.67 c | 433.86 ± 1.94 a | 363.28 ± 4.60 b | |
| 2 | 224.44 ± 2.64 c | 463.39 ± 2.54 a | 352.96 ± 6.16 b | |
| Leucine | 0 | 133.04 ± 1.17 a | 75.18 ± 1.14 c | 98.13 ± 0.13 b |
| 1 | 139.06 ± 1.77 a | 81.77 ± 0.28 c | 124.31 ± 1.66 b | |
| 2 | 141.77 ± 1.52 a | 79.97 ± 0.92 c | 129.32 ± 3.03 b | |
| Isoleucine | 0 | 169.45 ± 0.87 a | 83.12 ± 1.01 c | 142.35 ± 0.83 b |
| 1 | 194.37 ± 1.75 a | 102.10 ± 0.17 b | 192.46 ± 3.33 a | |
| 2 | 218.71 ± 1.61 a | 113.11 ± 1.00 b | 228.27 ± 4.46 a |
| Volatile Compounds 1 | Week | CON (Peak Area) | JA1 (Peak Area) | JA2 (Peak Area) |
|---|---|---|---|---|
| (2-Isothiocyanatoethyl) benzene | 0 | 2.12 ± 0.20 a | 1.84 ± 0.08 b | 2.18 ± 0.11 a |
| 1 | 0.73 ± 0.05 b | 0.91 ± 0.04 a | 0.64 ± 0.05 b | |
| 2 | 0.26 ± 0.06 b | 0.26 ± 0.00 b | 0.36 ± 0.02 a | |
| 2,3-Butanediol | 0 | 0.06 ± 0.01 NS | 0.07 ± 0.03 | 0.07 ± 0.01 |
| 1 | 0.16 ± 0.03 b | 0.17 ± 0.00 b | 0.32 ± 0.07 a | |
| 2 | 1.09 ± 0.13 c | 1.60 ± 0.10 b | 2.39 ± 0.69 a | |
| 3,7-Dimethyl-2,6-octadien-1-ol | 0 | 1.67 ± 0.21 a | 1.13 ± 0.07 b | 1.01 ± 0.09 b |
| 1 | 0.50 ± 0.05 a | 0.59 ± 0.02 a | 0.39 ± 0.03 b | |
| 2 | 0.21 ± 0.02 b | 0.19 ± 0.02 | 0.25 ± 0.03 | |
| 4-Isothiocyanato-1-butene | 0 | 2.50 ± 0.38 b | 2.96 ± 0.19 a | 2.44 ± 0.05 b |
| 1 | 0.57 ± 0.01 b | 0.78 ± 0.00 a | 0.48 ± 0.00 c | |
| 2 | 0.05 ± 0.01 NS | 0.04 ± 0.00 | 0.06 ± 0.00 | |
| Acetic acid | 0 | 0.12 ± 0.02 NS | 0.13 ± 0.01 | 0.09 ± 0.01 |
| 1 | 0.51 ± 0.04 a | 0.34 ± 0.02 c | 0.42 ± 0.03 b | |
| 2 | 1.63 ± 0.16 a | 1.44 ± 0.06 b | 1.78 ± 0.02 a | |
| Benzenepropanenitrile | 0 | 0.75 ± 0.13 NS | 0.86 ± 0.08 | 0.88 ± 0.02 |
| 1 | 1.01 ± 0.04 a | 0.91 ± 0.04 a | 0.62 ± 0.02 b | |
| 2 | 1.04 ± 0.08 a | 0.76 ± 0.06 b | 0.77 ± 0.06 b | |
| Diallyl_disulphide | 0 | 1.50 ± 0.18 NS | 1.44 ± 0.09 | 1.44 ± 0.09 |
| 1 | 3.95 ± 0.28 a | 2.82 ± 0.16 b | 2.63 ± 0.15 c | |
| 2 | 3.68 ± 0.50 a | 3.17 ± 0.25 c | 3.48 ± 0.01 b | |
| Methyl-2-propenyl disulfide | 0 | 0.95 ± 0.16 NS | 0.84 ± 0.04 | 0.97 ± 0.05 |
| 1 | 2.13 ± 0.07 a | 1.69 ± 0.03 b | 1.33 ± 0.04 c | |
| 2 | 1.20 ± 0.15 a | 0.76 ± 0.09 b | 0.82 ± 0.00 b | |
| Methyl-2-propenyl trisulfide | 0 | 1.78 ± 0.22 NS | 1.46 ± 0.12 | 1.86 ± 0.01 |
| 1 | 1.26 ± 0.10 a | 0.96 ± 0.04 b | 0.98 ± 0.05 b | |
| 2 | 1.07 ± 0.15 b | 1.01 ± 0.08 b | 1.16 ± 0.04 a | |
| Zingiberene | 0 | 1.63 ± 0.00 a | 1.64 ± 0.14 a | 1.18 ± 0.27 b |
| 1 | 1.36 ± 0.20 a | 0.72 ± 0.15 b | 0.62 ± 0.06 b | |
| 2 | 1.45 ± 0.27 a | 0.74 ± 0.01 c | 1.14 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Lee, M.J.; Choi, Y.-J.; Park, S.J.; Lee, M.-A.; Min, S.G.; Park, S.-H.; Seo, H.-Y.; Yun, Y.-R. Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi. Foods 2021, 10, 423. https://doi.org/10.3390/foods10020423
Lee HJ, Lee MJ, Choi Y-J, Park SJ, Lee M-A, Min SG, Park S-H, Seo H-Y, Yun Y-R. Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi. Foods. 2021; 10(2):423. https://doi.org/10.3390/foods10020423
Chicago/Turabian StyleLee, Hye Jin, Min Jung Lee, Yun-Jeong Choi, Sung Jin Park, Mi-Ai Lee, Sung Gi Min, Sung-Hee Park, Hye-Young Seo, and Ye-Rang Yun. 2021. "Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi" Foods 10, no. 2: 423. https://doi.org/10.3390/foods10020423
APA StyleLee, H. J., Lee, M. J., Choi, Y.-J., Park, S. J., Lee, M.-A., Min, S. G., Park, S.-H., Seo, H.-Y., & Yun, Y.-R. (2021). Free Amino Acid and Volatile Compound Profiles of Jeotgal Alternatives and Its Application to Kimchi. Foods, 10(2), 423. https://doi.org/10.3390/foods10020423







