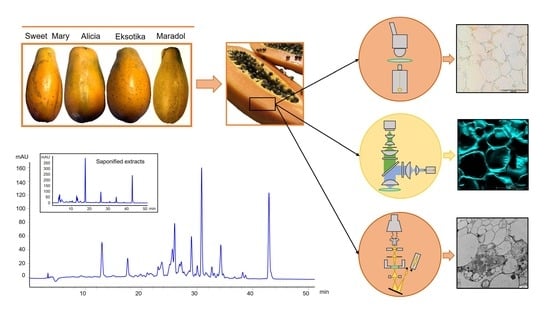
Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Papaya Fruits
2.3. Carotenoid Extraction and Saponification
2.3.1. Carotenoid Extraction from Papaya Tissues
2.3.2. Saponification of Carotenoid Extracts
2.4. Carotenoid Analysis by HPLC with Diode Array Detector (DAD)
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
2.6. Microscopy Analysis of Papaya Tissues
2.6.1. Optical Light Microscopy
2.6.2. Confocal Laser Scanning Microscopy
2.6.3. Transmission Electron Microscopy
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fruits of Papaya: Origin and Comparison
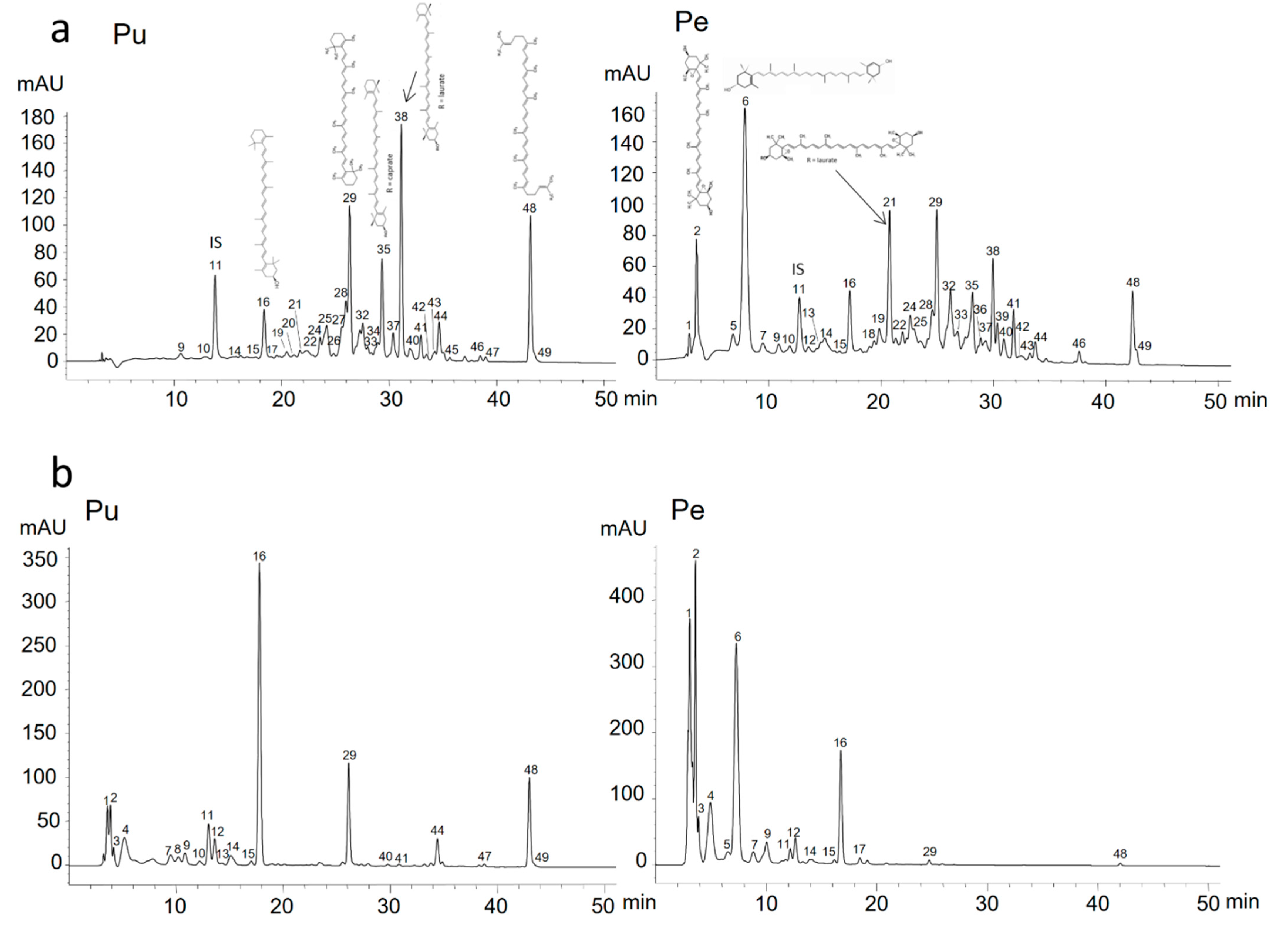
3.2. Characterization of Carotenoid and Carotenoid Ester Profile of Papaya Fruits
3.2.1. Free Xanthophylls in Papaya Fruits
3.2.2. Hydrocarbon Carotenes in Papaya Fruits
3.2.3. Xanthophyll Esters in Papaya Fruits
3.3. Carotenoid and Carotenoid Ester Content in Papaya Fruits
3.4. Carotenoid Deposition in Sweet Mary, Alicia, and Eksotika Papaya Fruits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandoval, K.V.; Ávila, D.D.; Gracia, T.J.H. Estudio del mercado de papaya mexicana: Un análisis de su competitividad (2001–2015). SUMNEG 2017, 8, 131–139. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Raya, V.; Lobo, M.G.; Ritter, A. Effect of climate conditions on growth and production of hydroponic papaya crops in the Canary Islands. In Proceedings of the XI International Symposium on Protected Cultivation in Mild Winter Climates and I International Symposium on Nettings and Screens in Horticulture, Canary Islands, Spain, 27–30 January 2019; pp. 77–84. [Google Scholar] [CrossRef]
- Hueso, J.J.; Salinas, I.; Pinillos, V.; Cuevas, J. Papaya greenhouse cultivation in south-east Spain. In Proceedings of the V International Symposium on Papaya, Mérida, Yucatán, Mexico, 24–27 October 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Ramona, C.; Ana, B.; Mihai, C.; Stãnicã, F. Carica papaya L. cultivated in greenhouse conditions. J. Hortic. Sci. Biotechnol. 2017, 21, 130–136. Available online: https://journal-hfb.usab-tm.ro/romana/lucraristiintifice2017_3.html/ (accessed on 23 March 2020).
- Ramos-Parra, P.A.; García-Salinas, C.; Rodríguez-López, C.E.; García, N.; García-Rivas, G.; Hernández-Brenes, C.; de la Garza, R.I.D. High hydrostatic pressure treatments trigger the novo carotenoid biosynthesis in papaya fruit (Carica papaya cv. Maradol). Food Chem. 2019, 277, 326–372. [Google Scholar] [CrossRef] [PubMed]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A.; Musa, K.H.; Douqan, E.M. Antioxidant activity and physicochemical properties of mature papaya fruit (Carica papaya L. cv. Eksotika). Adv. J. Food Sci. Technol. 2013, 7, 859–865. [Google Scholar] [CrossRef]
- Sancho, L.E.G.G.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Cano, M.P.; de Ancos, B.; Lobo, G.; Monreal, M. Carotenoid pigments and colour of hermaphrodite and female papaya fruits (Carica papaya L.) cv. Sunrise during post-harvest ripening. J. Agric. Food Chem. 1996, 71, 351–358. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Socaciu, C.; Pintea, A. Xanthophyll esters in fruits and vegetables. Not. Bot. Hort. Agrob. 2014, 42, 310–324. [Google Scholar] [CrossRef]
- Ampomah-Dwamena, C.; Driedonks, N.; Lewis, D.; Shumskaya, M.; Chen, X.; Wurtzel, E.T.; Allan, A.C. The Phytoene synthase gene family of apple (Malus x domestica) and its role in controlling fruit carotenoid content. BMC Plant. Biol. 2015, 15, 185. [Google Scholar] [CrossRef] [PubMed]
- Lux, P.E.; Carle, R.; Zacarias, L.; Rodrigo, M.J.; Schweiggert, R.M.; Steingass, C.B. Genuine carotenoid profiles in sweet orange [Citrus sinensis (L.) Osbeck cv. Navel] peel and pulp at different maturity stages. J. Agric. Food Chem. 2019, 67, 13164–13175. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.C.; Mercadante, A.Z. Impact of in vitro digestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Vargas, E.; Conrad, J.; Hempel, J.; Gras, C.C.; Ziegler, J.U.; Carle, R. Carotenoids, carotenoid esters, and anthocyanins of yellow-, orange-, and red-peeled cashew apples (Anacardium occidentale L.). Food Chem. 2016, 200, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Bandino, E.; Hormaza, J.I.; Cano, M.P. Characterization and the impact of in vitro simulated digestion on the stability and bioaccessibility of carotenoids and their esters in two Pouteria lucuma varieties. Food Chem. 2020, 316, 126369. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Carle, R. Carotenoid deposition in plant and animal foods and its impact on bioavailability. Crit. Rev. Food Sci. Nutr. 2017, 57, 1807–1830. [Google Scholar] [CrossRef]
- Ramos-Parra, P.A.; García-Salinas, C.; Díaz de la Garza, R.I. Folate levels and polyglutamylation profiles of papaya (Carica papaya cv. Maradol) during fruit development and ripening. J. Agric. Food Chem. 2013, 61, 3949–3956. [Google Scholar] [CrossRef]
- Plaza, L.; Colina, C.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Wirt, U.; Bamedi, A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta L.) and several fruits by liquid chromatography-mass spectrophotometry. J. Agric. Food Chem. 2002, 50, 66–70. [Google Scholar] [CrossRef]
- De Faria, A.F.; De Rosso, V.V.; Mercadante, A.Z. Carotenoid composition of jackfruit (Artocarpus heterophyllus), determined by HPLC-PDA MS/MS. Plant. Foods Hum. Nutr. 2009, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.; Rodrigues, E.; Mercadante, A.Z. Carotenoids from Byrsonima crassifolia: Identification, quantification and in vitro scavenging capacity against peroxyl radicals. J. Food Compos. Anal. 2013, 31, 155–160. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectrom. 2012, 312, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Micronutrients—Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Breithaupt, D.; Schwack, W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur. Food Res. Technol. 2000, 211, 52–55. [Google Scholar] [CrossRef]
- Molnár, P.; Martus, Z.; Szabolcs, J.; Körtvélyesi, T. Kinetic studies on the thermal Z/E-isomerization of C40-carotenoids. J. Chem. Res. Synop. 1997, 4, 120–121. [Google Scholar] [CrossRef]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Welti-Chanes, J.; García-Cayuela, T. Characterization of carotenoid and carotenoid esters of astringent persimmon tissues (Diospyros kaki Thunb. var. Rojo brillante). Effects of thermal and high pressure non-thermal processing. Preprints 2018, 2018110548. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. Carotenoid composition of two Brazilian genotypes of acerola (Malpighia punicifolia L.) from two harvests. Food Res. Int. 2005, 38, 1073–1077. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Jansz, E.R.; Wickramasinghe, S.N.; Warnasuriya, N.D. Carotenoids in yellow-and red-fleshed papaya (Carica papaya L). J. Sci. Food Agric. 2003, 83, 1279–1282. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Esquivel, P.; Carle, R. Chemical and morphological characterization of Costa Rican papaya (Carica papaya L.) hybrids and lines with particular focus on their genuine carotenoid profiles. J. Agric. Food Chem. 2012, 60, 2577–2585. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Yahia, E.M.; González-Aguilar, G.A. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J. Sci. Food Agric. 2010, 90, 2358–2365. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An in vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef]
- Britton, G.; Khachik, F. Carotenoids in food. In Carotenoids; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2009; Volume 5, pp. 45–66. [Google Scholar] [CrossRef]
- Becerra, M.O.; Contreras, L.M.; Lo, M.H.; Díaz, J.M.; Herrera, G.C. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods. 2020, 66, 103771. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Steingass, C.B.; Heller, A.; Esquivel, P.; Carle, R. Characterization of chromoplasts and carotenoids of red-and yellow-fleshed papaya (Carica papaya L.). Planta 2011, 234, 1031. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Ming, R. Exploring the differential mechanisms of carotenoid biosynthesis in the yellow peel and red flesh of papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.M. Ascorbic acid, vitamin A, and mineral composition of banana (Musa sp.) and papaya (Carica papaya) cultivars grown in Hawaii. J. Food Compos. Anal. 2006, 19, 434–445. [Google Scholar] [CrossRef]
- Vasquez-Caicedo, A.L.; Heller, A.; Neidhart, S.; Carle, R. Chromoplast morphology and β-carotene accumulation during postharvest ripening of mango Cv. ‘Tommy Atkins’. J. Agric. Food Chem. 2006, 54, 5769–5776. [Google Scholar] [CrossRef] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Schwartz, S.J. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Kopec, R.E.; Villalobos-Gutierrez, M.G.; Högel, J.; Quesada, S.; Esquivel, P.; Carle, R. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: A randomised cross-over study. Br. J. Nutr. 2014, 111, 490–498. [Google Scholar] [CrossRef]
- Zaripheh, S.; Erdman, J.W. Factors that influence the bioavailability of xanthopylls. J. Nutr. 2002, 132, 531S–534S. [Google Scholar] [CrossRef]
- Amar, I.; Abraham, A.; Nissim, G. Solubilization patterns of lutein and lutein esters in food grade nonionic microemulsions. J. Agric. Food Chem. 2013, 51, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.; Mercadante, A.Z. Carotenoid esters analysis and occurrence: What do we know so far? Arch. Bichem. Biophys. 2018, 648, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | cv. Sweet Mary | cv. Alicia | cv. Eksotika | cv. Maradol |
|---|---|---|---|---|
| Fruit appearance |  |  |  |  |
| Total weight of whole fruit (g) | 1124.0 ± 214.2 a | 1286.1 ± 155.3 a | 1148.2 ± 204.3 a | 1350.0 ± 251.1 b |
| Apical caliber (cm) | 19.1 ± 5.2 a | 20.5 ± 0.8 a | 19.4 ± 2.0 a | 26.5 ± 3.8 b |
| Equatorial caliber (cm) | 10.7 ± 0.7 a | 11.6 ± 0.3 a | 11.5 ± 0.4 a | 10.9 ± 0.3 a |
| Titratable acidity 1 | 0.184 ± 0.000 a | 0.182 ± 0.005 a | 0.190 ± 0.002 a | 0.179 ± 0.02 b |
| pH | 5.275 ± 0.007 a | 5.350 ± 0.028 b | 5.430 ± 0.000 c | 5.487 ± 0.019 c |
| Soluble solids (°Brix at 25 ⁰C) | 12.2 ± 0.2 b | 11.2 ± 0.1 a | 12.1 ± 0.2 b | 12.5 ± 0.1 b |
| Moisture content (% wet basis) | 83.4 ± 0.2 a | 84.6 ± 0.3 b | 85.1 ± 0.2 c | 83.7 ± 0.1 a |
| Pulp color parameters | ||||
| L* | 63.1 ± 1.1 a | 62.3 ± 0.9 a | 63.0 ± 3.7 a | 62.7 ± 1.6 a |
| a* | 28.1 ± 1.2 c | 23.9 ± 0.3 b | 19.8 ± 1.0 a | 28.1 ± 1.7 c |
| b* | 42.8 ± 2.1 b | 40.4 ± 1.1 b | 30.8 ± 0.8 a | 42.8 ± 2.1 b |
| Hue angle (h*) | 56.6 ± 0.3 b | 59.3 ± 1.0 c | 57.3 ± 0.7 b | 49.4 ± 0.7 a |
| Peel color parameters | ||||
| L* | 56.0 ± 0.7 a | 50.3 ± 1.1 a | 59.0 ± 2.2 a | 56.0 ± 0.7 a |
| a* | 13.7 ± 0.9 a | 15.2 ± 0.5 a | 14.1 ± 0.5 a | 17.7 ± 0.4 a |
| b* | 24.4 ± 0.6 a | 22.9 ± 1.4 a | 26.4 ± 3.6 a | 24.4 ± 0.6 a |
| hue angle (h*) | 60.8 ± 1.1 b | 56.4 ± 1.3 a | 61.7 ± 2.5 b | 54.1 ± 0.9 a |
| No. | Rt (min) | Compound Identity | HPLC-DAD UV/Vis Absorption Maxima (nm) | %III/II | %Ab/AII | [M + H]+ m/z | HPLC/APCI+ MS Fragmentation Pattern (m/z) Fragment ions (m/z) | STD c |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.4 | (13Z)-violaxanthin a | 326, 416, 440, 470 | 78 | 0 | 601 | 583 [M + H − 18]+, 565 [M + H − 36]+, 509 [M + H − 92]+, 491 [M + H − 92 − 18]+, | n.a. |
| 2 | 3.8 | (all-E)-violaxanthin | 414, 438, 468 | 98 | 0 | 601 | 583 [M + H − 18]+, 565 [M + H − 36]+, 521 [M + H − 80]+ | Y |
| 3 | 4.2 | not identified 1 | 400, 422, 448 | 0 | 0 | nd g | nd g | n.a. |
| 4 | 5.2 | (9Z)-neoxanthin | 327, 410, 432, 462 | 55 | 0 | 601 | 583 [M + H − 18]+, 565 [M + H − 36]+, 547 [M + H − 54]+, 521 [M + H − 80]+ | n.a. |
| 5 | 7.6 | (all-E)-neoxanthin | 416, 437, 469 | 99 | 0 | 601 | 583 [M + H − 18]+, 565 [M + H − 36]+, 547 [M + H − 54]+, 521 [M + H − 80]+ | Y |
| 6 | 7.9 | (all-E)-lutein | (420), 444, 472 | 62 | 0 | 569 | 551 [M + H − 18]+, 533 [M + H − 36]+ | Y |
| 7 | 9.5 | (all-E)-zeaxanthin | (426), 450, 470 | 18 | 0 | 569 | 551 [M + H − 18]+, 533 [M + H − 36]+ | Y |
| 8 | 10.3 | lutein-5,6-epoxide | (418), 440, 468 | 85 | nc f | 585 | 567 [M + H − 18]+, 549 [M + H − 36]+, 505 [M + H − 80]+ | n.a. |
| 9 | 10.9 | (all-E)-antheraxanthin | (422), 444, 472 | 66 | 0 | 585 | 567 [M + H − 18]+, 549 [M + H − 36]+, 505 [M + H − 80]+ | n.a. |
| 10 | 12.3 | (9Z)-violaxanthin | 327, 414, 436, 468 | 89 | nc f | 601 | 583 [M + H − 18]+, 565 [M + H − 36]+ | n.a. |
| 11 | 13.2 | (all-E)-β-apo-caroten-8’-al (internal standard) | 462 | 0 | 0 | 417 | 399 [M + H − 18]+, 325 [M + H − 74]+ | Y |
| 12 | 13.7 | β-cryptoxanthin-5,6-epoxide | (420), 445, 471 | 52 | nc f | 569 | 551 [M + H − 18]+, 459 [M + H − 18 − 92]+, 221 | n.a. |
| 13 | 14.4 | (9Z)-α-cryptoxanthin d | 412, 437, 466 | 0 | 0 | nd g | nd g | n.a. |
| 14 | 14.7 | not identified 2 | 437, 457, 488 | 0 | 0 | nd g | nd g | n.a. |
| 15 | 17.2 | (all-E)-α-cryptoxanthin b | (413), 435, 464 | 61 | nc f | 553 | 535 [M + H − 18]+, 479, 461 [M + H − 92]+, 439 | Y |
| 16 | 18.1 | (all-E)-β-cryptoxanthin | (426), 450, 476 | 18 | nc f | 553 | 535 [M + H − H2O]+, 461 [M + H − 92]+ | Y |
| 17 | 19.3 | α-carotene-5,6-epoxy- | (418), 441, 469 | 10 | 0 | 553 | 535 [M + H − 18]+, 495, 205 | n.a. |
| 18 | 19.7 | (all-E)-luteoxanthin | 400, 416, 444 | nc f | 0 | 601 | 583 [M + H − 18]+ | n.a. |
| 19 | 20.3 | (13Z)-α-carotene | 337, (413), 439, 467 | 31 | 0 | 537 | 481 [M + H − 56]+, 445 [M + H − 92]+ | n.a. |
| 20 | 21.2 | (13Z)-β-carotene c | 337, (414), 436, 464 | 14 | 0 | 537 | 457 [M + H − 80]+, 445 [M + H − 92]+, 400 [M + H − 137]+, 269 [M + H − 268]+, 177 [M + H − 360]+, 137 [M + H − 400]+ | n.a. |
| 21 | 21.8 | (all-E)-violaxanthin laurate d | 417, 441, 469 | nc f | 0 | 783 | 765 [M + H − 18]+, 747 [M + H − 18 − 18]+, 691 [M + H − 92]+, 673 [M + H − 92 − 18]+, 583 [M + H − 12:0]+, 565 [M + H − 12:0 − 18]+, 547 [M + H − 12:0 − 18 − 18]+ | n.a. |
| 22 | 22.1 | β-cryptoxanthin-5,8-epoxide | 412, 438, 464 | 50 | 0 | 569 | 551 [M + H − 18]+, 459 [M + H − 18 − 92]+,221 | n.a. |
| 23 | 22.5 | (all-E)-ζ-carotene | 378, 400, 423 | 108 | 0 | 541 | 472 [M + H − 69]+, 404 [M + H − 137]+,364,337 | n.a. |
| 24 | 23.4 | β-cryptoxanthin-5,8’-epoxide | 413, 438, 465 | 50 | 0 | 569 | nd g | n.a. |
| 25 | 24.0 | (all-E)-α-carotene | (420), 445, 470 | 66 | 0 | 537 | 457 [M + H − 80]+, 413 [M + H − 124]+, 177 [M + H − 360]+, 137 [M + H − 400]+, 123 [M + H − 414]+ | Y |
| 26 | 24.7 | (9Z)-α-carotene | 396, 420, 440, 469 | 60 | 0 | 537 | 457 [M + H − 80]+, 445 [M + H − 92]+ | n.a. |
| 27 | 25.6 | (9Z)-violaxanthin laurate | 388, 413, 436, 466 | 92 | 0 | 783 | 765 [M + H − H2O]+, [M + H − 18]+,747 [M + H − 2H2O]+, [M + H − 18 − H2O]+, 565 [M + H − 12:0 − H2O]+, [M + H − 12:0 − 18]+ | n.a. |
| 28 | 25.9 | (all-E)-lutein-3-O-myristate | 401, 426, 472 | 0 | 0 | nd g | 533 [M + H − 228 − 18]+, 495 [M + H − 228 − 56]+, 459 [M + H − 228 − 92]+, 429, 441 | n.a. |
| 29 | 26.2 | (all-E)-β-carotene c | (428), 450, 476 | 16 | 0 | 537 | 457 [M + H − 80]+, 445 [M + H − 92]+, 400 [M + H − 137]+, 269 [M + H − 268]+, 177 [M + H − 360]+, 137 [M + H − 400]+ | Y |
| 30 | 26.8 | (9Z)-β-carotene | 380, 400, 426, 454 | 23 | 0 | 537 | 457 [M + H − 80]+, 445 [M + H − 92]+, 400 [M + H − 137]+, 269 [M + H − 268]+, 177 [M + H − 360]+, 137 [M + H − 400]+ | n.a. |
| 31 | 27.2 | (all-E)-violaxanthin dimyristate | 413, 435, 464 | 91 | 0 | 10220 | 1004 [M + H − H2O]+, 793 [M + H − 14:0]+, 775 [M + H − 14:0 − H2O]+, 547 [M + H − 14:0 − 14:0 − H2O]+ | n.a. |
| 32 | 27.4 | (all-E)-antheraxanthin myristate palmitate | 421, 443, 467 | 31 | 0 | 1033 | 1015 [M + H − 18]+, 941 [M + H − 92]+, 805 [M + H − 14:0]+, 787 [M + H − 14:0 − 18]+, 771 [M + H − 16:0]+,759 [M + H − 16:0 − 18]+, 549 [M + H − 14:0 − 16:0]+, 531 [M + H − 14:0 − 16:0 − 18:0]+ | n.a. |
| 33 | 27.9 | (all-E)-violaxanthin palmitate d | 416, 441, 469 | nc f | nc f | 839 | 821 [M + H − 18]+, 803 [M + H − 18 − 18]+, 747 [M + H − 16:0]+, 729 [M + H − 92 − 18]+, 583 [M + H − 256]+, 565 [M + H − 18 − 16:0]+, 547 [M + H − 16:0 − 18 − 18]+ | n.a. |
| 34 | 29.0 | (9Z)-neoxanthin dibutyrate d | 327, 412, 436, 464 | 80 | 16 | 741 | 723 [M + H − 18]+, 653 [M + H − 4:0]+, 649 [M + H − 92]+, 635 [M + H − 4:0 − 18]+, 631 [M + H − 92 − 18]+, 565 [M + H − 4:0 − 4:0]+, 547 [M + H − 4:0 − 4:0 − 18]+ | n.a. |
| 35 | 29.2 | (all-E)-β-cryptoxanthin caprate d | 428, 450, 476 | nc f | nc f | 707 | 615 [M + H − 27]+, 535 [M + H − 100]+, 443 [M + H − 11]+, 442 [M + H − 16]+ | n.a. |
| 36 | 29.8 | (9Z)-violaxanthin myristate palmitate | 413, 439, 467 | 92 | 0 | 1050 | 1032 [M + H − H2O]+, 803 [M + H − 14:0 − H2O]+, 775 [M + H − 16:0 − H2O]+, 565 [M + H − 14:0 − 16:0]+, 547 [M+14:0 − 16:0 − H2O]+, | n.a. |
| 37 | 30.0 | (all-E)-lutein dimyristate | 422, 446, 474 | 38 | 0 | nd g | 761 M + H − 14:0]+, 669 [M + H − 92]+, 553 [M + H − 14:14:0]+ | n.a. |
| 38 | 30.3 | (all-E)-β-cryptoxanthin laurate | 421, 451, 478 | 25 | 0 | 735 | 643 [M + H − 92]+, 535 [M + H − 12:0]+, 479 [M + H − 56 − 12:0]+, 443 [M + H − 92 − 12:0]+ | |
| 39 | 30.4 | (all-E)-antheraxanthin-3-O palmitate | 422, 444, 472 | nc f | nc f | 823 | 805 [M + H − 18]+, 787 [M + H − 18 − 18]+, 731 [M + H − 92]+, 567 [M + H − 16:0]+, 549 [M + H − 16:0 − 18]+, 531 [M + H − 16:0 − 18 − 18]+ | n.a. |
| 40 | 31.7 | (all-E)-antheraxanthin laurate myristate d | 418, 442, 470 | 33 | 0 | 977 | 959 [M + H − 18]+, 777 [M + H − 12:0]+, 749 [M + H − 14:0]+, 759 [M + H − 12:0 − 18]+, 731 [M + H − 14:0 − 18]+, 549 [M + H − 12:0 − 14:0]+, 531 [M + H − 12:0 − 14:0 − 18]+ | n.a. |
| 41 | 32.1 | (all-E)-β-cryptoxanthin myristate | 424, 448, 476 | 9 | 0 | 763 | 671 [M + H − 92]+, 535 [M + H − 14:0]+, 443 [M + H − 14:0 − 92]+ | n.a. |
| 42 | 33.3 | (Z)-lycopene isomer 1 d | 417, 442, 469, 499 | 0 | 0 | 537 | 481 [M + H − 51]+, 467 [M + H − 49]+, 455 [M + H − 100]+, 427 [M + H − 15]+, 413 [M + H − 43]+, 399 [M + H − 64]+, 387 [M + H − 11]+ | n.a. |
| 43 | 33.9 | (all-E)-β-cryptoxanthin palmitate d | 433, 460, 487 | 0 | 0 | 791 | 699 [M + H − 39]+, 535 [M + H − 100]+, 443 [M + H − 4]+, 413 [M + H − 46]+ | n.a. |
| 44 | 34.0 | (13Z)-lycopene isomer 2 d | 442, 465, 493 | 0 | 0 | 537 | 481 [M + H − 42]+, 467 [M + H − 35]+, 455 [M + H − 100]+, 427 [M + H − 61]+, 413 [M + H − 88]+, 399 [M + H − 24]+, 387 [M + H − 42]+ | n.a. |
| 45 | 34.9 | (13’Z)-lycopene isomer 3 d | 437, 460, 490 | 0 | 0 | 537 | 481 [M + H − 5]+, 467 [M + H − 42]+, 455 [M + H − 86]+, 427 [M + H − 22]+, 413 [M + H − 4]+, 399 [M + H − 86]+, 387 [M + H − 11]+ | n.a. |
| 46 | 38.4 | (9Z)-lycopene isomer 4 d | 440, 465, 496 | 0 | 0 | 537 | 481 [M + H − 11]+, 467 [M + H − 32]+, 455 [M + H − 79]+, 427 [M + H − 48]+, 413 [M + H − 30]+, 399 [M + H − 42]+, 387 [M + H − 38]+ | n.a. |
| 47 | 38.9 | (9’Z)-lycopene isomer 5 d | 413, 439, 465, 496 | 0 | 0 | 537 | 481 [M + H − 49]+, 467 [M + H − 20]+, 455 [M + H − 100]+, 427 [M + H − 24]+, 413 [M + H − 60]+, 399 [M + H − 21]+, 387 [M + H − 19]+, | n.a. |
| 48 | 43.1 | (all-E)-lycopene | 418, 443, 471, 502 | 6 | 0 | 537 | 457 [M + H − 80]+, 413 [M + H − 124]+, 177 [M + H − 360]+, 137 [M + H − 400]+, 121 [M + H − 416]+ | Y |
| 49 | 43.4 | (Z)-lycopene isomer 6 d | 443, 471, 502 | 0 | 0 | 537 | 481 [M + H − 17]+, 467 [M + H − 32]+, 455 [M + H − 100]+, 427 [M + H − 23]+, 413 [M + H − 61]+, 399 [M + H − 40]+, 387 [M + H − 17]+ | n.a. |
| No.2 | Carotenoid Compound | cv. Sweet Mary | cv. Alicia | cv. Eksotika | cv. Maradol | ||||
|---|---|---|---|---|---|---|---|---|---|
| Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified extract (SAP) | ||
| 1 | (13Z)-violaxanthin | 1.2 ± 0.1 a | 112.7 ± 9.4 c | n.d a | 11.2 ± 0.1 a | n.d a | 124.4 ± 7.7 b | 37.1 ± 1.8 b | 136.5 ± 0.6 c |
| 2 | (all-E)-violaxanthin | 3.4 ± 0.1 b | 115.3 ± 7.8 c | n.d a | 114.3 ± 3.8 a | n.d a | 151.6 ± 3.9 b | 52.8 ± 2.1 c | 147.6 ± 0.2 bc |
| 3 | not identified 1 | tr. | tr. | tr. | tr. | tr. | tr. | tr. | tr. |
| 4 | (9Z)-neoxanthin | n.d a | 176.8 ± 2.0 c | n.d a | 166.2 ± 3.6 a | n.d a | 151.1 ± 2.1 a | 5.8 ± 0.1 b | 150.4 ± 2.2 b |
| 5 | (all-E)-neoxanthin | n.d a | 37.5 ± 2.9 b | n.d a | n.d a | n.d a | n.d a | 30.7 ± 1.6 b | 91.4 c |
| 6 | (all-E)-lutein | n.d a | 72.8 ± 0.9 a | n.d a | 108.1 ± 2.7 c | n.d a | 116.6 ± 9.7 b | 142.2 ± 3.5 b | 162.1 ± 1.4 a |
| 7 | (all-E)-zeaxanthin | n.d a | 61.4 ± 1.1 a | 15.0 ± 1.0 b | 95.5 ± 1.8 c | 27.5 ± 1.8 c | 67.4 ± 4.7 b | 88.5 ± 0.6 d | 196.8 ± 3.6 c |
| 8 | lutein-5,6-epoxide | n.d a | 47.8 ± 1.3 a | n.d a | 81.4 ± 0.3 b | n.d a | 63.5 ± 0.1 a | 62.5 ± 4.8 b | 190.2 ± 2.5 c |
| 9 | (all-E)-antheraxanthin | 14.5 ± 0.8 b | 44.4 ± 3.6 b | n.d a | 118.5 ± 2.3 b | n.d a | 60.4 ± 1.0 a | 49.4 ± 1.7 c | 123.8 ± 2.2 c |
| 10 | (9Z)-violaxanthin | 6.0 ± 0.2 c | 29.0 ± 1.4 b | n.d a | 33.9 ± 0.7 d | n.d a | 5.7 ± 0.4 c | 20.7 ± 0.4 b | n.d a |
| 11 | (all-E)-β-apo-caroten-8’ al (IS3) | 147.0 ± 0.6 a | 133.4 ± 0.5 a | 129.5 ± 0.3 a | 133.4 ± 0.5 a | 135.2 ± 0.3 a | 135.8 ± 0.6 a | 147.0 ± 0.6 a | 147.0 ± 0.6 a |
| 12 | β-cryptoxanthin-5, 6-epoxide | n.d a | 46.8 ± 0.5 ab | n.d a | 59.1 ± 0.4 a | n.d a | 69.0 ± 3.2 c | 19.4 ± 1.2 b | 74.4 ± 0.3 bc |
| 13 | (9Z)-α-cryptoxanthin | n.d a | 10.8 ± 0.4 c | n.d a | n.d a | n.d a | n.d a | 13.3 ± 0.3 b | 9.9 ± 0.2 b |
| 14 | not identified 2 | tr. | tr. | tr. | tr. | tr. | tr. | tr. | tr. |
| 15 | (all-E)-α-cryptoxanthin | 2.5 ± 0.1 b | 16.9 ± 0.8 c | 5.4 ± 0.2 a | 15.9 ± 0.6 b | 6.6 ± 0.5 a | 15.2 ± 0.5 b | 25.1 ± 0.3 c | 13.6 ± 0.0 a |
| 16 | (all-E)-β-cryptoxanthin | 43.1 ± 1.1 b | 418.9 ± 1.5 c | 42.5 ± 2.8 a | 441.5 ± 1.0 b | 40.5 ± 1.4 a | 579.3 ± 2.7 d | 160.9 ± 2.2 c | 184.4 ± 0.4 a |
| 17 | α-carotene-5,6-epoxide | 2.6 ± 0.3 a | n.d a | 5.5 ± 0.3 a | n.d a | 8.5 ± 0.9 a | n.d a | 31.9 ± 0.9 a | n.d a |
| 18 | (all-E)-luteoxanthin | n.d a | n.d a | n.d a | n.d a | n.d a | n.d a | 64.7 ± 1.2 b | n.d a |
| 19 | (13Z)-α-carotene | 14.0 ± 0.1 b | 12.8 ± 0.0 b | 13.5 ± 0.3 a | 12.7 ± 0.0 a | 14.3 ± 1.1 ab | 12.5 ± 0.2 a | 33.3 ± 0.6 c | 31.6 ± 0.8 c |
| 20 | (13Z)-β-carotene | 3.9 ± 0.1 a | 3.1 ± 0.1 a | 5.1 ± 0.2 a | 4.4 ± 0.2 a | 4.9 ± 0.1 a | 4.2 ± 0.1 a | 17.0 ± 0.2 b | 15.6 ± 0.8 b |
| 21 | (all-E)-violaxanthin laurate | 11.3 ± 1.2 ab | n.d a | 8.1 ± 2.3 a | n.d a | 20.2 ± 1.9 b | n.d a | 89.5 ± 1.1 c | n.d a |
| 22 | β-cryptoxanthin-5,8-epoxide | 7.7 ± 0.7 b | n.d a | 3.2 ± 0.1 a | n.d a | 14.1 ± 1.0 c | n.d a | 57.8 ± 0.4 d | 46.8 ± 0.2 b |
| 23 | (all-E)-ζ-carotene | 4.2 ± 0.3 b | 1.6 ± 0.5 b | n.d a | n.d a | 55.7 ± 0.7 c | 32.9 ± 0.3 c | 77.5 ± 0.6 d | 75.1 ± 0.6 d |
| 24 | β-cryptoxanthin-5,8’-epoxide | 28.6 ± 0.3 b | 13.4 ± 0.2 a | 13.1 ± 1.8 a | 11.3 ± 0.2 a | 23.3 ± 1.2 a | 13.4 ± 1.9 a | 48.0 ± 3.5 b | 38.5 ± 2.8 b |
| 25 | (all-E)-α-carotene | 75.0 ± 0.0 c | 62.1 ± 0.1 c | 74.7 ± 0.5 a | 73.4 ± 0.1 a | 79.2 ± 0.5 ab | 72.5 ± 0.7 a | 95.9 ± 5.2 b | 93.2 ± 2.3 b |
| 26 | (9Z)-α-carotene | 3.2 ± 0.2 b | 2.4 ± 0.1 b | n.d a | n.d a | 4.1 ± 0.2 b | 3.3 ± 0.3 b | 10.3 ± 0.5 c | 8.7 ± 0.6 c |
| 27 | (9Z)-violaxanthin laurate | 50.9 ± 2.5 b | n.d a | n.d a | n.d a | 59.0 ± 1.3 b | n.d a | 107.5 ± 4.2 c | n.d a |
| 28 | (all-E)-lutein-3-O-myristate | 168.7 ± 0.1 a | n.d a | 210.7 ± 0.6 a | 57.4 ± 3.1 c | 261.6 ± 4.8 c | 35.1 ± 1.6 b | 278.0 ± 2.9 b | n.d a |
| 29 | (all-E)-β-carotene | 164.5 ± 6.6 c | 159.2 ± 0.7 c | 120.3 ± 2.3 a | 132.9 ± 3.8 a | 170.3 ± 8.6 b | 166.5 ± 1.4 b | 405.2 ± 10.7 d | 337.5 ± 14.3 d |
| 30 | (9Z)-β-carotene | 5.9 ± 0.1 b | 5.1 ± 0.1 b | 5.2 ± 0.1 b | 6.4 ± 0.1 b | n.d a | n.d a | 25.8 ± 0.7 c | 21.3 ± 1.4 c |
| 31 | (all-E)-violaxanthin dimyristate | 36.0 ± 2.6 a | n.d a | 41.3 ± 2.0 a | n.d a | 48.1 ± 2.6 a | n.d a | 97.5 ± 1.6 b | n.d a |
| 32 | (all-E)-antheraxanthin myristate palmitate | 43.2 ± 1.6 b | n.d a | 43.4 ± 0.4 a | n.d a | 52.6 ± 2.4 b | n.d a | 70.4 ± 3.3 c | n.d a |
| 33 | (all-E)-violaxanthin palmitate | 6.8 ± 0.7 a | n.d a | 10.7 ± 1.0 a | n.d a | 20.2 ± 0.6 b | n.d a | 46.0 ± 1.0 c | n.d a |
| 34 | (9Z)-neoxanthin dibutyrate | 7.5 ± 0.3 a | n.d a | 15.6 ± 0.6 b | n.d a | 27.6 ± 2.3 c | n.d a | 52.8 ± 1.8 d | n.d a |
| 35 | (all-E)-β-cryptoxanthin caprate | 81.8 ± 1.5 d | n.d a | 69.7 ± 2.1 a | n.d a | 90.4 ± 2.2 c | n.d a | 91.6 ± 1.2 b | 14.0 ± 0.3 b |
| 36 | (9Z)-violaxanthin myristate palmitate | n.d a | n.d a | 9.9 ± 0.2 b | n.d a | 12.0 ± 0.4 c | n.d a | 22.7 ± 0.5 d | n.d a |
| 37 | (all-E)-lutein dimyristate | 60.3 ± 4.0 b | n.d a | 61.6 ± 4.0 a | n.d a | 79.7 ± 1.7 a | n.d a | 92.1 ± 5.39 a | n.d a |
| 38 | (all-E)-β-cryptoxanthin laurate | 174.9 ± 10.4 b | n.d a | 167.8 ± 3.5 a | n.d a | 223.2 ± 8.2 b | n.d a | 281.7 ± 1.8 b | n.d a |
| 39 | (all-E)-antheraxanthin-3-O palmitate | n.d a | n.d a | n.d a | n.d a | n.d a | n.d a | 56.6 ± 1.0 b | n.d a |
| 40 | (all-E)-antheraxanthin laurate myristate | 21.9 ± 2.2 b | 8.2 ± 0.6 b | 17.2 ± 1.8 a | n.d a | 16.7 ± 0.9 a | n.d a | 36.5 ± 1.9 b | n.d a |
| 41 | (all-E)-β-cryptoxanthin myristate | 18.6 ± 1.6 b | 4.6 ± 0.3 b | 12.8 ± 0.2 a | n.d a | 21.3 ± 1.1 b | 22.6 ± 0.4 c | 28.3 ± 0.6 b | n.d a |
| 42 | (Z)-lycopene isomer 1 | 13.8 ± 0.6 b | 12.0 ± 0.5 b | 11.6 ± 0.5 a | 10.8 ± 0.4 a | 23.2 ± 1.8 c | 19.1 ± 1.3 c | 28.0 ± 0.6 c | 29.1 ± 1.0 d |
| 43 | (all-E)-β-cryptoxanthin palmitate | 13.9 ± 0.4 c | 5.5 ± 0.0 c | 5.2 ± 0.2 a | n.d a | 10.0 ± 0.2 b | 10.7 ± 1.2 d | 20.6 ± 0.1 c | 4.2 ± 0.2 b |
| 44 | (13Z)-lycopene isomer 2 | 112.6 ± 0.1 a | 98.3 ± 2.7 a | 194.3 ± 3.4 b | 189.0 ± 4.4 b | 198.1 ± 6.0 b | 203.7 ± 8.8 b | 256.9 ± 8.3 c | 141.9 ± 2.5 a |
| 45 | (13’Z)-lycopene isomer 3 | 22.4 ± 0.2 a | 16.3 ± 0.3 b | 24.7 ± 0.5 a | 21.5 ± 0.6 b | 28.5 ± 1.4 a | 30.2 ± 1.9 c | 43.9 ± 1.7 b | 14.3 ± 0.4 a |
| 46 | (9Z)-lycopene isomer 4 | 26.3 ± 0.5 a | 22.1 ± 0.7 b | 21.4 ± 2.0 a | 20.7 ± 0.4 a | 30.4 ± 2.3 a | 33.1 ± 1.2 c | 22.0 ± 0.9 a | 55.8 ± 0.6 d |
| 47 | (9’Z)-lycopene isomer 5 | 12.3 ± 0.3 a | 9.7 ± 0.7 a | 21.0 ± 1.1 b | 21.3 ± 0.3 c | 18.2 ± 0.5 a | 16.6 ± 0.1 b | 27.8 ± 0.9 c | 14.3 ± 0.5 a |
| 48 | (all-E)-lycopene | 378.2 ± 5.4 c | 342.9 ± 4.3 c | 328.4 ± 2.0 a | 320.3 ± 2.9 a | 421.2 ± 1.2 b | 401.9 ± 7.6 b | 496.7 ± 1.8 b | 459.5 ± 3.2 b |
| 49 | (Z)-lycopene isomer 6 | 22.6 ± 0.6 b | 20.4 ± 0.6 b | 16.1 ± 0.3 a | 18.7 ± 0.4 a | 36.5 ± 1.5 c | 33.3 ± 1.3 c | 85.7 ± 2.1 d | 46.9 ± 1.1 d |
| total free xanthophylls | 109.0. ± 4.0 a | 1204.5 ± 33.7 a | 84.6 ± 6.1 a | 1257.0 ± 17.5 a | 120.5 ± 6.4 a | 1417.6 ± 37.8 b | 879.0 ± 25.7 b | 1566.5 ± 18.0 c | |
| total xanthophyll esters | 695.8 ± 29.5 a | 18.2 ± 1.0 a | 673.9 ± 19.8 a | 57.4 ± 3.1 b | 942.5 ± 30.7 b | 68.5 ± 7.2 b | 1371.7 ± 24.6 c | 84.0 ± 2.6 c | |
| total hydrocarbon carotenoids | 859.0 ± 15.4 a | 767.9 ± 11.3 a | 836.2 ± 14.0 a | 832.2 ± 13.6 b | 1084.6 ± 26.6 b | 1029.9 ± 25.2 c | 1657.7 ± 33.7 c | 1344.8 ± 30.0 d | |
| total carotenoids | 1663.7 ± 48.9 a | 1990.7 ± 46.1 a | 1594.7 ± 39.7 a | 2146.6 ± 34.2 b | 2147.5 ± 63.7 b | 2515.9 ± 70.2 c | 3908.4 ± 84.0 c | 2995.3 ± 50.6 d | |
| RAE 4 | 22.7 ± 0.7 b | 25.7 ± 0.1 a | 18.0 ± 0.5 a | 22.6 ± 0.1 a | 23.4 ± 0.9 b | 25.5 ± 0.2 a | 41.1 ± 0.9 c | 43.3 ± 1.7 a | |
| No.2 | Carotenoid Compound | cv. Sweet Mary | cv. Alicia | cv. Eksotika | cv. Maradol | ||||
|---|---|---|---|---|---|---|---|---|---|
| Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified Extract (SAP) | Direct Extract (C) | Saponified Extract (SAP) | ||
| 1 | (13Z)-violaxanthin | 36.7 ± 2.4 b | 152.5 ± 9.4 a | 24.0 ± 1.3 a | 275.2 ± 12.6 c | 76.8 ± 9.8 d | 165.9 ± 8.1 a | 49.9 ± 0.3 c | 160.2 ± 4.2 b |
| 2 | (all-E)-violaxanthin | 186.5 ± 0.3 a | 441.4 ± 15.9 b | 116.5 ± 1.4 a | 411.6 ± 8.2 a | 284.0 ± 2.3 b | 349.0 ± 22.5 a | 142.9 ± 0.3 a | 421 ± 12.4 b |
| 3 | not identified 1 | tr | tr. | tr. | tr. | tr. | tr. | tr. | tr. |
| 4 | (9Z)-neoxanthin | 7.8 ± 0.7 b | 472.0 ± 4.3 d | n.d a | 62.4 ± 4.8 b | 62.4 ± 4.8 c | 124.5 ± 1.7 a | 4.8 ± 0.6 ab | 162.5 ± 5.6 c |
| 5 | (all-E)-neoxanthin | 32.4 ± 0.9 a | 152.4 ± 4.1 c | 36.0 ± 2.2 b | 123.3 ± 0.9 a | 38.7 ± 2.6 c | 148.3 ± 9.9 b | 46.0 ± 0.4 bc | 151.2 ± 2.7 c |
| 6 | (all-E)-lutein | 922.5 ± 9.1 b | 782.9 ± 6.2 a | 1381.1 ± 3.9 c | 1238.4 ± 10.1 a | 1094.1 ± 5.2 c | 1499.9 ± 34.9 a | 492.4 ± 1.2 a | 852.6 ± 6.1 a |
| 7 | (all-E)-zeaxanthin | 44.6 ± 3.2 a | 120.0 ± 8.4 a | 127.1 ± 10.1 c | 195.3 ± 0.8 b | 81.3 ± 2.2 b | 133.9 ± 2.3 a | 59.0 ± 1.9 a | 129.4 ± 2.3 a |
| 8 | lutein-5,6-epoxide | n.d a | 273.5 ± 7.3 b | n.d a | n.d a | n.d a | n.d a | 38.8 ± 1.0 b | 160.0 ± 6.0 b |
| 9 | (all-E)-antheraxanthin | 25.2 ± 1.1 a | 116.9 ± 9.6 a | 20.9 ± 1.7 a | 139.7 ± 5.1 b | 40.0 ± 3.1 b | 102.6 ± 5.8 b | 64.7 ± 5.5 c | 140.9 ± 7.6 b |
| 10 | (9Z)-violaxanthin | 20.3 ± 0.1 c | n.d a | 7.8 ± 0.2 b | n.d a | n.d a | n.d a | n.d a | n.d a |
| 11 | (all-E)-β-apo-caroten-8 al (IS3) | 139.7 ± 0.9 b | 138.7 ± 0.4 b | 130.4 ± 0.9 b | 133.3 ± 0.6 a | 119.9 ± 13.4 b | 110.0 ± 4.4 a | 147.0 ± 0.6 a | 141.2 ± 2.3 b |
| 12 | β-cryptoxanthin-5,6-epoxide | 5.1 ± 0.5 b | 71.3 ± 3.0 b | n.d a | 89.9 ± 0.2 b | 64.5 ± 4.8 d | 32.8 ± 1.0 a | 12.1 ± 0.2 c | 36.2 ± 0.9 a |
| 13 | (9Z)-α-cryptoxanthin | 10.9 ± 0.6 b | 7.2 ± 0.1 b | n.d a | 21.5 ± 0.2 c | n.d a | n.d a | 18.3 ± 0.0 c | 27.9 ± 0.3 c |
| 14 | not identified 2 | tr. | n.d | n.d | tr. | n.d | tr. | n.d | tr. |
| 15 | (all-E)-α-cryptoxanthin | 3.5 ± 0.5 b | 29.8 ± 3.2 b | n.d a | 30.7 ± 0.3 b | n.d a | 8.3 ± 0.7 a | 7.7 ± 0.0 c | 12 ± 0.2 a |
| 16 | (all-E)-β-cryptoxanthin | 65.6 ± 1.4 a | 188.9 ± 9.0 b | 68.5 ± 3.9 a | 170.7 ± 1.4 a | 70.7 ± 2.1 a | 183.8 ± 14.1 c | 88.2 ± 0.7 a | 197.8 ± 8.2 d |
| 17 | α-carotene-5,6-epoxide | 32.7 ± 0.1 b | 23.8 ± 0.2 a | 8.2 ± 1.2 a | 42.8 ± 3.1 b | 7.2 ± 1.4 a | 22.5 ± 1.4 a | 34.9 ± 0.4 b | 32.1 ± 1.0 b |
| 18 | (all-E)-luteoxanthin | 103.2 ± 5.1 c | 195.3 ± 5.2 c | n.d a | n.d a | n.d a | 16.2 ± 0.0 b | 36.3 ± 1.2 b | 34.7 ± 0.7 b |
| 19 | (13Z)-α-carotene | 89.6 ± 0.8 c | 64.8 ± 0.8 b | 22.7 ± 1.6 b | n.d a | 13.6 ± 1.0 a | n.d a | 18.2 ± 0.8 a | 17.8 ± 0.7 b |
| 20 | (13Z)-β-carotene | 9.8 ± 0.7 a | 6.6 ± 0.1 a | 47.5 ± 1.5 c | 46.2 ± 3.2 c | 20.4 ± 0.9 b | 15.1 ± 0.4 b | 29.5 ± 0.2 b | 20.4 ± 0.2 b |
| 21 | (all-E)-violaxanthin laurate | 141.3 ± 0.1 c | n.d a | 4.7 ± 0.1 a | n.d a | 31.9 ± 1.3 b | n.d a | 39.8 ± 1.9 b | n.d a |
| 22 | β-cryptoxanthin-5,8-epoxide | 25.7 ± 0.9 c | n.d a | 16.6 ± 0.2 b | n.d a | 43.9 ± 2.2 d | 70.7 ± 6.4 b | n.d a | n.d a |
| 23 | (all-E)-ζ-carotene | 7.7 ± 2.0 a | 5.7 ± 0.7 b | 90.2 ± 3.9 b | 58.4 ± 1.9 d | 109.7 ± 11.4 c | 33.9 ± 0.1 c | n.d a | n.d a |
| 24 | β-cryptoxanthin-5,8’-epoxide | 35.6 ± 1.0 c | n.d a | n.d a | n.d a | 35.5 ± 3.2 d | 32.4 ± 1.3 c | 11.9 ± 0.1 b | 20.8 ± 0.3 b |
| 25 | (all-E)-α-carotene | 22.5 ± 1.4 b | 14.8 ± 0.2 b | n.d a | n.d a | 40.7 ± 1.3 c | n.d a | 105.1 ± 0.4 d | 95.6 ± 2.1 c |
| 26 | (9Z)-α-carotene | 9.8 ± 1.0 b | 6.5 ± 0.1 b | n.d a | n.d a | 6.1 ± 0.4 b | n.d a | 14.7 ± 0.3 c | 12.5 ± 0.5 c |
| 27 | (9Z)-violaxanthin laurate | 19.9 ± 1.1 b | n.d a | n.d a | n.d a | 69.4 ± 4.2 c | n.d a | 141.7 ± 0.7 d | n.d a |
| 28 | (all-E)-lutein-3-O-myristate | 117.5 ± 2.4 c | n.d a | 70.9 ± 3.3 b | n.d a | n.d a | n.d a | 229.0 ± 0.4 d | n.d a |
| 29 | (all-E)-β-carotene | 233.2 ± 5.4 b | 200.1 ± 2.6 b | 157.1 ± 5.9 a | 144.0 ± 4.1 a | 223.6 ± 9.5 c | 207.8 ± 13.8 c | 251.4 ± 0.8 b | 239.8 ± 1.3 d |
| 30 | (9Z)-β-carotene | n.d a | n.d a | n.d a | n.d a | n.d a | n.d a | 11.1 ± 1.0 c | 8.1 ± 0.1 b |
| 31 | (all-E)-violaxanthin dimyristate | 28.1 ± 0.8 c | n.d a | 11.8 ± 1.4 b | n.d a | n.d a | n.d a | 240.2 ± 8.0 d | n.d a |
| 32 | (all-E)-antheraxanthin myristate palmitate | 146.3 ± 6.2 c | n.d a | 95.4 ± 2.7 b | 52.1 ± 2.8 b | 125.6 ± 1.7 c | n.d a | 70.2 ± 1.0 a | 50.4 ± 4.6 b |
| 33 | (all-E)-violaxanthin palmitate | 53.7 ± 1.5 d | n.d a | 29.6 ± 3.2 c | n.d a | 8.9 ± 0.4 b | n.d a | n.d a | n.d a |
| 34 | (9Z)-neoxanthin dibutyrate | 44.3 ± 2.2 c | n.d a | 16.5 ± 2.8 b | n.d a | 6.7 ± 0.2 a | n.d a | 38.7 ± 2.5 c | n.d a |
| 35 | (all-E)-β-cryptoxanthin caprate | 54.1 ± 0.1 b | n.d a | 28.9 ± 2.7 a | n.d a | 45.2 ± 3.1 b | n.d a | 69.0 ± 0.4 c | n.d a |
| 36 | (9Z)-violaxanthin myristate palmitate | 28.1 ± 0.1 b | n.d a | 12.4 ± 1.7 a | n.d a | 11.1 ± 1.0 a | n.d a | 47.0 ± 4.0 c | n.d a |
| 37 | (all-E)-lutein dimyristate | 49.0 ± 2.3 a | n.d a | 39.9 ± 4.5 a | n.d a | 39.1 ± 3.1 a | n.d a | 91.8 ± 0.2 b | n.d a |
| 38 | (all-E)-β-cryptoxanthin laurate | 74.4 ± 5.7 b | n.d a | 66.8 ± 0.0 a | n.d a | 64.0 ± 0.4 a | n.d a | 194.9 ± 8.0 c | n.d a |
| 39 | (all-E)-antheraxanthin-3-O-palmitate | 74.7 ± 3.2 c | n.d a | 23.3 ± 0.4 a | n.d a | 120.2 ± 7.1 c | n.d a | 54.8 ± 0.2 b | n.d a |
| 40 | (all-E)-antheraxanthin laurate myristate | 36.9 ± 2.0 b | n.d a | 24.0 ± 0.2 a | n.d a | 26.3 ± 1.0 a | n.d a | 61.5 ± 0.7 c | 42.6 ± 5.4 a |
| 41 | (all-E)-β-cryptoxanthin myristate | 35.6 ± 1.7 b | n.d a | 24.4 ± 2.0 a | n.d a | 22.7 ± 1.8 a | 4.4 ± 0.2 b | 57.4 ± 0.3 c | n.d a |
| 42 | (Z)-lycopene isomer 1 | 17.6 ± 0.2 b | 12.8 ± 0.3 b | n.d a | n.d a | 21.4 ± 1.3 b | 15.6 ± 1.1 c | 25.3 ± 1.0 b | 22.5 ± 0.3 d |
| 43 | (all-E)-β-cryptoxanthin palmitate | n.d a | n.d a | n.d a | n.d a | n.d a | n.d a | 11.2 ± 0.3 b | n.d a |
| 44 | (13Z)-lycopene isomer 2 | 17.9 ± 1.0 a | 15.9 ± 0.4 a | 41.5 ± 0.4 b | 41.1 ± 1.3 b | 57.7 ± 2.0 c | 41.8 ± 1.2 c | 258.2 ± 1.1 d | 254.3 ± 1.2 d |
| 45 | (13’Z)-lycopene isomer 3 | n.d a | n.d a | n.d a | n.d a | n.d a | n.d a | 20.4 ± 1.2 b | 18.9 ± 1.1 b |
| 46 | (9Z)-lycopene isomer 4 | 22.0 ± 0.2 a | 15.2 ± 0.2 a | 37.0 ± 0.8 b | 35.6 ± 0.5 b | 50.8 ± 2.1 c | 35.9 ± 1.2 c | 67.7 ± 1.5 c | 67.1 ± 0.8 d |
| 47 | (9’Z)-lycopene isomer 5 | n.d a | n.d a | n.d a | n.d a | 11.5 ± 0.9 b | 8.0 ± 0.5 b | 20.7 ± 2.1 c | 15.2 ± 0.4 c |
| 48 | (all-E)-lycopene | 306.6 ± 3.9 b | 251.3 ± 0.2 b | 230.0 ± 2.2 a | 231.3 ± 1.1 a | 318.8 ± 26.8 c | 238.4 ± 20.4 c | 441.0 ± 2.0 d | 420.5 ± 12.4 d |
| 49 | (Z)-lycopene isomer 6 | 41.8 ± 1.5 a | 32.5 ± 0.2 a | 48.9 ± 1.4 b | 45.9 ± 0.6 a | 58.2 ± 2.9 c | 44.2 ± 3.7 b | 50.0 ± 1.1 a | 42.6 ± 3.8 b |
| total free xanthophylls | 1525.6 ± 28.0 b | 3010.9 ± 95.2 b | 1806.7 ± 29.3 c | 2910.2 ± 51.3 b | 1899.1 ± 43.7 c | 2868.1 ± 224.6 b | 1073.1 ± 13.4 a | 2507.2 ± 83.2 a | |
| total xanthophyll esters | 903.9 ± 29.6 b | 0.0 ± 0.0 a | 448.7 ± 25.0 a | 52.1 ± 2.8 b | 571.1 ± 29.6 a | 4.4 ± 0.3 a | 1347.2 ± 20.8 c | 93.0 ± 10.0 b | |
| total hydrocarbon carotenoids | 811.2 ± 16.6 b | 649.9 ± 5.9 a | 675.0 ± 17.6 a | 645.2 ± 15.8 a | 964.5 ± 75.4 a | 663.3 ± 43.8 a | 1348.3 ± 14.1 c | 1222.4 ± 29.7 b | |
| total carotenoids | 3240.8 ± 74.3 b | 3660.8 ± 101.1 a | 2930.4 ± 71.8 a | 3607.5 ± 69.9 a | 3434.7 ± 148.7 a | 3535.8 ± 268.7 a | 3768.6 ± 48.3 c | 3822.6 ± 122.9 a | |
| RAE4 | 36.7 ± 3.2 b | 28.2 ± 1.5 ab | 28.7 ± 0.6 a | 24.7 ± 0.5 a | 36.7 ± 1.6 a | 31.8 ± 3.3 ab | 34.0 ± 0.4 ab | 35.7 ± 0.8 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Abia, S.; Lobo-Rodrigo, G.; Welti-Chanes, J.; Cano, M.P. Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands. Foods 2021, 10, 434. https://doi.org/10.3390/foods10020434
Lara-Abia S, Lobo-Rodrigo G, Welti-Chanes J, Cano MP. Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands. Foods. 2021; 10(2):434. https://doi.org/10.3390/foods10020434
Chicago/Turabian StyleLara-Abia, Sara, Gloria Lobo-Rodrigo, Jorge Welti-Chanes, and M. Pilar Cano. 2021. "Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands" Foods 10, no. 2: 434. https://doi.org/10.3390/foods10020434
APA StyleLara-Abia, S., Lobo-Rodrigo, G., Welti-Chanes, J., & Cano, M. P. (2021). Carotenoid and Carotenoid Ester Profile and Their Deposition in Plastids in Fruits of New Papaya (Carica papaya L.) Varieties from the Canary Islands. Foods, 10(2), 434. https://doi.org/10.3390/foods10020434