Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review
Abstract
:1. Introduction to Malnutrition in Older Adults
2. Protein Requirements and the Importance of Protein-Fortified Products in the Diet of Older Adults
3. The Use of Whey Protein to Fortify Foods for Older Adults
4. Mouthfeel and Mouthdrying Perception of Whey Protein-Fortified Products
4.1. Whey Protein-Derived Mouthdrying
4.2. Mucoadhesion and Mouthfeel Perception
5. Age and Individual Differences Likely to Influence Mouthfeel Perception
5.1. Whey Protein-Derived Mouthdrying and Changes in Perception with Age
5.2. Individual Differences That Could Influence Perception of Whey Protein-Fortified Products
5.3. Food Oral Processing and Mouthfeel Perception
5.4. Differences in Saliva Flow with Age and Their Potential Effect on Mouthfeel Perception
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 18 November 2020).
- Office for National Statistics. 2018. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/november2018 (accessed on 2 January 2019).
- World Health Organisation (WHO). 2015. Available online: https://www.who.int/ageing/events/world-report-2015-launch/en/ (accessed on 4 January 2019).
- World Health Organisation (WHO). Decade of Healthy Ageing 2020–2030. 2020. Available online: https://www.who.int/docs/default-source/decade-of-healthy-ageing/final-decade-proposal/decade-proposal-final-apr2020-en.pdf?sfvrsn=b4b75ebc_3 (accessed on 20 April 2020).
- Pout, V. Older adults. In Manual of Dietetic Practice, 5th ed.; Gandy, J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 92–103. [Google Scholar]
- Ahmed, T.; Haboubi, N. Assessment and management of nutrition in older people and its importance to health. Clin. Interv. Aging 2010, 5, 207–216. [Google Scholar]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Philips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence based recommendation for optimal dietary protein intake in older people: A position paper from the PROT-AGE study group. JAMA 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A. Protein intake and exercise for optimal muscle function with ageing: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Department of Health. 1992. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/743779/The_Nutrition_of_Elderly_People__1992_.pdf (accessed on 3 January 2019).
- Dorrington, N.; Fallaize, R.; Hobbs, D.A.; Weech, M.; Lovegrove, J.A. A review of nutritional requirements of adults aged ≥ 65 years in the UK. J. Nutr. 2020, 150, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Armarya, S.; Singh, K.; Sabharwal, M. Changes during ageing and their association with malnutrition. J. Clin. Gerontol. Geriatr. 2015, 6, 78–84. [Google Scholar] [CrossRef] [Green Version]
- BDA. 2017. Available online: https://www.bda.uk.com/publications/professional/NutritionHydrationDigest.pdf (accessed on 10 January 2019).
- BAPEN. 2018. Available online: https://www.bapen.org.uk/malnutrition-undernutrition/introduction-to-malnutrition?start=4 (accessed on 10 January 2019).
- Hickson, M. Malnutrition and ageing. Postgrad. Med. J. 2006, 82, 2–8. [Google Scholar] [CrossRef]
- Elia, M. Defining, recognizing and reporting malnutrition. Int. J. Low Extrem. Wonds 2017, 16, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Maleta, K. Undernutrition. Malawi. Med. J. 2006, 18, 189–205. [Google Scholar]
- Leiji-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults > 65 years. A systematic review and meta analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef]
- Todorovic, V.; Russell, C.; Elia, M. The MUST Explanatory Booklet. 2003. Available online: https://www.bapen.org.uk/pdfs/must/must_explan.pdf (accessed on 2 January 2019).
- National Institute for Health and Care Excellence. 2017. Available online: https://www.nice.org.uk/guidance/cg32/chapter/1-Guidance#indications-for-nutrition-support-in-hospital-and-the-community (accessed on 8 January 2019).
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Van Kan, G.A.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology and consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachex. Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Stevenson, E.; Brunstrom, J.; Johnstone, A.; Green, M.; Williams, L.; Corfe, B. Protein for Life Team. 2019. Available online: https://ktn-uk.co.uk/news/protein-for-life-a-framework-for-action (accessed on 20 July 2019).
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, S.; Graham, B. Taste and smell perception affect appetite and immunity in the elderly. Eur. J. Clin. Nutr. 2000, 54, 54–63. [Google Scholar] [CrossRef]
- Morley, J. Workshop: Anorexia during disease—From research to clinical practice anorexia, sarcopenia and ageing. Nutrition 2001, 17, 660–663. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Gil-Guerrero, L.; Iniesta, R. The anorexia of ageing: Physiopathology, prevalence, associated comorbidity and mortality. A systematic review. Maturitas 2013, 74, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe-Descamps, M.; Sulmont-Rosse, C.; Septier, C.; Feron, G.; Laboure, H. Using food comfortability to compare foods sensory characteristics expectations of elderly people with or without oral health problems. J. Texture Stud. 2017, 48, 280–287. [Google Scholar] [CrossRef]
- Gura, K.; Ciccone, R. Drugs and appetite. An overview of appetite stimulants in the paediatric patient. ICAN Infant Child Adolesc. Nutr. 2010, 2, 358–369. [Google Scholar] [CrossRef]
- Nieuwenhuizen, W.F.; Weenen, H.; Rigby, P.; Hetherington, M.M. Older adults and patients in need of nutritional support: Review of current treatment options and factors influencing nutritional intake. Clin. Nutr. 2010, 29, 160–169. [Google Scholar] [CrossRef]
- Stull, A.J.; Apolzan, J.W.; Thalacker-Mercer, A.E.; Iglay, H.; Campbell, W.W. Liquid and solid meal replacement products differentially affect postprandial appetite and food intake in older adults. J. Am. Diet. Assoc. 2008, 108, 1226–1230. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L. Food texture and the satiety cascade. Nutr. Bull. 2016, 41, 277–282. [Google Scholar] [CrossRef]
- Giezenaar, C.; Chapman, I.; Luscombe-Marsh, N.; Feinle-Bisset, C.; Horowitz, M.; Soenen, S. Ageing is associated with decreases in appetite and energy intake—A meta analysis in healthy adults. Nutrients 2016, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razak, P.A.; Richard, K.M.J.; Thankachan, R.P.; Hafiz, K.A.A.; Kumar, K.N.; Sameer, K.M. Geriatric oral health: A review article. J. Int. Oral Health 2014, 6, 110–116. [Google Scholar]
- Rathee, M.; Hooda, A. Nutritional status in denture wearers: A review. Int. J. Nutr. Wellness 2009, 10, 1–5. [Google Scholar]
- Watson, S.; McGowan, L.; McCrum, L.A.; Cardwell, C.R.; McGuinness, B.; Moore, C.; Woodside, J.V.; McKenna, G. The impact of dental status on perceived ability to eat certain foods and nutrient intake in older adults: Cross-sectional analysis of the UK National Diet and Nutrient survey 2008–2014. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, S.; Bult, J.H.F.; Mojet, J.; Kroeze, J.H.A. Food perception with age and its relationship to pleasantness. Chem. Senses 2007, 32, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methven, L.; Jimenez-Pranteda, M.L.; Lawlor, J. Sensory and consumer science methods used with older adults: A review of current methods and recommendations for the future. Food Qual. Pref. 2016, 48, 333–344. [Google Scholar] [CrossRef]
- Imoscopi, A.; Inelmen, E.M.; Sergi, G.; Miotto, F.; Manzato, E. Taste loss in the elderly: Epidemiology, causes and consequences. Aging Clin. Exp. Res. 2012, 24, 570–579. [Google Scholar]
- Solemadal, K.; Sandvik, L.; Willumsen, T.; Mowe, M.; Hummel, T. The impact of oral health on taste ability in acutely hospitalized elderly. PLoS ONE 2012, 7, e36557. [Google Scholar] [CrossRef]
- Moody, A.; Mindell, J.; Faulding, S. Health Survey for England 2016. 2017. Available online: http://healthsurvey.hscic.gov.uk/media/63790/HSE2016-pres-med.pdf (accessed on 7 January 2019).
- Ciancio, S.G. Medications impact on oral health. J. Am. Dent. Assoc. 2004, 135, 1440–1448. [Google Scholar] [CrossRef]
- Stratton, R.; Smith, T.; Gabe, S. Managing Malnutrition to Improve Lives and Save Money. 2018. Available online: https://www.bapen.org.uk/pdfs/reports/mag/managing-malnutrition.pdf (accessed on 3 January 2019).
- Hoffman, J.R.; Falvo, M.J. Protein—Which is best? J. Sci. Med. 2004, 3, 118–130. [Google Scholar]
- Pires, M.A.; Pastrana, L.M.; Fucinos, P.; Abreu, C.S.; Oliveira, S.M. Sensorial Perception of Astringency: Oral Mechanisms and Current Analysis Methods. Foods 2020, 9, 1124. [Google Scholar] [CrossRef]
- Carter, B.G.; Foegeding, E.A.; Drake, M.A. Invited review: Astringency in whey protein beverages. J. Dairy. Sci. 2020, 103, 5793–5804. [Google Scholar] [CrossRef]
- Department of Health. 1991. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/743790/Dietary_Reference_Values_-_A_Guide__1991_.pdf (accessed on 4 January 2019).
- Stevenson, E.J.; Watson, A.W.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Johnstone, A.M.; Williams, E.A. Protein for life: Towards a focused dietary framework for healthy ageing. Nutr. Bull. 2018, 43, 97–102. [Google Scholar] [CrossRef] [Green Version]
- PENG. 2011. Available online: https://www.peng.org.uk/publications-resources/pocket-guide.php (accessed on 10 January 2019).
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanism of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef]
- Giezenaar, C.; Trahair, L.G.; Rigda, R.; Hutchison, A.T.; Feinle-Bisset, C.; Luscombe-Marsh, N.D.; Hausken, T.; Jones, K.L. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, 845–854. [Google Scholar] [CrossRef]
- Giezenaar, C.; Trahair, L.G.; Luscombe-Marsh, N.D.; Hausken, T.; Standfield, S.; Jones, K.L.; Lange, K.; Horowitz, M.; Chapman, I.; Soenen, S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut hormone concentrations in older men and women. Am. J. Clin. Nutr. 2017, 106, 865–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BAPEN. 2016. Available online: https://www.bapen.org.uk/nutrition-support/nutrition-by-mouth/oral-nutritional-supplements (accessed on 8 January 2019).
- Wang, Z.; Chang, S.; Li, Y.; Kong, L.; Wu, D.; Qin, L.; Yu, C.; Wu, C.; Du, M. Effects of ball milling treatment on physiochemical properties and digestibility of Pacific oyster (Crassostrea gigas) protein powder. Food Sci. Nutr. 2018, 6, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croissant, A.E.; Kang, E.J.; Campbell, R.E.; Bastian, E.; Drake, M.A. The effect of bleaching agent on the flavour of liquid whey and whey protein concentrate. J. Dairy Sci. 2009, 92, 5917–5927. [Google Scholar] [CrossRef]
- Evans, J.; Zulewska, J.; Newbold, M.; Drake, M.A.; Barbano, D.M. Comparison of composition and sensory properties of 80% whey protein and milk serum protein concentrates. J. Dairy Sci. 2010, 93, 1824–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, S.R.; Wilcox, C.R.; Ibrahim, K.; Roberts, H.C. Can fortified foods and snacks increase the energy and protein intake of hospitalised older patients? A systematic review. J. Hum. Nutr. Diet. 2018, 31, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Morilla-Herrera, J.C.; Martin-Santos, F.J.; Caro-Bautista, J.; Saucedo-Figueredo, C.; Garcia-Mayor, S.; Morales-Asencio, J.M. Effectiveness of food based fortification in older people a systematic review and meta-analysis. J. Nutr. Health Aging 2016, 20, 178–184. [Google Scholar] [CrossRef]
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta analysis of the effects of high protein oral nutritional supplements. Ageing Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.L.; Stratton, R.J.; Cawood, A.L.; Smith, T.R.; Elia, M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin. Nutr. 2017, 36, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: A randomised, double blind, placebo controlled trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Munk, T.; Beck, A.M.; Holst, M.; Rosenbom, E.; Rasmussen, H.H.; Nielsen, M.A.; Thomsen, T. Positive effect of protein supplementation hospital food on protein intake in patients at nutritional risk: A randomised controlled trial. J. Hum. Nutr. Diet. 2014, 27, 122–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleton, K.M.; Smith, E. A role for identification in the gradual decline in the pleasantness of flavours with age. J. Gerontol. Psychol. Sci. 2015, 71, 987–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beelen, J.; de Roos, N.M.; de Groot, L.C.P.G.M. Protein enrichment of familiar foods as an innovative strategy to increase protein intake in institutionalized elderly. J. Nutr. Health Aging 2017, 21, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Beelen, J.; de Roos, N.M.; de Groot, L.C.P.G.M. A 12 week intervention with protein enriched foods and drinks improved protein intake but not physical performance of older patients during the first 6 months after hospital release: A randomised controlled trial. Br. J. Nutr. 2017, 117, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Devries, M.C.; Sithamparapillai, A.; Brimble, K.S.; Banfield, L.; Morton, R.W.; Philips, S.M. Changes in kidney function do not differ between healthy adults consuming higher compared with lower or normal protein diets: A systematic review and meta analysis. J. Nutr. 2018, 148, 1760–1775. [Google Scholar] [CrossRef]
- Mitchell, S.M.; McKenzie, E.J.; Mitchell, C.J.; Milan, A.M.; Zeng, N.; D’Souza, R.F.; Ramzan, F.; Sharma, P.; Rettedal, E.; Knowles, S.O.; et al. A period of 10 weeks of increased protein consumption does not alter faecal microbiota or volatile metabolites in healthy older men: A randomised controlled trial. J. Nutr. Sci. 2020, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Elia, M. A review of reviews: A new look at the evidence for oral nutritional supplements in clinical practice. Clin. Nutr. Supp. 2007, 2, 5–23. [Google Scholar] [CrossRef]
- Parker, A.M.; Watson, R.R. Lactose Intolerance. In Nutrients in Dairy and Their Implications on Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: New York, NY, USA, 2017; pp. 205–211. [Google Scholar]
- Anema, S.G. The whey proteins in milk: Thermal denaturation, physical interactions, and effects on the functional properties of milk. In Milk Proteins: From Expression to Food, 2nd ed.; Boland, M., Singh, H., Thompson, Eds.; Academic Press: New York, NY, USA, 2014; pp. 270–311. [Google Scholar]
- O’Mahony, J.A.; Fox, P.F. Milk: An overview. In Milk Proteins: From Expression to Food, 2nd ed.; Boland, M., Singh, H., Thompson, Eds.; Academic Press: New York, NY, USA, 2014; pp. 20–61. [Google Scholar]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Bansal, N.; Bhandari, B. Functional Milk Proteins: Production and Utilization-Whey Based Ingredients. In Advanced Dairy Chemistry. Volume 1B: Proteins: Applied Aspects, 4th ed.; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: New York, NY, USA, 2016; pp. 67–99. [Google Scholar]
- Frankowski, K.M.; Miracle, R.E.; Drake, M.A. The role of sodium in the salty taste of permeate. J. Dairy Sci. 2014, 97, 5356–5370. [Google Scholar] [CrossRef] [PubMed]
- Etzel, M.R. Manufacture and use of dairy protein fractions. J. Nutr. 2004, 134, 996S–1002S. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—from ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Dangin, M.; Guillet, C.; Garcia-Rodenas, C.; Gachon, P.; Bouteloup-Demange, C.; Reiffers-Magnani, K.; Fauquant, J.; Ballevre, O.; Beaufrere, B. The rate of protein digestion affects protein gain differently during ageing in humans. J. Physiol. 2003, 549, 635–644. [Google Scholar] [CrossRef]
- Sahathevan, S.; Se, C.H.; Ng, S.H.; Khor, B.H.; Chinna, K.; Goh, B.L.; Gafor, H.A.; Bavanandan, S.; Ahmad, G.; Karupaiah, T. Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: A multicentre, parallel, open-label randomised controlled trial. Clin. Nutr. ESPEN 2018, 25, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solak, B.B.; Akin, N. Health benefits of whey protein: A review. J. Food Sci. Eng. 2012, 2, 129–137. [Google Scholar]
- Gosney, M. Are we wasting our money on food supplements in elder care wards? J. Adv. Nurs. 2003, 43, 275–280. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Elia, M.; Holdoway, A.; Stratton, R.J. A systematic review of compliance to oral nutritional supplements. Clin. Nutr. 2012, 31, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.L.; Drake, M.A. Consumer perception of astringency in clear acidic whey protein beverages. J. Food Sci. 2010, 75, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, O.; Law, C.; Methven, L.; Mottram, D.; Gosney, M. Investigating age related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing 2010, 39, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Methven, L.; Rahelu, K.; Economou, N.; Kinneavy, L.; Ladbrooke-Davis, L.; Kennedy, O.B.; Mottram, D.S.; Gosney, M.A. The effect of consumption volume of profile and liking of oral nutritional supplements of varied sweetness: Sequential profiling and boredom tests. Food Qual. Prefer. 2010, 21, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Bull, S.P.; Hong, Y.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Whey protein mouth drying influenced by thermal denaturation. Food Qual. Prefer. 2017, 56, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; van der Stelt, A.J.; Prokop, J.; Lawlor, J.B.; Schlich, P. Alternating temporal dominance of sensations and liking scales during the intake of a full portion of an oral nutritional supplement. Food Qual. Prefer 2016, 53, 159–167. [Google Scholar] [CrossRef]
- Thomas, A.; van der Stelt, A.J.; Schlich, P.; Lawlor, J.B. Temporal drivers of liking for oral nutritional supplements for older adults throughout the day with monitoring of hunger and thirst status. Food Qual. Prefer. 2018, 70, 40–48. [Google Scholar] [CrossRef]
- Norton, V.; Lignou, S.; Bull, S.P.; Gosney, M.A.; Methven, L. An investigation of the influence of age and saliva flow on the oral retention of whey protein and its potential effect on the perception and acceptance of whey protein beverages. Nutrients 2020, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Norton, V.; Lignou, S.; Bull, S.P.; Gosney, M.A.; Methven, L. Consistent effects of whey protein fortification on consumer perception and liking of solid food matrices (cakes and biscuits) regardless of age and saliva flow. Foods 2020, 9, 1328. [Google Scholar] [CrossRef]
- Oltman, A.E.; Lopetcharat, K.; Bastian, E.; Drake, M.A. Identifying key attributes for protein beverages. J. Food Sci. 2015, 80, S1383–S1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.T.; Jo, Y.; Lopetcharat, K.; Drake, M.A. Comparison of a central location test versus a home usage test for consumer perception of ready-to-mix protein beverages. J. Dairy Sci. 2020, 103, 3107–3124. [Google Scholar] [CrossRef] [PubMed]
- Wendin, K.; Hoglund, E.; Andersson, M.; Rothenberg, E. Protein enriched foods and healthy ageing: Effects of protein fortification on muffin characteristics. Agro Food Ind. Hi-Tech 2017, 28, 16–18. [Google Scholar]
- Song, X.; Perez-Cueto, F.J.A.; Bredie, W.L. Sensory-driven development of protein-enriched rye bread and cream cheese for the nutritional demands of older adults. Nutrients 2018, 10, 1006. [Google Scholar] [CrossRef] [Green Version]
- Whetstine, M.E.; Croissant, A.E.; Drake, M.A. Characterization of dried whey protein concentrate and isolate flavour. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef]
- Karagul-Yuceer, Y.; Drake, M.A.; Cadwallader, K.R. Aroma-active components of liquid cheddar whey. J. Food Chem. Toxicol. 2003, 68, 1215–1219. [Google Scholar] [CrossRef]
- Wright, J.M.; Whetstine, M.E.C.; Miracle, R.E.; Drake, M.A. Characterization of a cabbage off flavour in whey protein isolate. J. Food Sci. 2006, 71, 86–90. [Google Scholar] [CrossRef]
- Russell, T.A.; Drake, M.A.; Gerard, P.D. Sensory properties of whey and soy proteins. J. Food Sci. 2006, 71, S447–S455. [Google Scholar] [CrossRef]
- Tsikritzi, R.; Wang, J.; Collins, V.J.; Allen, V.J.; Mavrommatis, Y.; Moynihan, P.J.; Gosney, M.A.; Kennedy, O.B.; Methven, L. The effect of nutrient fortification of sauces on product stability, sensory properties, and subsequent liking by older adults. J. Food Sci. 2015, 80, 1100–1110. [Google Scholar] [CrossRef]
- Tsikritzi, R.; Moynihan, P.J.; Gosney, M.A.; Allen, V.J.; Methven, L. The effect of macro- and micro-nutrient fortification of biscuits on their sensory properties and on hedonic liking of older people. J. Sci. Food Agric. 2014, 94, 2040–2048. [Google Scholar] [CrossRef] [Green Version]
- Vandenberghe-Descamps, M.; Laboure, H.; Prot, A.; Septier, C.; Tournier, C.; Feron, G.; Sulmont-Rosse, C. Salivary flow decreases in healthy elderly people independently of dental status and drug intake. J. Texture Stud. 2016, 47, 353–360. [Google Scholar] [CrossRef]
- Thomson, W.M. Dry mouth and older people. Aust. Dent. J. 2015, 60, 54–63. [Google Scholar] [CrossRef]
- Guinard, J.X.; Mazzucchelli, R. The sensory perception of texture and mouthfeel. Trends Food Sci. Technol. 1996, 7, 213–219. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Texture is a sensory property. Food Qual. Prefer. 2002, 13, 215–225. [Google Scholar] [CrossRef]
- Szczesniak, A.S.; Kahn, E.L. Consumer awareness of and attitudes to food texture I: Adults. J. Texture Stud. 1971, 2, 280–295. [Google Scholar] [CrossRef]
- Lemieux, L.; Simard, R.E. Astringency, a textural defect in dairy products. Lait 1994, 74, 217–240. [Google Scholar] [CrossRef] [Green Version]
- ASTM E253–20. Available online: http://www.astm.org/cgi-bin/resolver.cgi?E253 (accessed on 1 July 2020).
- Green, B.G. Oral astringency: A tactile component of flavor. Acta Psychol. 1993, 84, 119–125. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Gilmore, M.M.; Beauchamp, G.K.; Green, B.G. Psychophysical evidence that oral astringency is a tactile sensation. Chem. Senses 1993, 18, 405–417. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Carpenter, G.H. Alternative mechanisms of astringency—what is the role of saliva? J. Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and perception. Crit. Rev. Food Sci. Nutr. 2008, 48, 858–875. [Google Scholar] [CrossRef]
- Damodaran, S.; Arora, A. Off flavour precursors in soy protein isolate and novel strategies for their removal. Annu. Rev. Food Sci. Technol. 2013, 4, 327–346. [Google Scholar] [CrossRef]
- Cosson, A.; Souchon, I.; Richard, J.; Descamps, N.; Saint-Eve, A. Using Multiple Sensory Profiling Methods to Gain Insight into Temporal Perceptions of Pea Protein-Based Formulated Foods. Foods 2020, 9, 969. [Google Scholar] [CrossRef]
- Jobstl, E.; O’Connell, J.; Fairclough, P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Lyman, B.J.; Green, B.G. Oral astringency: Effects of repeated exposure and interactions with sweeteners. Chem. Senses 1990, 15, 151–164. [Google Scholar] [CrossRef]
- Thorngate, J.H.; Noble, A.C. Sensory evaluation of bitterness and astringency of 3R(-)-Epicatechin and 3S(+)-Catechin. J. Sci. Food Agric. 1995, 67, 531–535. [Google Scholar] [CrossRef]
- Linne, B.; Simons, C.T. Quantification of oral roughness perception and comparison with mechanism of astringency perception. Chem. Senses 2017, 42, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Bajec, M.R.; Pickering, G.J. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol. Behav. 2008, 95, 581–590. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Tuorila, H.; Monteleone, E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite 2011, 56, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.E.; Ziegler, G.R.; Hayes, J.E. Salivary protein levels as a predictor of perceived astringency in model systems and solid foods. Physiol. Behav. 2016, 163, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Olaf Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency Is a Trigeminal Sensation That Involves the Activation of G Protein–Coupled Signaling by Phenolic Compounds. Chem. Senses 2014, 39, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Withers, C.A.; Lewis, M.J.; Gosney, M.A.; Methven, L. Potential sources of mouth drying in beverages fortified with dairy proteins: A comparison of casein- and whey-rich ingredients. J. Dairy Sci. 2014, 97, 1233–1247. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Vardhanabhuti, B.; Luck, P.; Drake, M.A.; Osborne, J.; Foegeding, E.A. Role of protein concentration and protein–saliva interactions in the astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1900–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beecher, J.W.; Drake, M.A.; Luck, P.J.; Foegeding, E.A. Factors regulating astringency of whey protein beverages. J. Dairy Sci. 2008, 91, 2553–2560. [Google Scholar] [CrossRef] [Green Version]
- Vardhanabhuti, B.; Kelly, M.A.; Luck, P.J.; Drake, M.A.; Foegeding, E.A. Roles of charge interactions on astringency of whey proteins at low pH. J. Dairy Sci. 2010, 93, 1890–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, A.; Streicher, C.; Singh, H. Interactions between whey proteins and salivary proteins as related to astringency of whey protein beverages at low pH. J. Dairy Sci. 2011, 94, 5842–5850. [Google Scholar] [CrossRef]
- Andrewes, P.; Kelly, M.; Vardhanabhuti, B.; Foegeding, E.A. Dynamic modelling of whey protein-saliva interactions in the mouth and relation to astringency in acidic beverages. Int. Dairy J. 2011, 21, 523–530. [Google Scholar] [CrossRef]
- Vardhanabhuti, B.; Cox, P.W.; Norton, I.T.; Foegeding, E.A. Lubricating properties of human whole saliva as affected by beta-lactoglobulin. Food Hydrocoll. 2011, 25, 1499–1506. [Google Scholar] [CrossRef]
- Wang, G.; Liu, N.; Guo, M. Use of whey protein as a natural polymer for tissue adhesive: Preliminary formulation and evaluation in vitro. Polymers 2018, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Hsein, H.; Garrait, G.; Beyssac, E.; Hoffart, V. Whey protein mucoadhesive properties for oral drug delivery: Mucin whey protein interaction and mucoadhesive bond strength. Coll. Surf. B 2015, 136, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Withers, C.A.; Cook, M.T.; Methven, L.; Gosney, M.A.; Khutoryanskiy, V.V. Investigation of milk proteins binding to the oral mucosa. Food Funct. 2013, 4, 1668–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of whey proteins during thermal processing: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Josephson, R.V.; Thomas, E.L.; Morr, C.V.; Coulter, S.T. Relation of heat-induced changes in protein-salt constituents to astringency in milk systems. J. Dairy Sci. 1967, 50, 1376–1383. [Google Scholar] [CrossRef]
- Bull, S.P.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Oral retention of whey protein: Measurement and mechanisms. Food Chem. 2020. submitted for publication. [Google Scholar]
- Çelebioğlu, H.Y.; Lee, S.; Chronakis, I.S. Interactions of salivary mucins and saliva with food proteins: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 64–83. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.L.; Woods, S.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesive polysaccharides modulate sodium retention, release and taste perception. Food Chem. 2018, 240, 482–489. [Google Scholar] [CrossRef]
- Lucas, P.W.; Prinz, J.F.; Agrawal, K.R.; Bruce, I.C. Food texture and its effect on ingestion, mastication and swallowing. J. Texture Stud. 2004, 35, 159–170. [Google Scholar] [CrossRef]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Sano, H.; Egashira, T.; Kinekawa, Y.; Kitabatake, N. Astringency of bovine milk whey protein. J. Dairy Sci. 2005, 88, 2312–2317. [Google Scholar] [CrossRef]
- Ye, A.; Zheng, T.; Ye, J.Z.; Singh, H. Potential role of the binding of whey proteins to human buccal cells on the perception of astringency in whey proteins beverages. Physiol. Behav. 2012, 106, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tan, S.Y.; Mutilangi, W.; Plans, M.; Rodriguez-Saona, L. Application of infrared portable sensor technology for predicting perceived astringency of acidic whey protein beverages. J. Dairy Sci. 2016, 99, 9461–9470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.A.; Vickers, Z.M. The astringency of whey protein beverages is caused by their acidity. Int. Dairy J. 2008, 18, 1153–1156. [Google Scholar] [CrossRef]
- Withers, C.; Gosney, M.A.; Methven, L. Perception of thickness, mouth coating and mouth drying of dairy beverages by younger and older volunteers. J. Sens. Stud. 2013, 28, 230–237. [Google Scholar] [CrossRef]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremiao, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing mucoadhesion in polymer gels: The effect of method type and instrument variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnurch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582. [Google Scholar] [CrossRef]
- Tortora, G.J.; Nielsen, M.T. Principles of Human Anatomy, 11th ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wickremaratchi, M.M.; Llewelyn, J.G. Effects of ageing on touch. Postgrad. Med. J. 2006, 82, 301–304. [Google Scholar] [CrossRef]
- Methven, L.; Allen, V.; Withers, C.; Gosney, M.A. Ageing and Taste. Proc. Nutr. Soc. 2012, 71, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Doty, R.L.; Kamath, V. The influence of age on olfaction: A review. Front Psychol. 2014, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Schiffman, S.S.; Zervakis, J. Taste and smell perception in the elderly: Effect of medications and disease. Adv. Food Nutr. Res. 2002, 44, 248–345. [Google Scholar]
- Kremer, S.; Mojet, J.; Kroeze, J.H.A. Perception of texture and flavor in soups by elderly and young subjects. J. Texture Stud. 2005, 36, 255–272. [Google Scholar] [CrossRef]
- Kremer, S.; Mojet, J.; Kroeze, J.H.A. Differences in perception of sweet and savoury waffles between elderly and young subjects. Food Qual. Prefer. 2007, 38, 106–116. [Google Scholar] [CrossRef]
- Hutchings, S.C.; Foster, K.D.; Grigor, J.M.V.; Bronlund, J.E.; Morgenstern, M.P. Temporal dominance of sensations: A comparison between younger and older subjects for the perception of food texture. Food Qual. Prefer. 2014, 31, 106–115. [Google Scholar] [CrossRef]
- Forde, C.G.; Delahunty, C.M. Understanding the role cross-modal sensory interactions play in food acceptability in younger and older consumers. Food Qual. Prefer. 2004, 15, 715–727. [Google Scholar] [CrossRef]
- Engelen, L. Oral processing: Implications for consumer choice and preference. In Methods in Consumer Research, Volume 1: New Approaches to Classic Methods; Ares, G., Varela, P., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 401–421. [Google Scholar]
- Engelen, L.; Van Der Bilt, A. Oral physiology and texture perception of semisolids. J. Texture Stud. 2008, 39, 83–113. [Google Scholar] [CrossRef]
- Laureati, M.; Sandvik, P.; Almli, V.L.; Sandell, A.M.; Zeinstra, G.G.; Methven, L.; Wallner, M.; Jilani, H.; Alfaro, B.; Proserpio, C. Individual differences in texture preferences among European children: Development and validation of child food texture preference questionnaire (CFTPQ). Food Qual. Prefer. 2020, 80, 103828. [Google Scholar] [CrossRef]
- Ketal, E.V.; Aguayo-Mendoza, M.G.; de Wijk, R.A.; de Graaf, C.; Piqueras-Fiszman, B.; Stieger, M. Age, gender, ethnicity and eating capability influence oral processing behaviour of liquid, semi-solid and solid foods differently. Food Res. Int. 2019, 119, 143–151. [Google Scholar] [CrossRef]
- Ketal, E.V.; de Wijk, R.A.; de Graaf, C.; Stieger, M. Relating oral physiology and anatomy of consumers varying in age, gender and ethnicity to food oral processing behavior. Physiol. Behav. 2020, 215, 112766. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, E.; Newbury, A.; Appleton, K.M. The psychology of nutrition with advancing age: Focus on food neophobia. Nutrients 2019, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Jeltema, M.; Beckley, J.; Vahalik, J. Model for understanding consumer textural food choice. Food Sci. Nutr. 2015, 3, 202–212. [Google Scholar] [CrossRef]
- Jeltema, M.; Beckley, J.; Vahalik, J. Food texture assessment and preference based on mouth behavior. Food Qual. Prefer. 2016, 52, 160–171. [Google Scholar] [CrossRef]
- Pereira, L.J. Oral cavity. In Food Oral Processing: Fundamentals of Eating and Sensory Perception, 1st ed.; Chen, J., Engelen, L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 139–156. [Google Scholar]
- Hand, A.R.; Frank, M.E. Fundamentals of Oral Histology and Physiology; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Engelen, L. Oral Receptors. In Food Oral Processing: Fundamentals of Eating and Sensory Perception, 1st ed.; Chen, J., Engelen, L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 15–45. [Google Scholar]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Stokes, J.R.; Boehm, M.W.; Baier, S.K. Oral processing, texture and mouthfeel: From rheology to tribology and beyond. Curr. Opin. Colloid Interface Sci. 2013, 18, 349–359. [Google Scholar] [CrossRef] [Green Version]
- de Wijk, R.A.; Prinz, J.F. Mechanisms underlying the role of friction in oral texture. J. Texture Stud. 2006, 37, 413–427. [Google Scholar] [CrossRef]
- Krop, E.M.; Hetherington, M.M.; Miquel, S.; Sarkar, A. The influence of oral lubrication on food intake: A proof-of-concept study. Food Qual. Prefe.r 2019, 74, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Mioche, L.; Bourdiol, P.; Monier, S.; Martin, J.F.; Cormier, D. Changes in jaw muscles activity with age: Effects on food bolus properties. Physiol. Behav. 2004, 82, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Crow, H.C.; Ship, J.A. Tongue strength and endurance in different aged individuals. J. Gerontol. Med. Sci. 1996, 51, M247–M250. [Google Scholar] [CrossRef] [PubMed]
- Health and Social Care Information Centre. 2011. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/adult-dental-health-survey/adult-dental-health-survey-2009-summary-report-and-thematic-series (accessed on 12 January 2019).
- Ikebe, K.; Matsuda, K.; Kagawa, R.; Enoki, K.; Yoshida, M.; Maeda, Y.; Nokubi, T. Association of masticatory performance with age, gender, number of teeth, occlusal force and salivary flow in Japanease older adults: Is ageing a risk factor for masticatory dysfunction? Arch. Oral Biol. 2011, 56, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.G.; Ayatollahi, S.M.T.; Walls, A.W.G.; Murray, J.J. Clinical factors related to reported satisfaction with oral function amongst dentate older adults in England. Community Dent. Oral Epidemiol. 1997, 25, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Jeltema, M.; Morgenstern, M.P.; Motoi, L.; Kim, E.; Hedderley, D. Comparison of physical chewing measures to consumer typed mouthbehaviour. J. Texture Stud. 2018, 49, 262–273. [Google Scholar] [CrossRef]
- Mioche, L.; Bourdiol, P.; Peyron, M. Influence of age on mastication: Effects on eating behaviour. Nutr. Res. Rev. 2004, 17, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Shinkawa, T.; Hayashida, N.; Mori, K.; Washio, K.; Hashiguchi, K.; Taira, Y.; Morishita, M.; Takamura, N. Poor chewing ability is associated with lower mucosal moisture in elderly individuals. Tohoku J. Exp. Med. 2009, 219, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, P.; Gregio, A.M.; Machado, M.A.; de Lima, A.A.; Azevedo, L.R. Saliva composition and functions: A comprehensive review. J. Contemp. Dent. Prac. 2008, 9, 72–80. [Google Scholar]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Gupta, A.; Epstein, J.B.; Sroussi, H. Hyposalivation in elderly patients. J. Can. Dent. Assoc. 2006, 72, 841–846. [Google Scholar]
- May, A.J.; Chatzeli, L.; Proctor, G.B.; Tucker, A.S. Salivary gland dysplasia in Fgf10 heterozygous mouse model of xerostomia. Curr. Mol. Med. 2015, 15, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Marton, K.; Madlena, M.; Banoczy, J.; Varga, G.; Fejerdy, P.; Sreebny, L.M.; Nagy, G. Unstimulated whole saliva flow rate in relation to sicca symptoms in Hungary. Oral Dis. 2008, 14, 472–477. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Vandenberghe-Descamps, M.; Feron, G.; Canon, F.; Laboure, H.; Sulmont-Rosse, C. Association between salivary hypofunction and food consumption in the elderlies. A systematic literature review. J. Nutr. Health Aging 2018, 22, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Laguna, L.; Sarkar, A. Ageing-related changes in quantity and quality of saliva: Where do we stand in our understanding? J. Texture Stud. 2019, 50, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Whelton, H. Introduction: The anatomy and physiology of salivary glands. In Saliva and Oral Health: An Essential Overview for the Health Professional, 4th ed.; Edgar, M., Dawes, C., O’Mullane, D., Eds.; Stephen Hancocks Limited: Oxford, UK, 2012; pp. 1–36. [Google Scholar]
- Lee, S.K.; Lee, S.W.; Chung, S.C.; Kim, Y.K.; Kho, H.S. Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch. Oral Biol. 2002, 47, 637–641. [Google Scholar] [CrossRef]
- Turner, M.D.; Ship, J.A. Dry mouth and its effects on the oral health of elderly people. J. Am. Dent. Assoc. 2007, 138, 15–20. [Google Scholar] [CrossRef]
- Chaudhury, N.M.A.; Shirlaw, P.; Pramanik, R.; Carpenter, G.H.; Proctor, G.B. Changes in saliva rheological properties and mucin glycosylation in dry mouth. J. Dent. Res. 2015, 94, 1660–1667. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral Biol. 2005, 50, 7–16. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-saliva interactions: Mechanisms and implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Munoz-Gonzalez, C.; Feron, G.; Canon, F. Main effects of human saliva on flavour perception and the potential contribution to food consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Bolus formation and swallowing. In Food Oral Processing: Fundamentals of Eating and Sensory Perception, 1st ed.; Chen, J., Engelen, L., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 139–156. [Google Scholar]
- Feron, G. Unstimulated saliva: Background noise in taste molecules. J. Texture Stud. 2019, 50, 6–18. [Google Scholar] [CrossRef] [Green Version]
- Vijay, A.; Inui, T.; Dodds, M.; Proctor, G.; Carpenter, G. Factors that influence the extensional rheological property of saliva. PLoS ONE 2015, 10, e0135792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushpass, R.A.G.; Daly, B.; Kelly, C.; Proctor, G.; Carpenter, G.H. Altered salivary flow, protein composition, and rheology following taste and TRP stimulation in older adults. Front. Physiol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gonzalez, C.; Brule, M.; Feron, G.; Canon, F. Does interindividual variability of saliva affect the release and metabolization of aroma compounds ex vivo? The particular case of elderly suffering or not from hyposalivation. J. Texture Stud. 2019, 50, 36–44. [Google Scholar] [CrossRef] [PubMed]
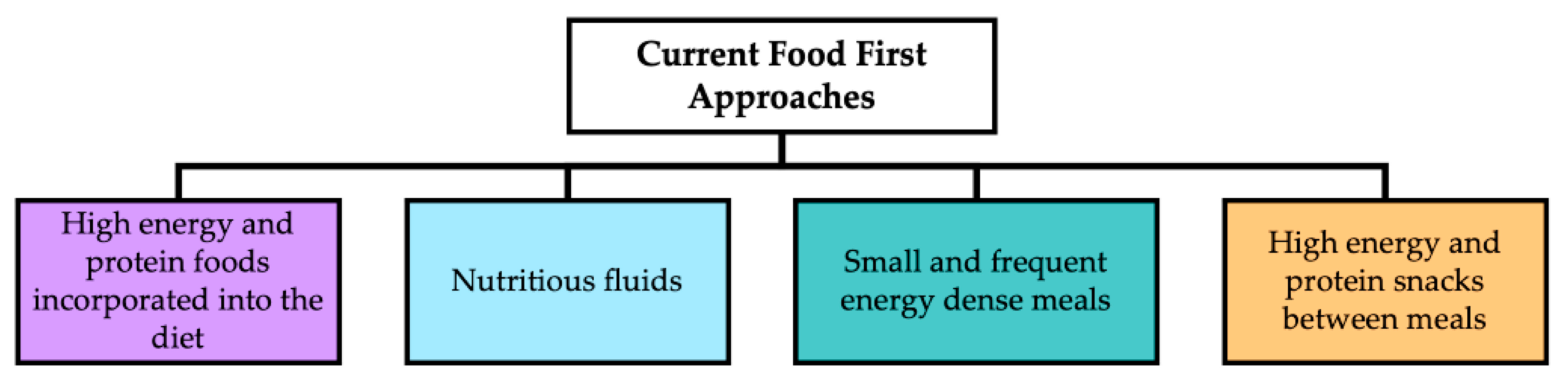
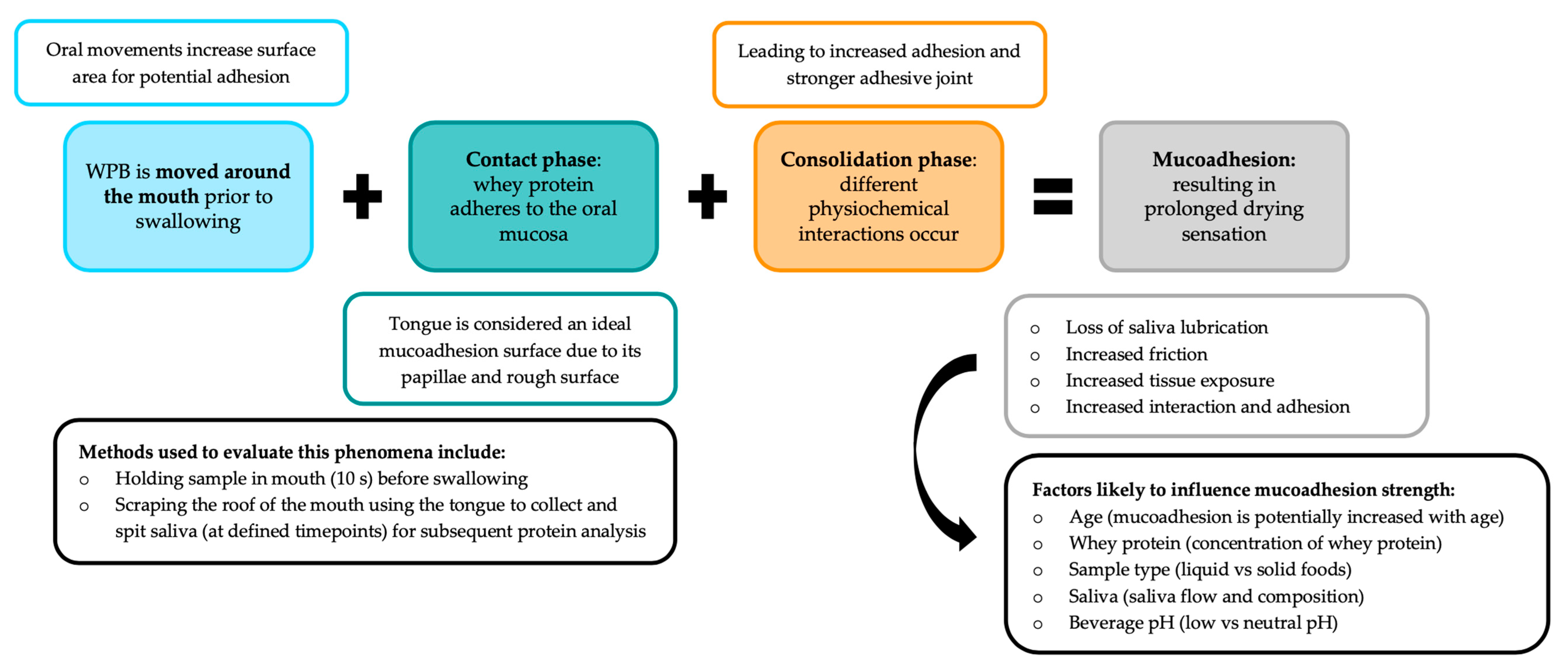
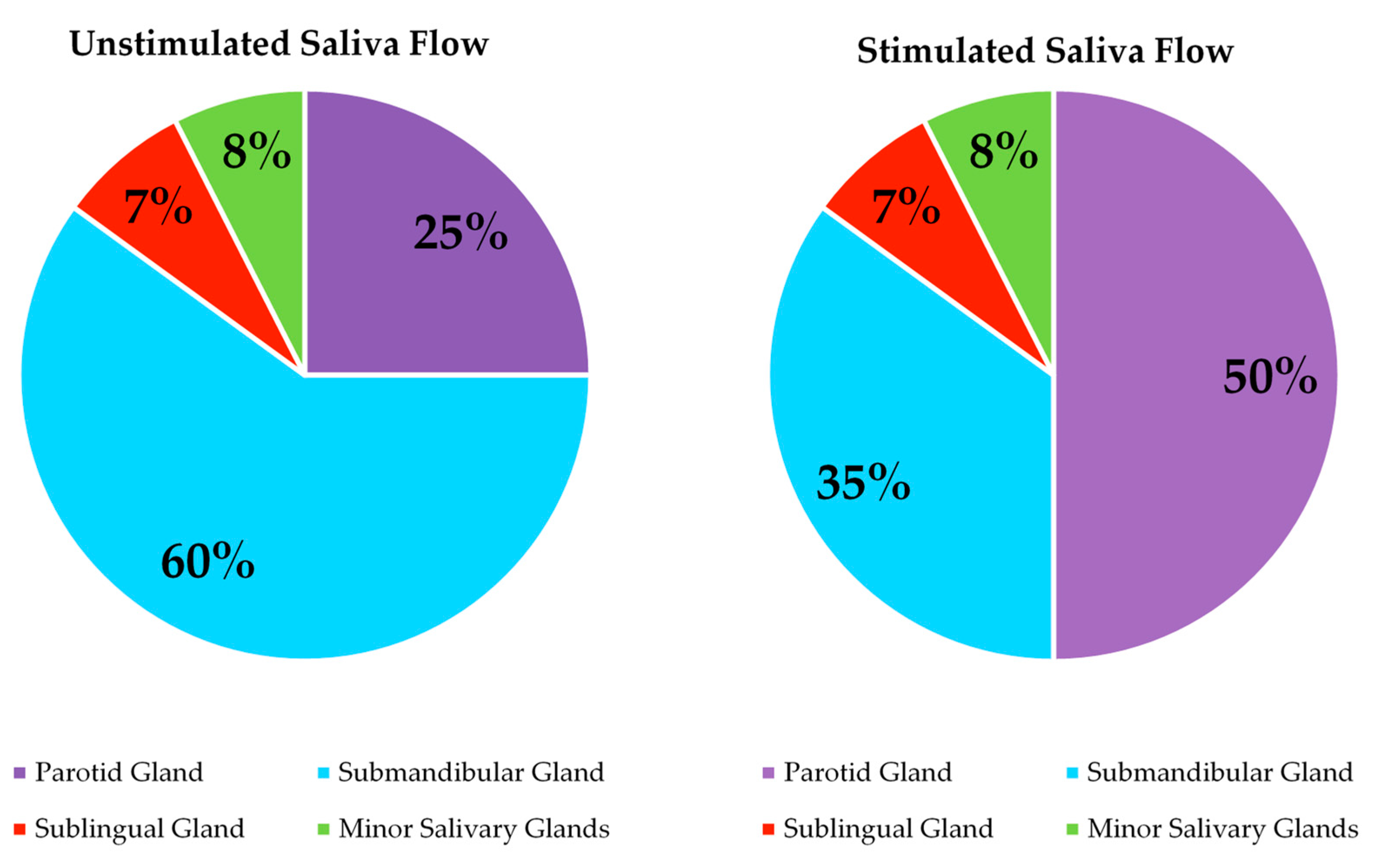
| Social | Physical | Medical | Psychological |
|---|---|---|---|
| Living and eating alone | Physical disabilities | Swallowing difficulties (dysphagia) | Anxiety |
| Poverty | Reduced appetite | Eating disorders | Depression |
| Difficulty in shopping or preparing food | Poor dentition | Medication | Dementia |
| Limited nutrition knowledge and cooking skills | Conditions leading to reduced appetite and absorption/utilisation of nutrients | Bereavement |
| Proposed Cause | WPB Model 1 | Description | Limitations |
|---|---|---|---|
| pH of WPB | WPC [86,122], WPI [124,139], WPI, β-LG and LF [125,126] |
|
|
| Saliva and protein interactions | β-LG [123], WPI [124,127], WPI, β-LG and LF [125,126] |
|
|
| Reduced lubrication from saliva | β-LG [128] |
|
|
| Adhesion and binding properties | WPC [89,134], β-LG and LF [140], WPI [130], β-LG [131] |
|
|
| Heating time | WPC [86], RW [133] |
|
|
| (a) Sensory methods commonly used to investigate whey protein-derived mouthdrying. | |||
| Method | Food Matrix | Description | Limitations |
| Sensory methods using trained panel or consumers. Key limitation: unable to explain the cause of mouthdrying | |||
| Descriptive analysis using a trained sensory panel 1,2 | Cakes and biscuits [90], WPB [83,86,91,92,123,124,125,126,140,141,142], rye bread and cream cheese [94] |
|
|
| Threshold using a trained sensory panel 1 | WPB [123,139,140] |
|
|
| Sequential profiling and time intensity methods using trained sensory panels 1 | WPB [86,122,123,124] |
|
|
| Sensory methods using consumers 1,2 | WPB [83,89,91,92,143], cakes and biscuits [90], muffins [93], rye bread and cream cheese [94] |
|
|
| 1 Refers to studies using a whey protein liquid model (whey protein beverage: WPB); 2 refers to studies using a whey protein solid model. | |||
| (b) Physiochemical analysis commonly used to investigate whey protein-derived mouthdrying. | |||
| Method | WPB Model | Description | Limitations |
| Physiochemical analysis. Key limitation: requires sensory data to provide correlations | |||
| Taste sensor 1* | WPI, PWP, aPWP [139] |
|
|
| Turbidity 1*# | β-LG [123], WPI [124], β-LG and LF 126] |
|
|
| Electrophoresis analysis 1*# | β-LG and LF [125,126] |
|
|
| Dynamic Light Scattering 1*# | WPC [86], β-LG and LF [126] |
|
|
| Zeta potential 1*# | WPC [86], β-LG and LF [126,140] |
|
|
| Portable infrared spectrometer 1* | WPI, WPC, WPH [141] |
|
|
| Tribology 1* | β-LG [128] |
|
|
| 1 Refers to studies using a whey protein beverage (WPB) model (whey protein isolate (WPI), process whey protein (PWP), acidic process whey protein (aPWP), whey protein concentration (WPC), whey protein hydrolysate (WPH), β-lactoglobulin (β-LG) and lactoferrin (LF); 2 refers to studies using a whey protein solid model; *denotes studies using a low pH WPB model; #denotes studies using a neutral pH WPB model. | |||
| (c) In vivo analysis commonly used to investigate whey protein-derived mouthdrying. | |||
| Method | WPB Model | Description | Limitations |
| In vivo analysis. Key limitation: requires sensory data to provide correlations | |||
| Saliva flow 1* | β-LG [123] |
|
|
| Animal models 1# | β-LG [131] |
|
|
| Oral retention 1# | WPC [89,134] |
|
|
| Dynamic in vivo models 1* | WPI [127] |
|
|
| 1 Refers to studies using a whey protein beverage (WPB) model (whey protein concentration (WPC), whey protein isolate (WPI), β-lactoglobulin (β-LG); 2 refers to studies using a whey protein solid model; * denotes studies using a low pH WPB model; # denotes studies using a neutral pH WPB model. | |||
| Category | Factors | Effect of Age | Effect on Mouthdrying | Food Matrix | Methodology Limitations |
|---|---|---|---|---|---|
| Physiology | Age 1* | n/a |
| WPB [89,143], cakes and biscuits [90] |
|
| Appetite 1,2*#† | ↓ |
| Cupcakes [90], ONS [87,88] |
| |
| Dental status 1,2*† | ↓ |
| Cakes and biscuits [90], meat and cereal [28] |
| |
| Saliva flow 1,2*# | ↓ |
| WPB [89,123], cakes and biscuits [90], meat and cereal [28] |
| |
| Detection thresholds to sensory stimuli 1# | ↑ |
| WPB [83,123,139,140] |
| |
| Social | Culture 2* | n/a |
| 18 different food products varying in physical properties [162] carrot, cheese and sausage [163] |
|
| Preferences | Food preference and neophobia 2‡ | No set direction |
| n/a [161,164] |
|
| Mouth behaviour 1* | Not known |
| Cakes and biscuits [90] |
|
| Proposed Mechanism | Description | Sensory Perception |
|---|---|---|
| Surface coating and wetting |
|
|
| Colloidal interactions |
|
|
| Complexation |
|
|
| Enzymatic breakdown |
|
|
| Binding of aroma compounds |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norton, V.; Lignou, S.; Methven, L. Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review. Foods 2021, 10, 433. https://doi.org/10.3390/foods10020433
Norton V, Lignou S, Methven L. Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review. Foods. 2021; 10(2):433. https://doi.org/10.3390/foods10020433
Chicago/Turabian StyleNorton, Victoria, Stella Lignou, and Lisa Methven. 2021. "Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review" Foods 10, no. 2: 433. https://doi.org/10.3390/foods10020433
APA StyleNorton, V., Lignou, S., & Methven, L. (2021). Influence of Age and Individual Differences on Mouthfeel Perception of Whey Protein-Fortified Products: A Review. Foods, 10(2), 433. https://doi.org/10.3390/foods10020433






