The Effect of Microfluidization Pressure and Tocopherol Content on the Retention of Vitamin A in Oil-In-Water Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Emulsions Preparation
2.3. Colloidal Stability
2.4. Droplet Size Distribution
2.5. Quantitative Analysis of Vitamin A by High-Performance Liquid Chromatography
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of the Emulsions
3.1.1. Colloidal Stability
3.1.2. Droplet Size Distribution
3.2. Effect of Microfluidization Process on Vitamin A Loss
3.3. Long-Term Chemical Stability of Vitamin A Encapsulated in O/W Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Sauvant, P.; Cansell, M.; Hadj Sassi, A.; Atgié, C. Vitamin A enrichment: Caution with encapsulation strategies used for food applications. Food Res. Int. 2012, 46, 469–479. [Google Scholar] [CrossRef]
- Yoshida, K.; Sekine, T.; Matsuzaki, F.; Yanaki, T.; Yamaguchi, M. Stability of vitamin A in oil-in-water-in-oil-type multiple emulsions. J. Am. Oil Chem. Soc. 1999, 76, 1–6. [Google Scholar] [CrossRef]
- Semenova, E.; Cooper, A.; Wilson, C.; Converse, C. Stabilization of All-trans-retinol by Cyclodextrins: A Comparative Study Using HPLC and Fluorescence Spectroscopy. J. Incl. Phenom. 2002, 44, 155–158. [Google Scholar] [CrossRef]
- Jafari, S.M.; Sedaghat Doost, A.; Nikbakht Nasrabadi, M.; Boostani, S.; Van der Meeren, P. Phytoparticles for the stabilization of Pickering emulsions in the formulation of novel food colloidal dispersions. Trends Food Sci. Technol. 2020, 98, 117–128. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Tong, Q.; Karim, A.; Mahdi, A.A.; Iqbal, M.W.; Aadil, R.M.; Ali, A.; Manzoor, M.F. Role of peppermint oil in improving the oxidative stability and antioxidant capacity of borage seed oil-loaded nanoemulsions fabricated by modified starch. Int. J. Biol. Macromol. 2020, 153, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.R.; Lim, S.J.; Park, J.S.; Kim, C.K. Phospholipid-based microemulsion formulation of all-trans-retinoic acid for parenteral administration. Int. J. Pharm 2004, 276, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tanglao, E.; Kumar, A.; Noriega, R.; Punzalan, M.E.; Marcelo, P. Development and physico-chemical characterization of virgin coconut oil-in-water emulsion using polymerized whey protein as emulsifier for Vitamin A delivery. Matec Web Conf. 2019, 268, 01002. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll. 2008, 22, 1191–1202. [Google Scholar] [CrossRef]
- Mahdi Jafari, S.; He, Y.; Bhandari, B. Nano-Emulsion Production by Sonication and Microfluidization—A Comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; McClements, D.J. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010, 24, 560–569. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Effectiveness of encapsulating biopolymers to produce sub-micron emulsions by high energy emulsification techniques. Food Res. Int. 2007, 40, 862–873. [Google Scholar] [CrossRef]
- Hebishy, E.; Buffa, M.; Guamis, B.; Blasco-Moreno, A.; Trujillo, A.-J. Physical and oxidative stability of whey protein oil-in-water emulsions produced by conventional and ultra high-pressure homogenization: Effects of pressure and protein concentration on emulsion characteristics. Innov. Food Sci. Emerg. Technol. 2015, 32, 79–90. [Google Scholar] [CrossRef]
- Mao, L.; Yang, J.; Xu, D.; Yuan, F.; Gao, Y. Effects of Homogenization Models and Emulsifiers on the Physicochemical Properties of β-Carotene Nanoemulsions. J. Dispers. Sci. Technol. 2010, 31, 986–993. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, Y.; Zhao, J.; Mao, L. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res. Int. 2008, 41, 61–68. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lefèvre, T.; Subirade, M.; Paquin, P. Effects of ultra-high pressure homogenization on the properties and structure of interfacial protein layer in whey protein-stabilized emulsion. Food Chem. 2009, 113, 191–195. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Preparation of curcumin-loaded emulsion using high pressure homogenization: Impact of oil phase and concentration on physicochemical stability. LWT 2017, 84, 34–46. [Google Scholar] [CrossRef]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; McClements, D.J. Vitamin E bioaccessibility: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Encapsulation of vitamin E in edible emulsions fabricated using a natural surfactant. Food Hydrocoll. 2013, 30, 712–720. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of vitamin E-enriched nanoemulsions: Factors affecting particle size using spontaneous emulsification. J. Colloid Interface Sci. 2013, 391, 95–102. [Google Scholar] [CrossRef]
- Raikos, V. Encapsulation of vitamin E in edible orange oil-in-water emulsion beverages: Influence of heating temperature on physicochemical stability during chilled storage. Food Hydrocoll. 2017, 72, 155–162. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; McClements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Golfomitsou, I.; Mitsou, E.; Xenakis, A.; Papadimitriou, V. Development of food grade O/W nanoemulsions as carriers of vitamin D for the fortification of emulsion based food matrices: A structural and activity study. J. Mol. Liq. 2018, 268, 734–742. [Google Scholar] [CrossRef]
- Ozturk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability: Nanoemulsion delivery systems for lipophilic vitamins. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical properties of high-pressure treated lentil protein-based nanoemulsions. LWT 2019, 101, 590–598. [Google Scholar] [CrossRef]
- McClements, D.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity; Taylor & Francis: Oxfordshire, UK, 2011; Volume 51, pp. 285–330. [Google Scholar]
- Jacobsen, C.; Let, M.B.; Nielsen, N.S.; Meyer, A.S. Antioxidant strategies for preventing oxidative flavour deterioration of foods enriched with n-3 polyunsaturated lipids: A comparative evaluation. Trends Food Sci. Technol. 2008, 19, 76–93. [Google Scholar] [CrossRef] [Green Version]
- Bush, L.; Stevenson, L.; Lane, K.E. The oxidative stability of omega-3 oil-in-water nanoemulsion systems suitable for functional food enrichment: A systematic review of the literature. Crit. Rev. Food Sci. Nutr. 2019, 59, 1154–1168. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.; McClements, D. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2014, 6, 41–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrentino, G.; Ramezani, M.; Morozova, K.; Hafner, D.; Pedri, U.; Pixner, K.; Scampicchio, M. Fining of Red Wine Monitored by Multiple Light Scattering. J. Agric. Food Chem. 2017, 65, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Gatti, R.; Gioia, M.G.; Cavrini, V. Analysis and stability study of retinoids in pharmaceuticals by LC with fluorescence detection. J. Pharm. Biomed. Anal. 2000, 23, 147–159. [Google Scholar] [CrossRef]
- Horn, A.F.; Nielsen, N.S.; Jensen, L.S.; Horsewell, A.; Jacobsen, C. The choice of homogenisation equipment affects lipid oxidation in emulsions. Food Chem. 2012, 134, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.F.; Barouh, N.; Nielsen, N.S.; Baron, C.P.; Jacobsen, C. Homogenization Pressure and Temperature Affect Protein Partitioning and Oxidative Stability of Emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1541–1550. [Google Scholar] [CrossRef] [Green Version]
- Bouaouina, H.; Desrumaux, A.; Loisel, C.; Legrand, J. Functional properties of whey proteins as affected by dynamic high-pressure treatment. Int. Dairy J. 2006, 16, 275–284. [Google Scholar] [CrossRef]
- Kim, H.J.; Decker, E.A.; McClements, D.J. Influence of Protein Concentration and Order of Addition on Thermal Stability of β-Lactoglobulin Stabilized n-Hexadecane Oil-in-Water Emulsions at Neutral pH. Langmuir 2005, 21, 134–139. [Google Scholar] [CrossRef]
- Sørensen, A.-D.; Baron, C.; Let, M.; Brüggemann, D.; Pedersen, L.; Jacobsen, C. Homogenization Conditions Affect the Oxidative Stability of Fish Oil Enriched Milk Emulsions: Oxidation Linked to Changes in Protein Composition at the Oil−Water Interface. J. Agric. Food Chem. 2007, 55, 1781–1789. [Google Scholar] [CrossRef]
- Let, M.B.; Jacobsen, C.; Sørensen, A.-D.M.; Meyer, A.S. Homogenization Conditions Affect the Oxidative Stability of Fish Oil Enriched Milk Emulsions: Lipid Oxidation. J. Agric. Food Chem. 2007, 55, 1773–1780. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barnaba, C.; Barbosa-Cánovas, G.V. Effects of high pressure processing on lipid oxidation: A review. Innov. Food Sci. Emerg. Technol. 2014, 22, 1–10. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cunha, R.L. Flaxseed oil—Whey protein isolate emulsions: Effect of high pressure homogenization. J. Food Eng. 2012, 111, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Atarés, L.; Marshall, L.J.; Akhtar, M.; Murray, B.S. Structure and oxidative stability of oil in water emulsions as affected by rutin and homogenization procedure. Food Chem. 2012, 134, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Flores, D.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef]
- Berton-Carabin, C.; Ropers, M.H.; Genot, C. Lipid Oxidation in Oil-in-Water Emulsions: Involvement of the Interfacial Layer. Compr. Rev. Food Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Jana, A.K.; Agarwal, S.; Chatterjee, S.N. Membrane lipid peroxidation by ultrasound: Mechanism and implications. J. Biosci. 1990, 15, 211–215. [Google Scholar] [CrossRef]
- Mao, L.; Yang, J.; Yuan, F.; Gao, Y.; Zhao, J. Effects of Small and Large Molecule Emulsifiers on the Characteristics of b-Carotene Nanoemulsions Prepared by High Pressure Homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Nakaya, K.; Ushio, H.; Matsukawa, S.; Shimizu, M.; Ohshima, T. Effects of droplet size on the oxidative stability of oil-in-water emulsions. Lipids 2005, 40, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.M.; Sasaki, S.; McClements, D.; Decker, E.A. Antioxidant Activity of Whey in a Salmon Oil Emulsion. J. Food Sci. 2000, 65, 1325–1329. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Lethuaut, L.; Métro, F.; Genot, C. Effect of droplet size on lipid oxidation rates of oil-in-water emulsions stabilized by protein. J. Am. Oil Chem. Soc. 2002, 79, 425–430. [Google Scholar] [CrossRef]
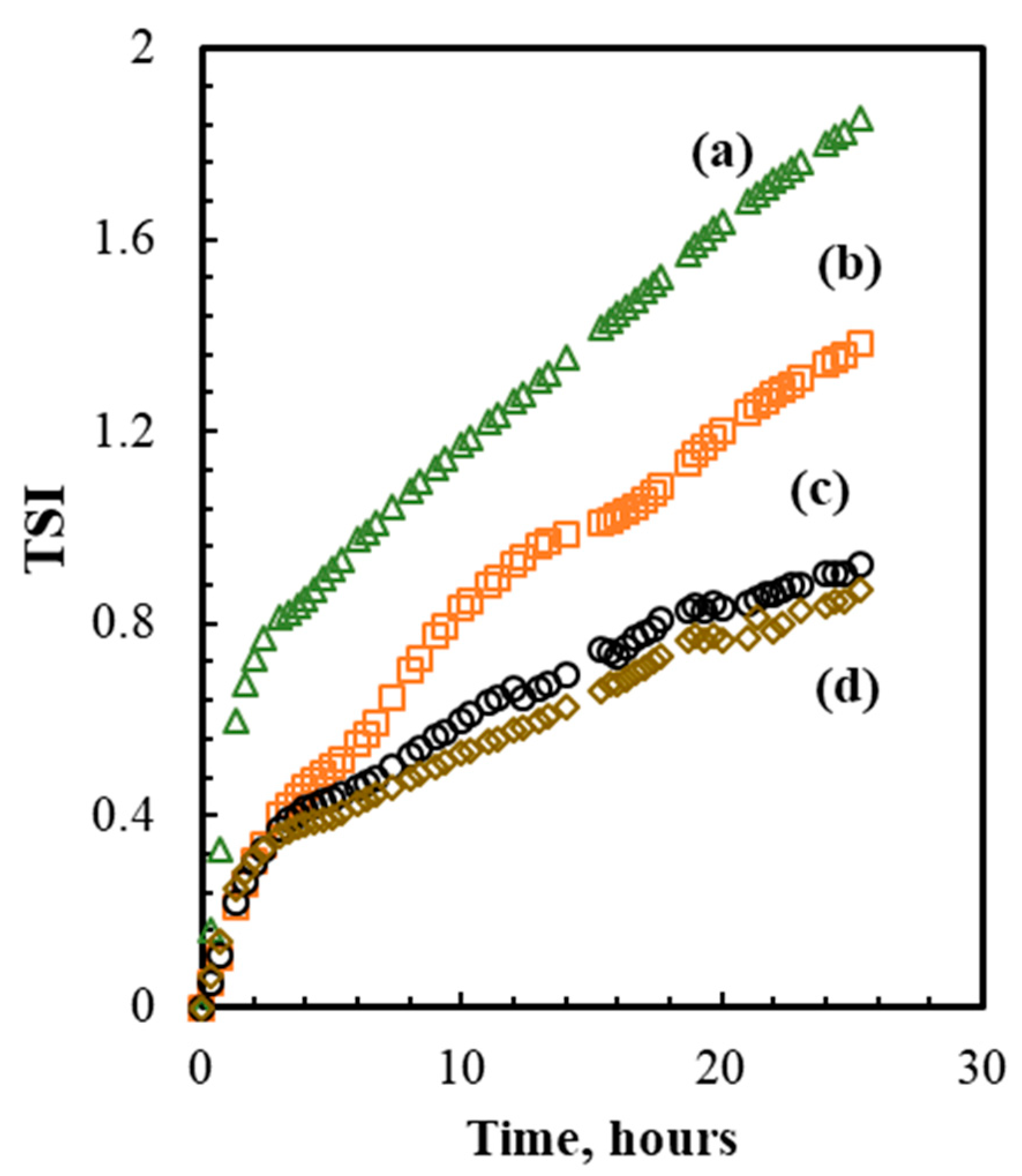
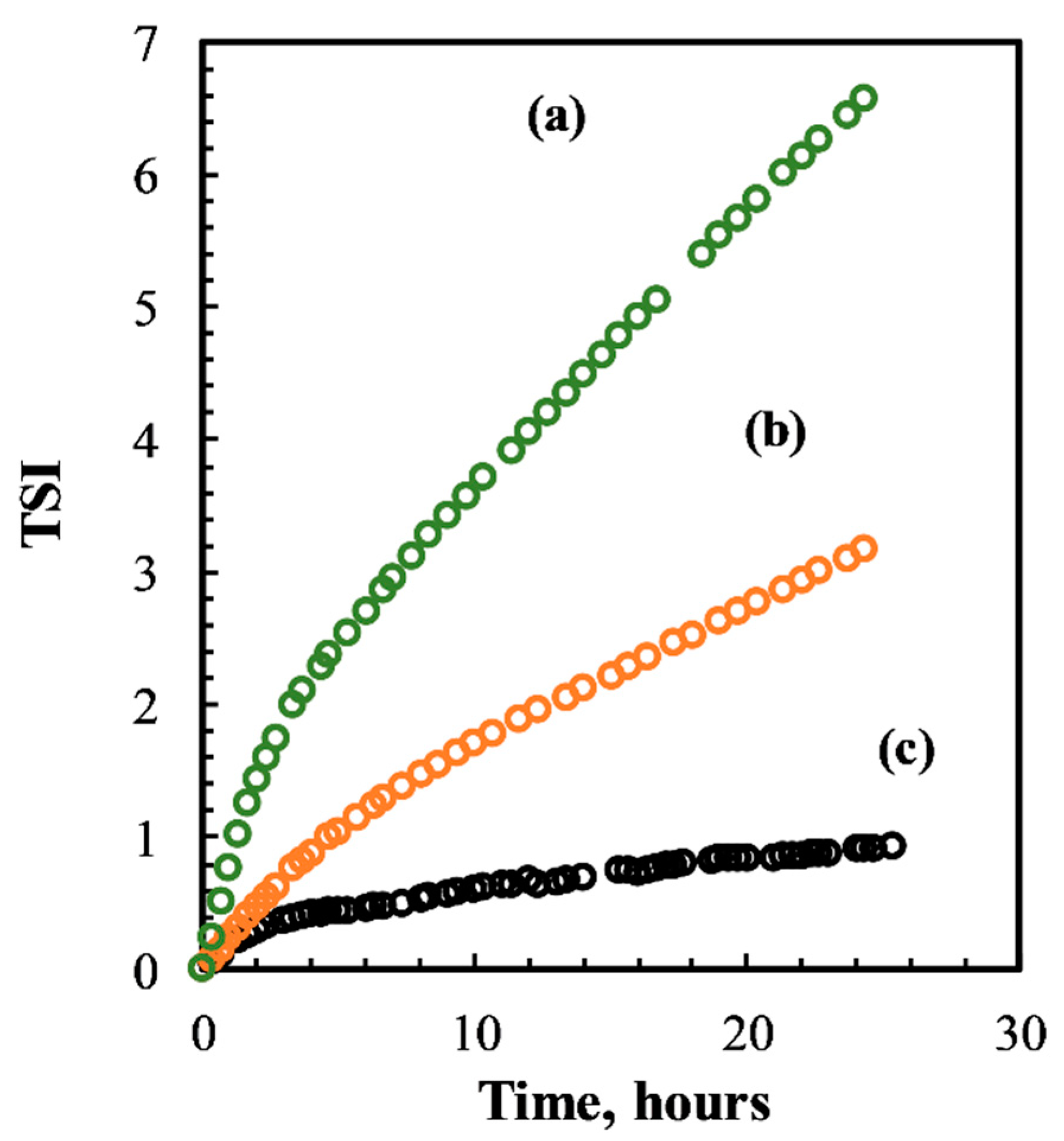
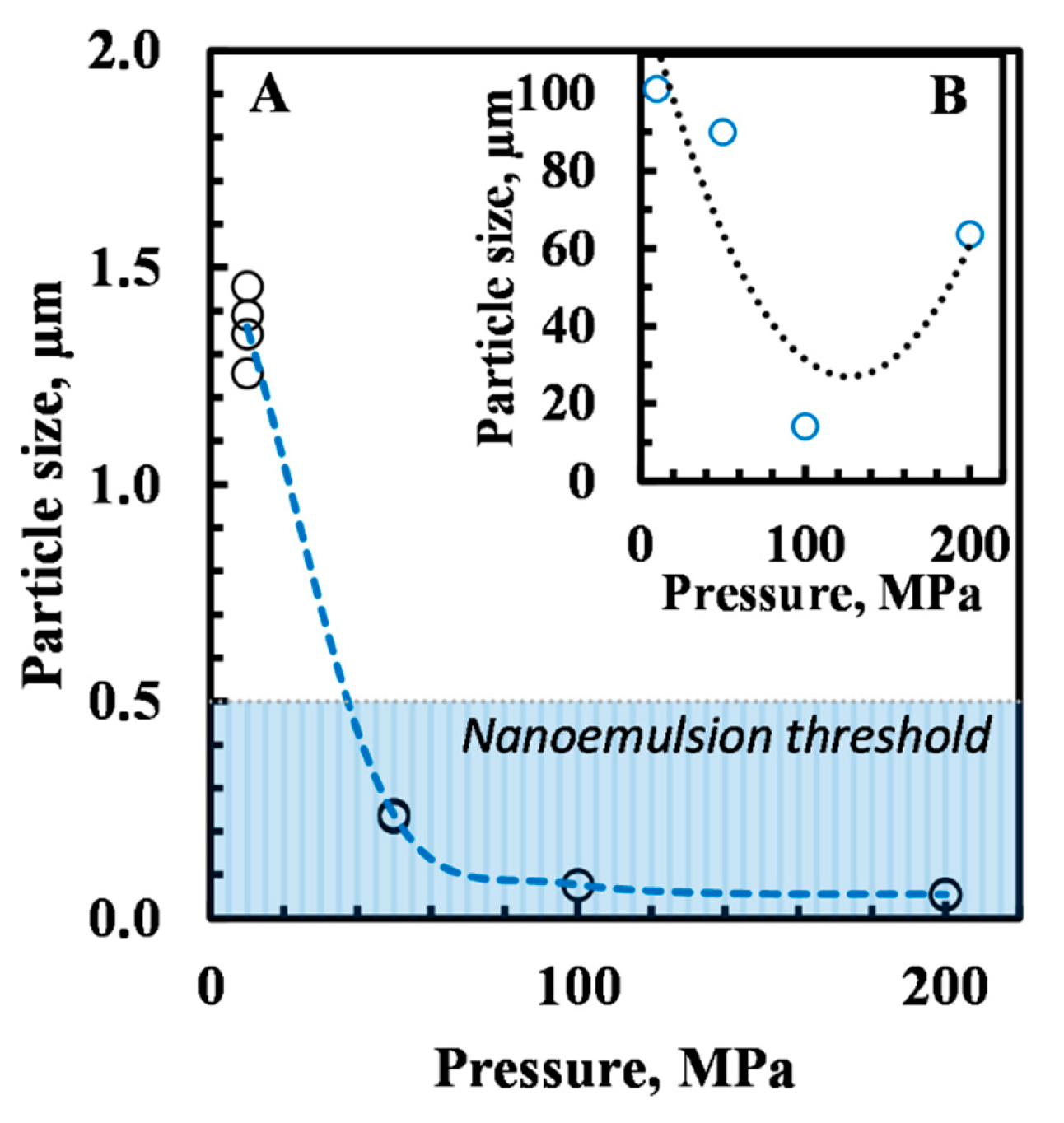
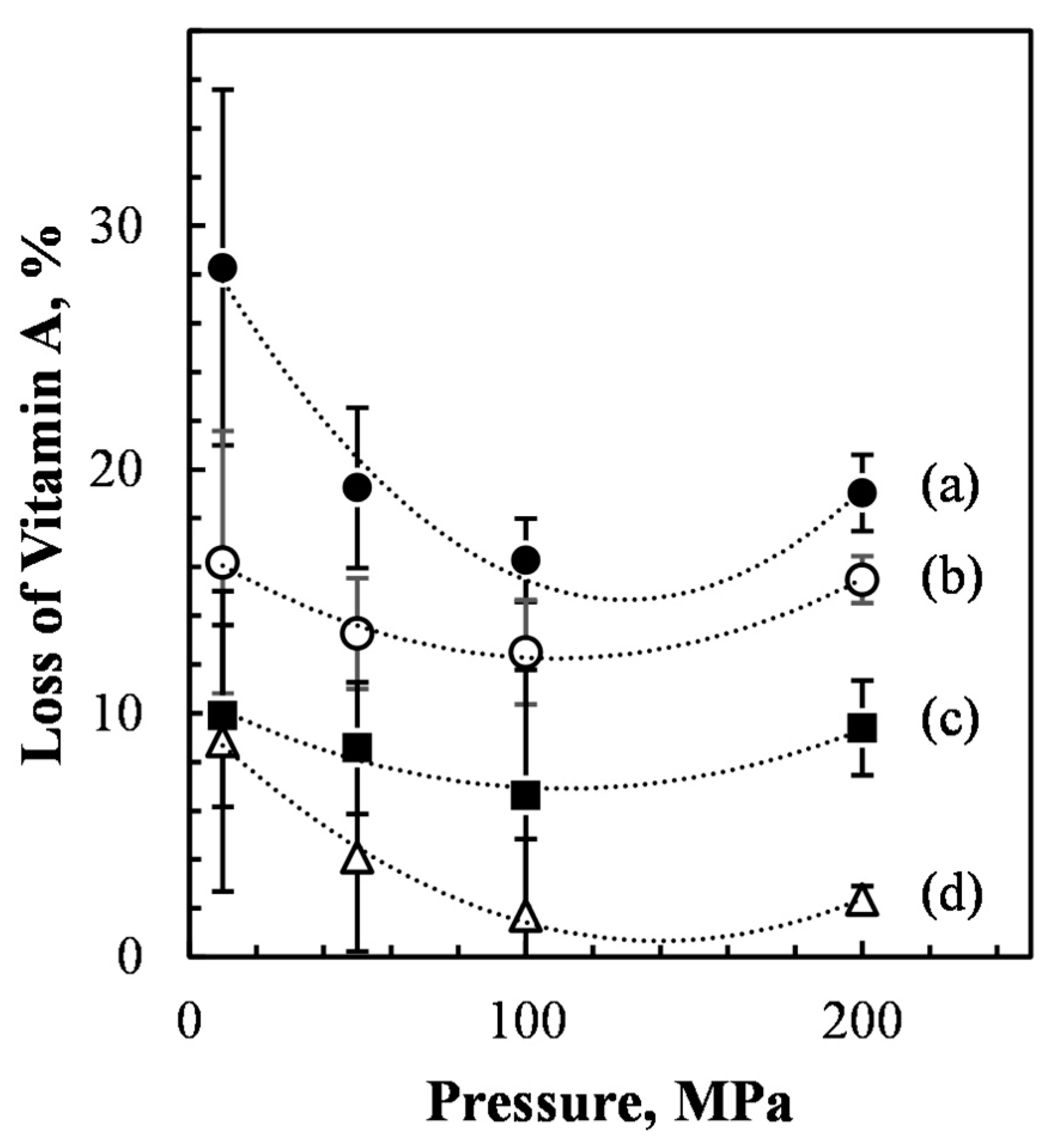
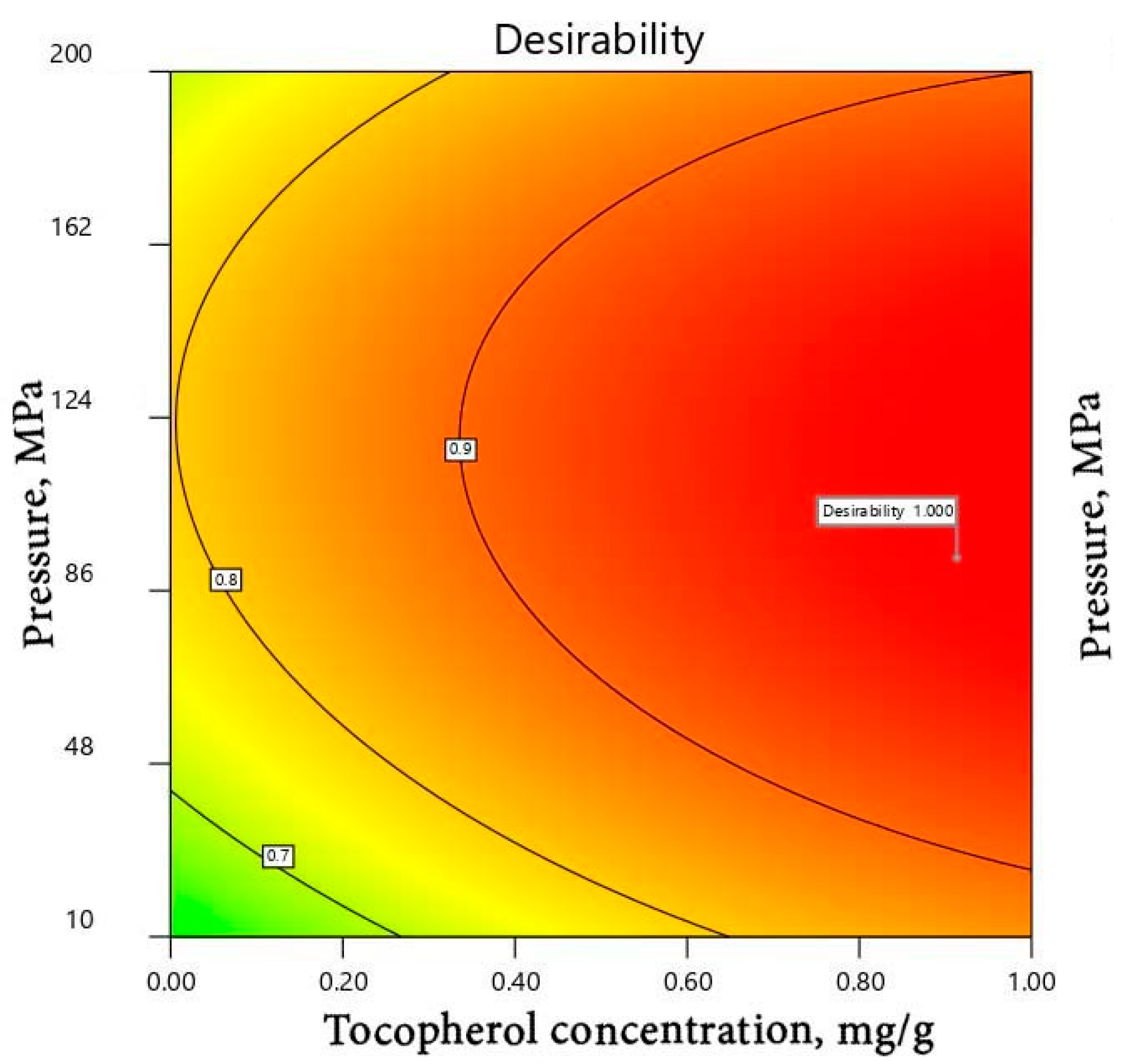
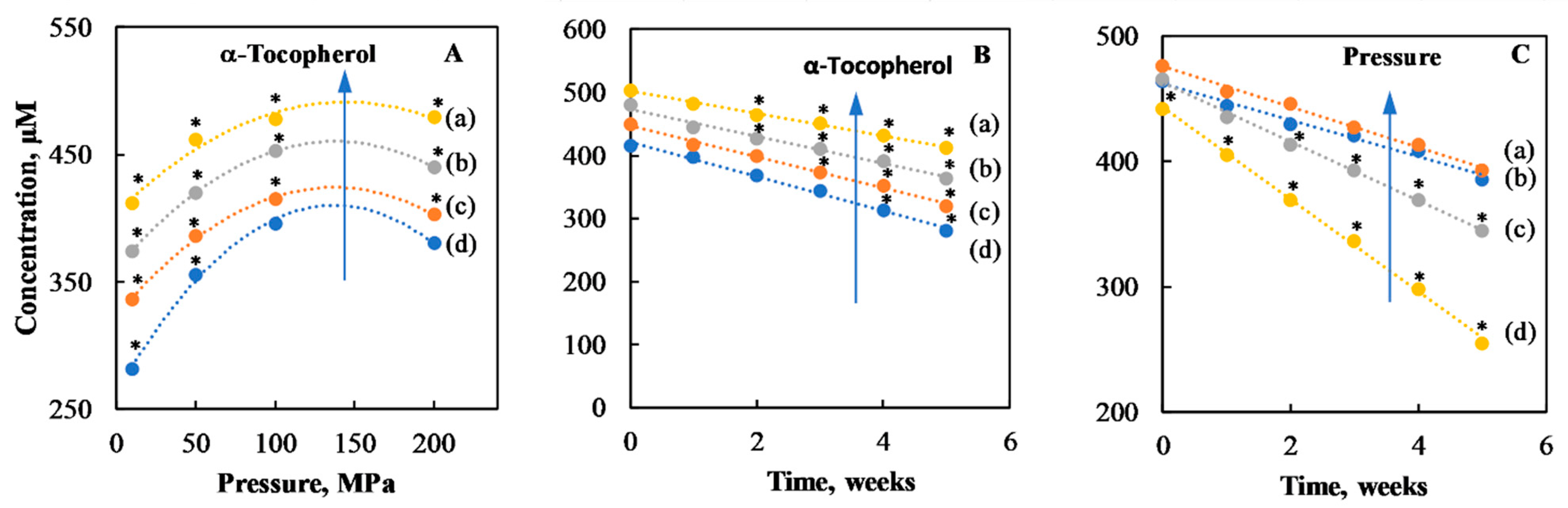
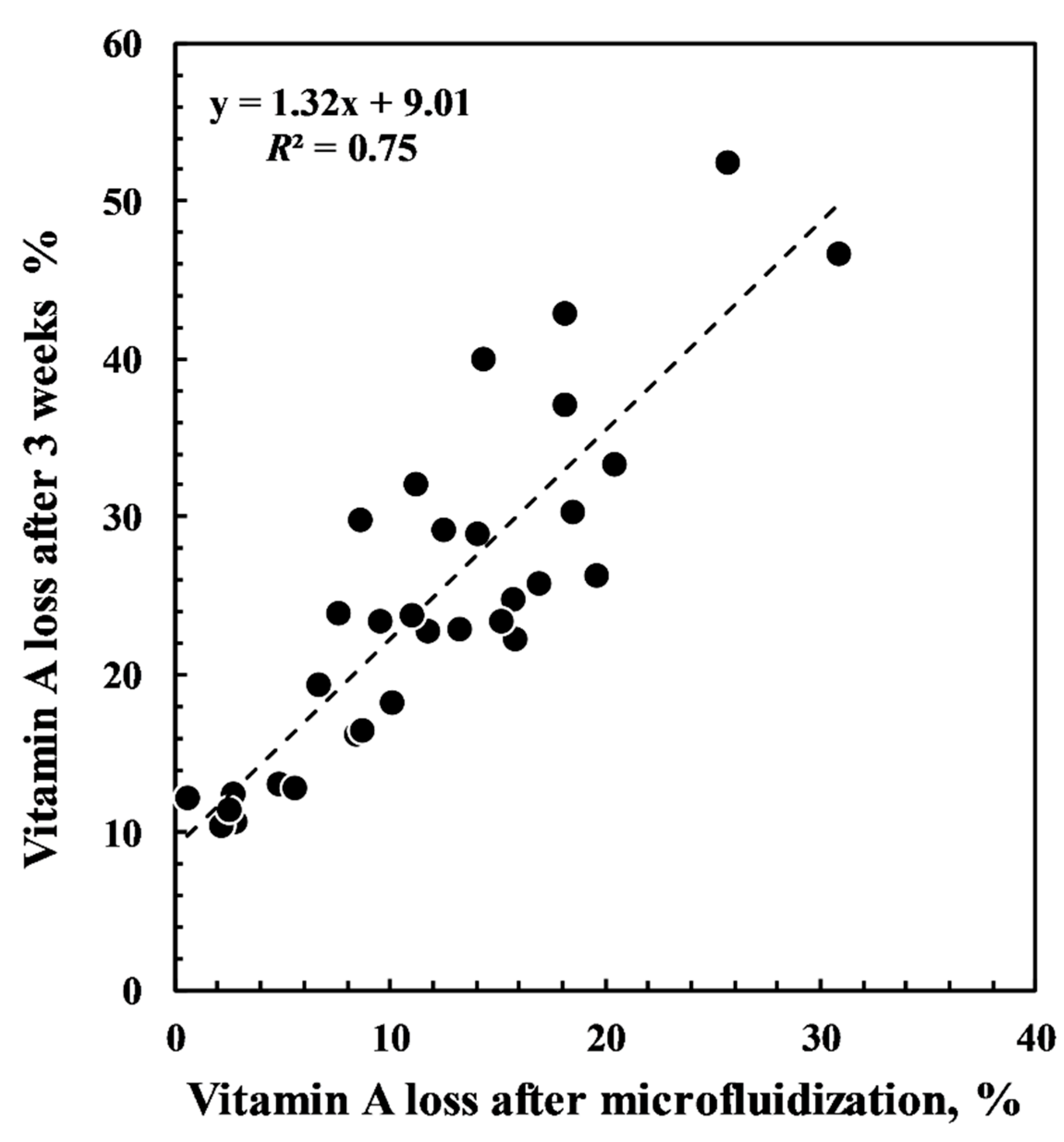
| Time (Weeks) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | T | 0 | 1 | 2 | 3 | 4 | 5 | K | r2 |
| MPa | mg/g | µM | µM | µM | µM | µM | µM | µM/day | |
| 10 | 0.00 | 377 ± 19 | 357 ± 24 | 319 ± 26 | 265 ± 21 | 210 ± 14 | 167 ± 24 | 44 ± 12 | 0.982 |
| 50 | 0.00 | 424 ± 9 | 399 ± 1 | 372 ± 17 | 340 ± 14 | 315 ± 7 | 282 ± 10 | 28 ± 3 | 0.998 |
| 100 | 0.00 | 440 ± 4 | 423 ± 1 | 411 ± 6 | 393 ± 4 | 370 ± 14 | 340 ± 14 | 19 ± 6 | 0.975 |
| 200 | 0.00 | 425 ± 4 | 410 ± 3 | 374 ± 12 | 376 ± 15 | 361 ± 16 | 335 ± 7 | 17 ± 9 | 0.941 |
| 10 | 0.25 | 440 ± 14 | 392 ± 1 | 363 ± 13 | 340 ± 13 | 289 ± 15 | 226 ± 5 | 40 ± 14 | 0.974 |
| 50 | 0.25 | 455 ± 6 | 418 ± 2 | 398 ± 6 | 373 ± 1 | 347 ± 4 | 328 ± 15 | 25 ± 5 | 0.991 |
| 100 | 0.25 | 459 ± 6 | 439 ± 1 | 419 ± 7 | 405 ± 1 | 399 ± 1 | 367 ± 6 | 17 ± 6 | 0.971 |
| 200 | 0.25 | 444 ± 3 | 418 ± 3 | 417 ± 5 | 405 ± 4 | 376 ± 5 | 359 ± 0 | 16 ± 8 | 0.946 |
| 10 | 0.50 | 473 ± 10 | 422 ± 9 | 382 ± 3 | 383 ± 8 | 336 ± 12 | 290 ± 6 | 33 ± 14 | 0.960 |
| 50 | 0.50 | 480 ± 7 | 435 ± 14 | 413 ± 1 | 401 ± 2 | 381 ± 2 | 339 ± 11 | 25 ± 10 | 0.963 |
| 100 | 0.50 | 490 ± 14 | 469 ± 9 | 465 ± 14 | 448 ± 12 | 432 ± 5 | 412 ± 16 | 15 ± 4 | 0.981 |
| 200 | 0.50 | 476 ± 5 | 454 ± 5 | 446 ± 14 | 434 ± 6 | 434 ± 2 | 396 ± 9 | 13 ± 9 | 0.898 |
| 10 | 1.00 | 479 ± 16 | 451 ± 17 | 419 ± 13 | 412 ± 17 | 376 ± 4 | 336 ± 8 | 27 ± 9 | 0.976 |
| 50 | 1.00 | 504 ± 10 | 491 ± 11 | 470 ± 5 | 459 ± 2 | 435 ± 10 | 420 ± 3 | 17 ± 3 | 0.993 |
| 100 | 1.00 | 516 ± 8 | 492 ± 7 | 488 ± 2 | 465 ± 6 | 453 ± 11 | 444 ± 3 | 14 ± 5 | 0.973 |
| 200 | 1.00 | 513 ± 1 | 497 ± 9 | 483 ± 4 | 468 ± 4 | 463 ± 4 | 453 ± 4 | 12 ± 4 | 0.975 |
| Analysis of variance with the model: Yijkl = Ajkl + Bikl + Cijl + ABkl + ACjl + BCil + ABCl + Eijkl | |||||
| Source | SS | df | MS | F | Sig. |
| A 1 | 290269 | 3 | 96756 | 964 | 0.001 |
| B | 188085 | 3 | 62695 | 625 | 0.001 |
| C | 291593 | 5 | 58318 | 581 | 0.001 |
| AB | 13988 | 9 | 1554 | 15 | 0.001 |
| AC | 8866 | 15 | 591 | 5 | 0.001 |
| BC | 41265 | 15 | 2751 | 27 | 0.001 |
| ABC | 5987 | 45 | 133 | 1 | 0.001 |
| Error | 9629 | 96 | 100 | ||
| Total | 849685 | 191 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. The Effect of Microfluidization Pressure and Tocopherol Content on the Retention of Vitamin A in Oil-In-Water Emulsions. Foods 2021, 10, 504. https://doi.org/10.3390/foods10030504
Banasaz S, Morozova K, Ferrentino G, Scampicchio M. The Effect of Microfluidization Pressure and Tocopherol Content on the Retention of Vitamin A in Oil-In-Water Emulsions. Foods. 2021; 10(3):504. https://doi.org/10.3390/foods10030504
Chicago/Turabian StyleBanasaz, Shahin, Ksenia Morozova, Giovanna Ferrentino, and Matteo Scampicchio. 2021. "The Effect of Microfluidization Pressure and Tocopherol Content on the Retention of Vitamin A in Oil-In-Water Emulsions" Foods 10, no. 3: 504. https://doi.org/10.3390/foods10030504








