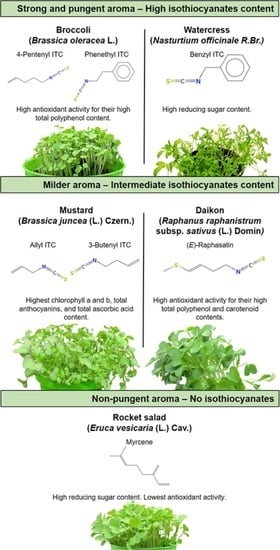
Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Biochemical Analyses
2.3. Reducing and Total Soluble Sugars Quantification
2.4. Essential Oil Hydrodistillation and Analysis by Gas-Chromatography-Mass Spectrometry (GC-MS)
2.5. Statistical Analysis
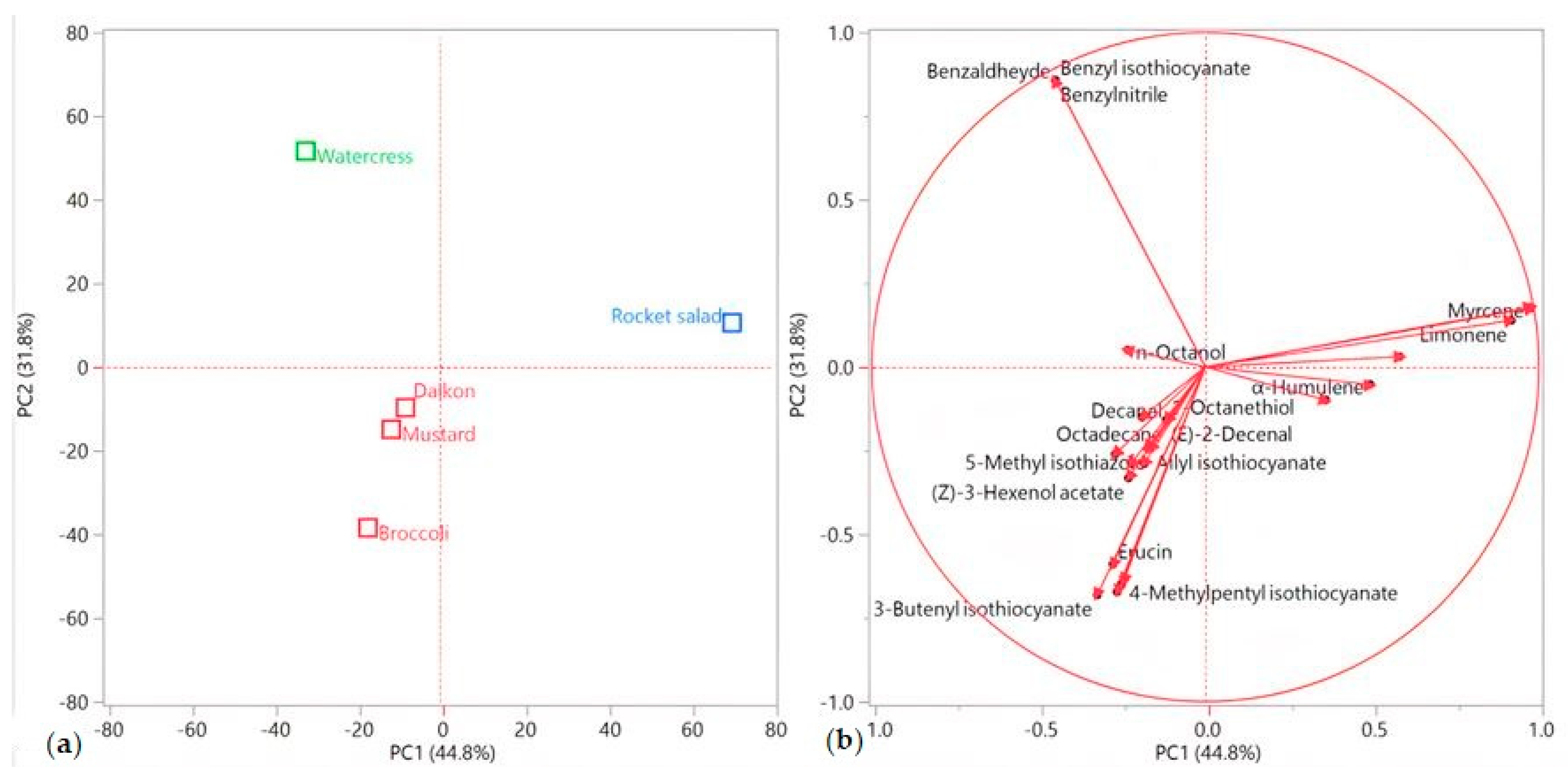
3. Results
3.1. Biochemical Analyses
3.2. Essential Oil Compositions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choe, U.; Yu, L.L.; Wang, T.T.Y. The Science behind Microgreens as an Exciting New Food for the 21st Century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Microgreens: Production, shelf life, and bioactive components. Crit. Rev. Food Sci. Nutr. 2017, 57, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Park, E.; Saftner, R.A.; Luo, Y.; Wang, Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: Microgreens. Postharvest Biol. Technol. 2015, 110, 140–148. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M. Comparison between the mineral profile and nitrate content of microgreens and mature lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Branca, F.; Chiarenza, G.L.; Cavallaro, C.; Gu, H.; Zhao, Z.; Tribulato, A. Diversity of Sicilian broccoli (Brassica oleracea var. italica) and cauliflower (Brassica oleracea var. botrytis) landraces and their distinctive bio-morphological, antioxidant, and genetic traits. Genet. Resour. Crop. Evol. 2017, 65, 485–502. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B Irradiation Changes Specifically the Secondary Metabolite Profile in Broccoli Sprouts: Induced Signaling Overlaps with Defense Response to Biotic Stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Renna, M.; Castellino, M.; Leoni, B.; Paradiso, V.M.; Santamaria, P. Microgreens Production with Low Potassium Content for Patients with Impaired Kidney Function. Nutritients 2018, 10, 675. [Google Scholar] [CrossRef] [Green Version]
- Khoja, K.K.; Buckley, A.; Aslam, M.F.; Sharp, P.A.; Latunde-Dada, G.O. In Vitro Bioaccessibility and Bioavailability of Iron from Mature and Microgreen Fenugreek, Rocket and Broccoli. Nutritients 2020, 12, 1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.F. Broccoli Microgreens: A Mineral-Rich Crop That Can Diversify Food Systems. Front. Nutr. 2017, 4, 7. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the Value of Cruciferous Sprouts as Sources of Bioactive Compounds for Nutrition and Health. Nutritients 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Scialabba, A.; Salvini, L.; Faqi, A.S.; Bellani, L.M. Tocopherol, fatty acid and phytosterol content in seeds of nine wild taxa of Sicilian Brassica (Cruciferae). Plant Biosyst. 2010, 144, 626–633. [Google Scholar] [CrossRef]
- Falk, K. Glucosinolate biosynthesis: Demonstration and characterization of the condensing enzyme of the chain elongation cycle in Eruca sativa. Phytochemistry 2004, 65, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Rask, L.; Andréasson, E.; Ekbom, B.; Eriksson, S.; Pontoppidan, B.; Meijer, J. Myrosinase: Gene family evolution and herbivore defense in Brassicaceae. Plant Mol. Biol. 2000, 42, 93–114. [Google Scholar] [CrossRef]
- Wittstock, U.; Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J. Chapter five Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores. In The Chemistry and Biochemistry of Plant Hormones—Recent Advances in Phytochemistry; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 101–125. [Google Scholar]
- Hecht, S.S. Inhibition of carcinogenesis by isothiocyanates. Drug Metab. Rev. 2000, 32, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace Leaf Samples Using GC, GC−MS, and Olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Marchioni, I.; Pistelli, L.; Ferri, B.; Cioni, P.; Copetta, A.; Pistelli, L.; Ruffoni, B. Preliminary studies on edible saffron bio-residues during different post-harvest storages. Bulg. Chem. Commun. 2019, 51, 131–136. [Google Scholar]
- Szôllôsi, R.; Szôllôsi Varga, I. Total antioxidant power in some species of Labiatae (adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Cheng, G.W.; Breen, P.J. Activity of Phenylalanine Ammonia-Lyase (PAL) and Concentrations of Anthocyanins and Phenolics in Developing Strawberry Fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Guidi, L.; Pardossi, A.A.; Tognoni, F. Biochemical Study of Leaf Browning in Minimally Processed Leaves of Lettuce (Lactuca sativaL. Var.Acephala). J. Agric. Food Chem. 2005, 53, 9980–9984. [Google Scholar] [CrossRef]
- Teixeira, R.S.S.; Da Silva, A.S.; Ferreira-Leitão, V.S.; Bon, E.P.D.S. Amino acids interference on the quantification of reducing sugars by the 3,5-dinitrosalicylic acid assay mislead carbohydrase activity measurements. Carbohydr. Res. 2012, 363, 33–37. [Google Scholar] [CrossRef]
- Li, P.; Gao, J.; Hu, H.; Luo, S.; Zhang, L. Postharvest senescence of fresh lotus pods and seeds is delayed by treatment with 1-methylcyclopropene. Ann. Appl. Biol. 2016, 169, 440–452. [Google Scholar] [CrossRef]
- Das, B.; Choudhury, B.; Kar, M. Quantitative estimation of changes in biochemical constituents of mahua (madhuca indica syn. bassia latifolia) flowers during postharvest storage. J. Food Process. Preserv. 2010, 34, 831–844. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic Toxicol. 2017, 36, 243–260. [Google Scholar] [CrossRef]
- Price, T. Seed Sprout Production for Human Consumption—A Review. Can. Inst. Food Sci. Technol. J. 1988, 21, 57–65. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and Iron Agronomic Biofortification of Brassicaceae Microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Renna, M.; Di Gioia, F.; Leoni, B.; Mininni, C.; Santamaria, P. Culinary Assessment of Self-Produced Microgreens as Basic Ingredients in Sweet and Savory Dishes. J. Culin. Sci. Technol. 2017, 15, 126–142. [Google Scholar] [CrossRef]
- Kopsell, D.A.; McElroy, J.S.; Sams, C.E.; Kopsell, D.E. Genetic Variation in Carotenoid Concentrations among Diploid and Amphidiploid Rapid-cycling Brassica Species. HortScience 2007, 42, 461–465. [Google Scholar] [CrossRef] [Green Version]
- Kyriacou, M.C.; De Pascale, S.; Kyratzis, A.; Rouphael, Y. Microgreens as a Component of Space Life Support Systems: A Cornucopia of Functional Food. Front. Plant Sci. 2017, 8, 1587. [Google Scholar] [CrossRef]
- De La Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Polash, M.; Sakil, M.; Hossain, M. Post-harvest biodegradation of bioactive substances and antioxidant activity in microgreens. J. Bangladesh Agril. Univ. 2018, 16, 250–253. [Google Scholar] [CrossRef]
- Singh, B.; Koley, T.; Karmakar, P.; Tripathi, A.; Singh, B.; Singh, M. Pigmented radish (Raphanus sativus): Genetic variability, heritability and inter-relationships of total phenolics, anthocyanins and antioxidant activity. Indian J. Agric. Sci. 2017, 87, 1600–1606. [Google Scholar]
- Paradiso, V.M.; Castellino, M.; Renna, M.; Gattullo, C.E.; Calasso, M.; Terzano, R.; Allegretta, I.; Leoni, B.; Caponio, F.; Santamaria, P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018, 9, 5629–5640. [Google Scholar] [CrossRef] [Green Version]
- Di Bella, M.C.; Niklas, A.; Toscano, S.; Picchi, V.; Romano, D.; Scalzo, R.L.; Branca, F. Morphometric Characteristics, Polyphenols and Ascorbic Acid Variation in Brassica oleracea L. Novel Foods: Sprouts, Microgreens and Baby Leaves. Agronomy 2020, 10, 782. [Google Scholar] [CrossRef]
- Singh, J.; Upadhyay, A.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in Brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Bianchi, G.; Genna, A.; Summa, C. Antioxidant properties and lipidic profile as quality indexes of cauliflower (Brassica oleracea L. var. botrytis) in relation to harvest time. Food Chem. 2007, 100, 1019–1025. [Google Scholar] [CrossRef]
- Amiri, H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2011, 26, 109–115. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Theodor, A.M.; Ismail, P.A. Effects of Zinc and Nickel on Antioxidative Enzyme Activities of Hairy Roots of Brassica juncea L. Czern (Indian Mustard). Int. J. Biotechnol. Res. 2013, 3, 53–60. [Google Scholar]
- Hichri, A.O.; Mosbah, H.; Majouli, K.; Hlila, M.B.; Ben Jannet, H.; Flamini, G.; Aouni, M.; Selmi, B. Chemical composition and biological activities of Eruca vesicaria subsp. longirostris essential oils. Pharm. Biol. 2016, 54, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Valette, L.; Fernandez, X.; Poulain, S.; Lizzani-Cuvelier, L.; Loiseau, A.-M. Chemical composition of the volatile extracts fromBrassica oleracea L. var.botrytis ‘Romanesco’ cauliflower seeds. Flavour Fragr. J. 2005, 21, 107–110. [Google Scholar] [CrossRef]
- Rangkadilok, N.E.; Nicolas, M.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W. Developmental changes of sinigrin and glucoraphanin in three Brassica species (Brassica nigra, Brassica juncea and Brassica oleracea var. italica). Sci. Hortic. 2002, 96, 11–26. [Google Scholar] [CrossRef]
- The Good Scents Company. Phytol, 150-86-7. Available online: http://www.thegoodscentscompany.com/data/rw1040391.html (accessed on 25 January 2021).
- The Good Scents Company. (Z)-3-hexen-1-ol, 928-96-1. Available online: http://www.thegoodscentscompany.com/data/rw1005932.html (accessed on 25 January 2021).
- Yu, J.; Jiang, Z.-T.; Li, R.; Chan, S. Chemical composition of the essential oils of Brassica juncea (L.) Coss. grown in different regions, Hebei, Shaanxi and Shandong, of China. J. Food Drug Anal. 2003, 11, 6. [Google Scholar] [CrossRef]
- Shin, S.-W.; Kang, C.-A. Studies on compositions and antifungal activities of essential oils from cultivars of Brassica juncea L. Saengyak Hakhoechi/Korean J. Pharmacogn. 2001, 32, 140–144. [Google Scholar]
- The Good Scents Company. Myrcene, 123-35-3. Available online: http://www.thegoodscentscompany.com/data/rw1016531.html (accessed on 25 January 2021).
- Miyazawa, M.; Maehara, T.; Kurose, K. Composition of the essential oil from the leaves of Eruca sativa. Flavour Fragr. J. 2002, 17, 187–190. [Google Scholar] [CrossRef]
- Badee, A.; Hallabo, S.; Abdel Aal, M. Antioxidant and antimicrobial activities of Egyptian Eruca sativa seed volatile oil. Egypt J. Food Sci. 2003, 31, 79–88. [Google Scholar]
- Mahdavi, S.; Kheyrollahi, M.; Sheikhloei, H.; Isazadeh, A. Antibacterial and Antioxidant Activities of Nasturtium officinale Essential Oil on Food Borne Bacteria. Open Microbiol. J. 2019, 13, 81–85. [Google Scholar] [CrossRef] [Green Version]


| Parameters | B. oleracea | R. raphanistrum | B. juncea | E. vesicaria | N. officinale |
|---|---|---|---|---|---|
| Broccoli | Daikon | Mustard | Rocket Salad | Watercress | |
| Water content (%) | 91.06 | 93.59 | 86.75 | 93.31 | 88.94 |
| Dry matter (%) | 8.94 | 6.41 | 13.25 | 6.69 | 11.06 |
| Reducing sugars (mg GLU/g FW) | 4.66 ± 0.24 b | 4.47 ± 0.21 b | 6.40 ± 0.59 ab | 7.98 ± 0.35 a | 8.44 ± 0.95 a |
| Total sugars (mg GLU/g FW) | 16.48 ± 1.26 ca | 28.27 ± 1.30 b | 58.11 ± 4.19 a | 16.99 ± 0.75 c | 18.87 ± 1.26 bc |
| Chl a (μg/g FW) | 737.83 ± 32.84 b | 623.55 ± 30.69 bc | 982.29 ± 24.51 a | 681.83 ± 21.04 bc | 584.76 ± 14.06 c |
| Chl b (μg/g FW) | 223.92 ± 20.14 b | 170.29 ± 7.14 c | 409.15 ± 29.78 a | 131.81 ± 6.66 d | 233.04 ± 5.57 b |
| ChlTOT (μg/g FW) | 961.75 ± 45.45 b | 793.83 ± 26.48 c | 1391.44 ± 41.15 a | 813.65 ± 23.50 bc | 817.80 ± 18.18 bc |
| Chl a/Chl b | 3.37 ± 0.22b b | 3.66 ± 0.19 b | 2.51 ± 0.18 c | 5.22 ± 0.27 a | 2.51 ± 0.05 c |
| TCar (μg/g FW) | 217.30 ± 12.00 a | 190.58 ± 8.21 a | 175.04 ± 15.83 a | 213.30 ± 5.90 a | 96.87 ± 6.29 b |
| TCar/ChlTOT | 0.23 ± 0.01 b | 0.24 ± 0.01 ab | 0.12 ± 0.01 c | 0.26 ± 0.00 a | 0.12 ± 0.01 c |
| TPC (mg GAE/g FW) | 3.63 ± 0.11 a | 3.25 ± 0.12 ab | 1.02 ± 0.05 c | 2.98 ± 0.11 b | 3.08 ± 0.27 b |
| TAnth (μg ME/g FW) | 172.51 ± 24.37 b | 57.56 ± 4.52 cd | 405.52 ± 31.55 a | 42.26 ± 7.71 d | 52.28 ± 4.32 cd |
| Reduced ascorbic acid (μg AsA/g FW) | 98.27 ± 10.7 b | 93.9 ± 5.12 b | 366.07 ± 100 a | 25.86 ± 3.12 d | 38.55 ± 3.66 c |
| Total ascorbic acid (μg AsATOT/g FW) | 124.1 ± 10.77 b | 125.58 ± 8.15 b | 606.87 ± 71.89 a | 29.67 ± 3.86 c | 137.52 ± 14.59 b |
| AsA/AsATOT | 0.79 ± 0.03 a | 0.76 ± 0.09 ab | 0.57 ± 0.09 b | 0.87 ± 0.10 a | 0.29 ± 0.04 c |
| DPPH radical scavenging assay (IC50 mg/mL) | 3.93 ± 0.27 b | 2.93 ± 0.33 b | 10.54 ± 1.00 a | 12.11 ± 0.50 a | 4.26 ± 0.42 b |
| Antioxidant activity—FRAP assay (mmol Fe2/g FW) | 8.6 ± 0.3 a | 7.6 ± 0.1 a | 3.2 ± 0.2 b | 2.1 ± 0.1 b | 8.6 ± 0.3 a |
| Compounds | l.r.i. a | Relative Abundance (%) ± SD | ||||
|---|---|---|---|---|---|---|
| Broccoli | Daikon | Mustard | Rocket Salad | Watercress | ||
| Hexanal * | 802 | - b | 0.3 ± 0.46 | - | - | - |
| 5-Cyano-1-pentene | 853 | 1.3 ± 0.03 | - | - | - | - |
| (Z)-3-Hexen-1-ol * | 857 | - | 11.0 ± 1.37 | 7.2 ± 0.25 | - | 0.2 ± 0.3 |
| 5-Methyl isothiazole | 862 | - | - | 3.1 ± 0.75 | - | - |
| Hexanol * | 871 | - | 0.2 ± 0.32 | - | - | - |
| Allyl isothiocyanate * | 892 | - | - | 22.7 ± 0.41 | - | - |
| Methyl allyl disulfide | 920 | - | - | 2.9 ± 0.22 | - | - |
| 2-Butyl isothiocyanate | 931 | 0.1 ± 0.01 | - | - | - | - |
| α-Thujene | 933 | - | - | - | 0.1 ± 0.15 | - |
| Benzaldehyde * | 965 | - | - | - | - | 1.5 ± 0.03 |
| 3-Butenyl isothiocyanate | 980 | 11.7 ± 0.02 | - | 14.1 ± 1.00 | - | - |
| β-Pinene * | 981 | - | - | - | 0.6 ± 0.05 | - |
| Heptanonitrile | 985 | - | 0.9 ± 0.18 | - | - | - |
| Myrcene * | 993 | - | 1.5 ± 1.56 | - | 83.7 ± 0.51 | - |
| (Z)-3-Hexenol acetate | 1007 | - | 1.7 ± 1.05 | 2.4 ± 0.26 | - | - |
| 3-Ethyl-1-hexanol | 1031 | - | 0.9 ± 0.08 | - | - | - |
| Limonene * | 1032 | - | 2.6 ± 0.03 | 3.6 ± 0.50 | 7.5 ± 0.03 | 1.0 ± 0.00 |
| Phenylacetaldehyde * | 1047 | 0.1 ± 0.08 | - | 1.7 ± 0.07 | - | - |
| γ-Terpinene * | 1062 | - | - | - | 0.9 ± 0.03 | - |
| 1-Octanol * | 1071 | - | 2.9 ± 0.00 | - | - | 0.7 ± 0.04 |
| Diallyl disulphide * | 1083 | - | - | 5.6 ± 1.24 | - | - |
| 4-Pentenyl isothiocyanate | 1086 | 50.2 ± 1.22 | - | 1.8 ± 0.45 | - | - |
| Nonanal * | 1104 | 0.1 ± 0.00 | 5.3 ± 0.16 | 0.8 ± 0.13 | - | 0.4 ± 0.01 |
| 2-Nonen-4-one | 1128 | - | 1.9 ± 0.35 | 0.6 ± 0.16 | - | - |
| 1-Octanethiol * | 1132 | - | 0.3 ± 0.44 | - | - | - |
| Benzylnitrile | 1140 | - | - | - | - | 26.0 ± 0.04 |
| 4-Methylpentyl isothiocyanate | 1166 | 0.2 ± 0.03 | - | - | - | - |
| Hexyl isothiocyanate * | 1199 | 0.3 ± 0.01 | - | - | - | - |
| Decanal * | 1206 | - | 0.3 ± 0.37 | - | - | - |
| 4,5-Dimethyl-2-isobutylthiazole | 1220 | - | 4.4 ± 0.31 | - | - | - |
| β-Cyclocitral * | 1222 | - | 0.7 ± 0.28 | - | - | - |
| (E)-2-Decenal | 1263 | - | 0.2 ± 0.22 | - | - | - |
| Heptyl isothiocyanate | 1265 | 0.3 ± 0.01 | - | - | - | |
| p-Vinylguaiacol | 1313 | - | 0.3 ± 0.36 | - | - | - |
| Benzyl isothiocyanate * | 1363 | - | - | - | 66.4 ± 0.11 | |
| cis-Raphasatin | 1419 | - | 4.4 ± 0.12 | - | - | - |
| β-Caryophyllene * | 1430 | - | 5.5 ± 0.01 | 0.7 ± 0.4 | 4.4 ± 0.43 | 0.5 ± 0.00 |
| Erucin | 1431 | 0.7 ± 0.01 | 0.9 ± 0.05 | - | - | - |
| trans-Raphasatin | 1440 | - | 12.2 ± 0.97 | - | - | - |
| α-Humulene * | 1456 | - | 2.0 ± 0.08 | 0.1 ± 0.19 | 1.3 ± 0.03 | - |
| Phenethyl isothiocyanate * | 1465 | 33.2 ± 0.94 | - | 3.4 ± 0.76 | - | - |
| (E)-β-Ionone | 1486 | - | 0.9 ± 0.11 | - | - | - |
| Berteroin | 1554 | 0.7 ± 0.08 | - | - | - | - |
| Caryophyllene oxide * | 1582 | - | 1.9 ± 0.16 | 0.2 ± 0.23 | 1.0 ± 0.04 | - |
| Humulene epoxide II | 1608 | - | 0.7 ± 0.17 | - | - | - |
| α-Bisabolol * | 1684 | - | 2.2 ± 0.13 | - | - | - |
| Octadecane * | 1800 | - | 0.2 ± 0.27 | - | - | - |
| Isopropyl palmitate * | 2023 | - | 1.6 ± 0.44 | - | - | - |
| (E)-15-Heptadecenal | 2083 | - | 0.2 ± 0.25 | - | - | - |
| Phytol * | 2115 | 0.3 ± 0.00 | 29.0 ± 2.47 | 28.4 ± 4.90 | 0.5 ± 0.02 | 3.3 ± 0.22 |
| Monoterpene hydrocarbons | - | 2.8 ± 0.30 | 3.6 ± 0.50 | 92.8 ± 0.41 | 1.0 ± 0.00 | |
| Sesquiterpene hydrocarbons | - | 7.5 ± 0.08 | 0.9 ± 0.59 | 5.7 ± 0.40 | 0.5 ± 0.00 | |
| Oxygenated sesquiterpenes | - | 4.7 ± 0.45 | 0.2 ± 0.23 | 1.0 ± 0.04 | - | |
| Oxygenated diterpenes | 0.3 ± 0.00 | 29.0 ± 2.47 | 28.4 ± 4.90 | 0.5 ± 0.02 | 3.3 ± 0.22 | |
| Apocarotenes | - | 1.6 ± 0.18 | - | - | - | |
| Isothiocyanates | 97.4 ± 0.12 | 17.5 ± 1.14 | 42.0 ± 2.62 | - | 66.4 ± 0.11 | |
| Thiazole derivatives | - | 4.4 ± 0.31 | 3.1 ± 0.75 | - | - | |
| Other nitrogen compounds | 1.3 ± 0.03 | 0.9 ± 0.18 | - | - | 26.0 ± 0.04 | |
| Other sulphur compounds | - | 0.3 ± 0.44 | 8.5 ± 1.46 | - | - | |
| Other non-terpene derivatives | 0.2 ± 0.08 | 27.7 ± 0.29 | 12.7 ± 0.38 | - | 2.9 ± 0.29 | |
| Total identified (%) | 99.1 ± 0.07 | 96.4 ± 1.30 | 99.3 ± 0.14 | 100.0 ± 0.01 | 100.0 ± 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchioni, I.; Martinelli, M.; Ascrizzi, R.; Gabbrielli, C.; Flamini, G.; Pistelli, L.; Pistelli, L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods 2021, 10, 427. https://doi.org/10.3390/foods10020427
Marchioni I, Martinelli M, Ascrizzi R, Gabbrielli C, Flamini G, Pistelli L, Pistelli L. Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family. Foods. 2021; 10(2):427. https://doi.org/10.3390/foods10020427
Chicago/Turabian StyleMarchioni, Ilaria, Marco Martinelli, Roberta Ascrizzi, Costanza Gabbrielli, Guido Flamini, Luisa Pistelli, and Laura Pistelli. 2021. "Small Functional Foods: Comparative Phytochemical and Nutritional Analyses of Five Microgreens of the Brassicaceae Family" Foods 10, no. 2: 427. https://doi.org/10.3390/foods10020427