Technological Applications of Natural Colorants in Food Systems: A Review
Abstract
:1. Introduction
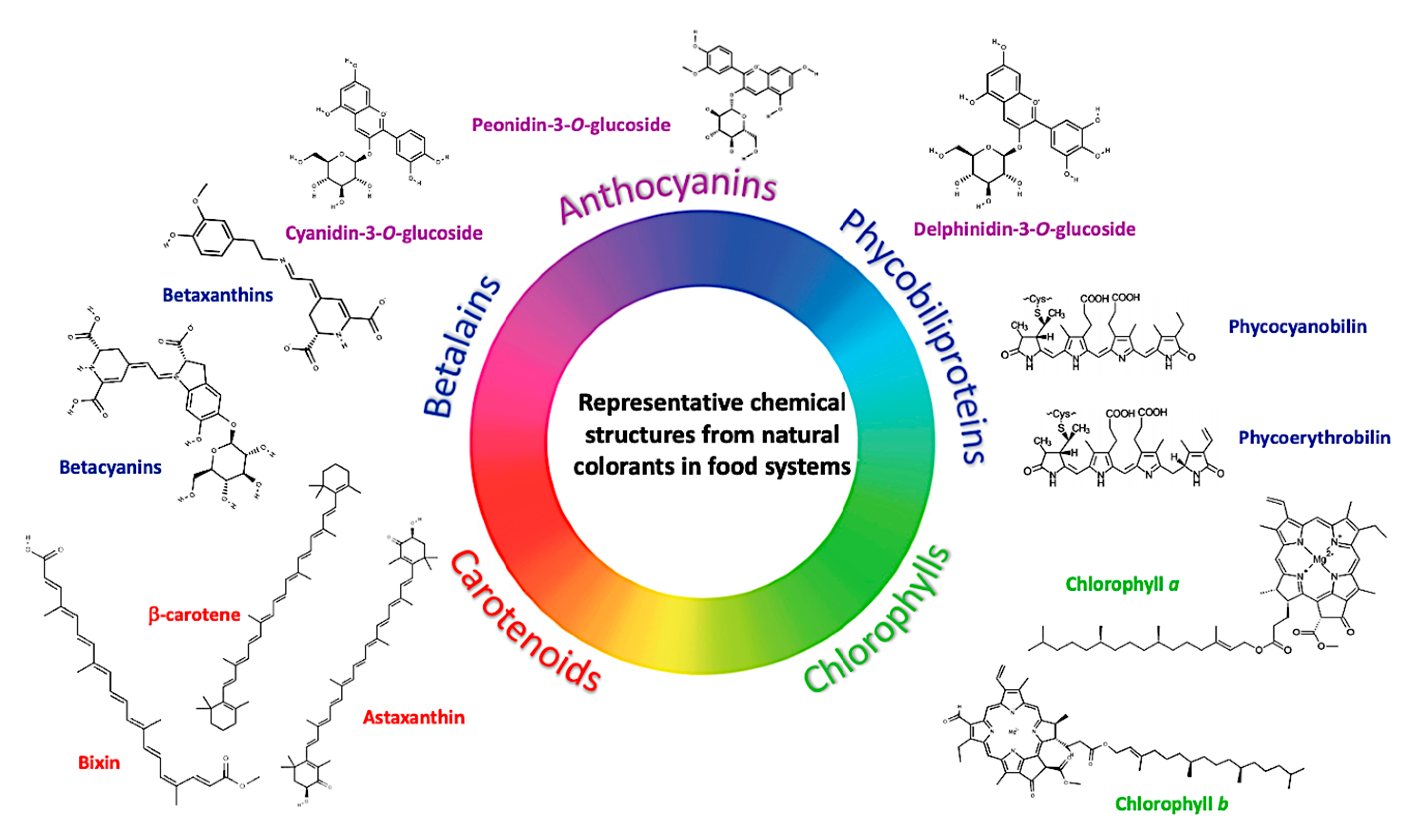
2. Natural Pigments Used in the Food Industry
2.1. Anthocyanins (ANC)
2.2. Carotenoids
2.3. Betalains
2.4. Other Pigments Potential Used in the Food Industry
3. Industrial Methods of Production
3.1. Electric Field-Based Technologies
3.2. Ohmic Heating
3.3. Pulsed Electric Fields (PEF)
3.4. High-Pressure-Assisted Extraction (HPE)
3.5. Supercritical Fluid Extraction (SFE)
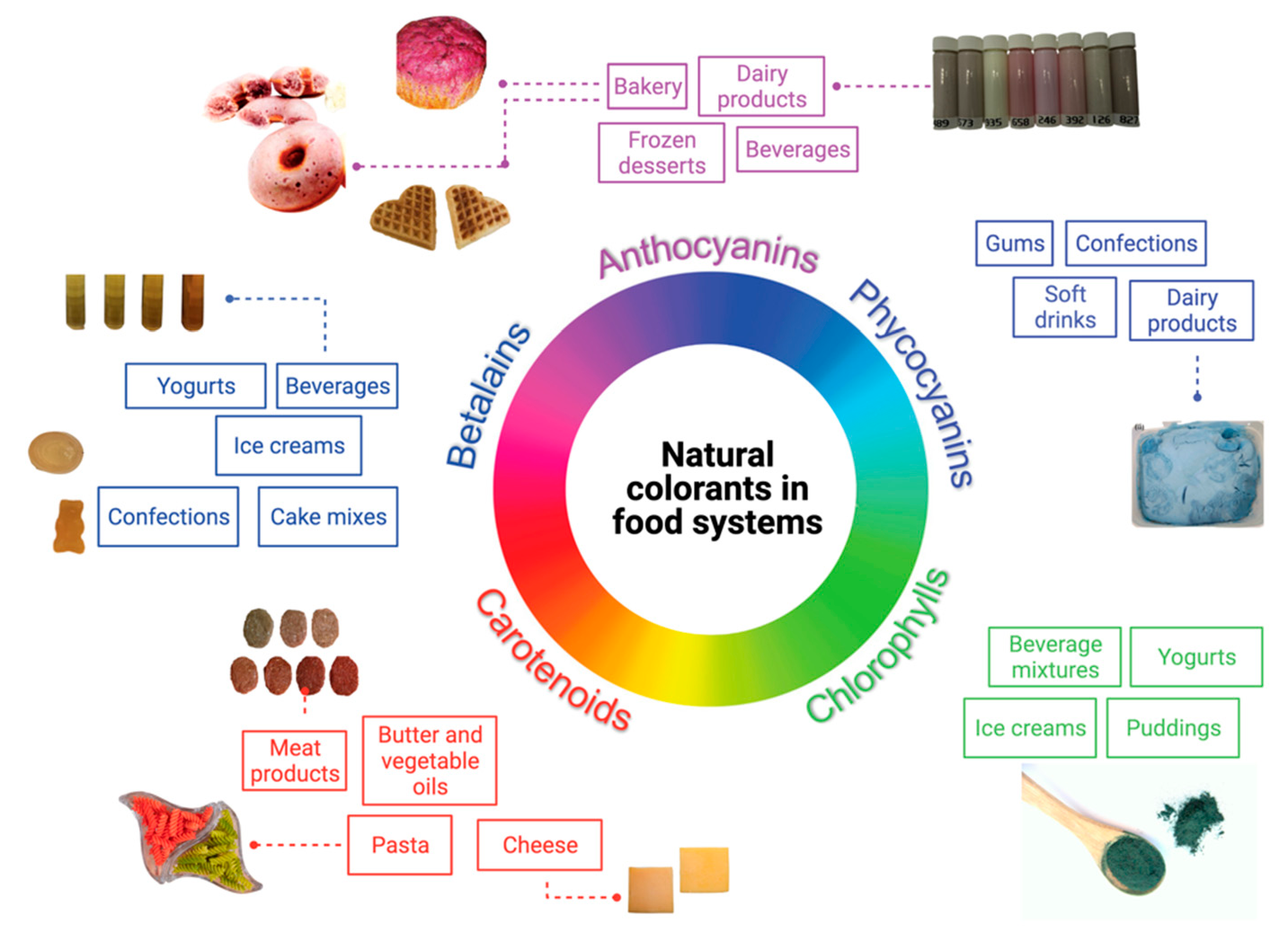
4. Technological Properties of Natural Food Colorants in Food Systems
4.1. Bakery Products
4.2. Beverages
4.3. Confectionery
4.4. Milk, Dairy, and Dairy-Like Products
4.5. Meat and Meat Products
4.6. Other Food Products
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ΔE | Color difference against control samples, in the L*C*h* color space |
| a* | Redness color change in the CIEL*a*b* space |
| ABTS | 2,2-azino-bis(ethylbenzothiazoline-6-sulfonic acid) |
| aw | Activity of water |
| ANC | Anthocyanins |
| b* | Yellowness color change in the CIEL*a*b* space |
| C* | Chroma |
| C3G | Cyanidin-3-O-glucoside |
| C3G-Mal | Cyanidin-3-(6′-malonylglucoside) |
| Caco-2 | Human colorectal adenocarcinoma cells |
| cfu | Colony forming units |
| D3G | Delphinidin-3-O-glucoside |
| DM | Dry matter |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC3G/L | Equivalents of C3G |
| EC50 | Half-maximal effective concentration |
| EF | Electric field |
| EGCG | Epigallocatechin gallate |
| FRAP | Ferric ion reducing antioxidant power |
| FT-IR | Fourier Transform Infrared |
| GAE | Gallic acid equivalents |
| GC | Gas chromatography |
| GRAS | Generally recognized as safe |
| HepG2 | Human hepatocellular carcinoma cells |
| HHPE | High hydrostatic pressure extraction |
| HPE | High-pressure-assisted extraction |
| IAT | Inlet air temperature |
| L* | Luminosity or lightness in the CIEL*a*b* space |
| MBC | Minimum bactericidal concentration |
| MCF-7 | Human mammary gland/breast adenocarcinoma cells (derived from metastatic site) |
| MDA | Malonaldehyde |
| MDA-MB-231 | Triple negative human mammary gland/breast adenocarcinoma cells |
| MIC | Minimum inhibitory concentration |
| OAT | Outlet air temperature |
| OH | Ohmic heating |
| P3G | Peonidin-3-(6′-malonylglucoside) |
| P3G-Mal | Pelargonidin-3-(6′-malonylglucoside) |
| PEF | Pulsed electric fields |
| Pr3G | Pelargonidin-3-O-glucoside |
| RSA | Radical scavenging activity (%) |
| SC-CO2 | Supercritical carbon dioxide |
| SFC | Supercritical fluid chromatography |
| SFE | Supercritical fluid extraction |
| TAC | Total anthocyanin content |
| TBARS | Thiobarbituric acid reactive substances |
| TEAC | Trolox equivalent antioxidant capacity |
| TPC | Total phenolic compounds |
| VPR | Vine-pruning residues |
| XR | X-ray |
References
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal Pigments: A Source of Natural Food Colors. In Microalgae Biotechnology for Food, Health and High Value Products; Asraful Alam, M., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 81–123. [Google Scholar]
- Albuquerque, B.R.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2020, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food colors: Existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Lin, W.-S.; He, P.H.; Chau, C.-F.; Liou, B.-K.; Li, S.; Pan, M.-H. The feasibility study of natural pigments as food colorants and seasonings pigments safety on dried tofu coloring. Food Sci. Hum. Wellness 2018, 7, 220–228. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas Šaponjac, V. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res. Int. 2015, 76, 761–768. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 867–901. [Google Scholar]
- Potera, C. Diet and nutrition: The Artificial Food Dye Blues. Environ. Health Perspect. 2010, 118, A428. [Google Scholar] [CrossRef] [Green Version]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; Routledge: Boca Raton, FL, USA, 2018. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- de Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef]
- Food-Info E163: Anthocyanins. Available online: http://www.food-info.net/uk/colour/anthocyanin.htm (accessed on 20 February 2020).
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vital, D.; Weiss, M.; Gonzalez de Mejia, E. Anthocyanins from purple corn ameliorated Tumor Necrosis Factor-α-induced inflammation and insulin resistance in 3T3-L1 adipocytes via activation of insulin signaling and enhanced GLUT4 translocation. Mol. Nutr. Food Res. 2017, 61, 1–13. [Google Scholar] [CrossRef]
- Arango-Varela, S.S.; Luzardo-Ocampo, I.; Maldonado-Celis, M.E.; Campos-Vega, R. Andean berry (Vaccinium meridionale Swartz) juice in combination with Aspirin modulated anti-inflammatory markers on LPS-stimulated RAW 264.7 macrophages. Food Res. Int. 2020, 137, 109541. [Google Scholar] [CrossRef] [PubMed]
- Eletr, A.A.; Siliha, H.A.E.; Elshobargy, G.A.; Galal, G.A. Evaluation of lycopene extracted from tomato processing waste as a natural antioxidant in some bakery products. Zagazig J. Agric. Res. 2017, 44, 1389–1401. [Google Scholar]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [Green Version]
- Hammond, B.R.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Queiroz, M.I.; Queiroz Zepka, L. Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-50970-5. [Google Scholar]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Food Carotenoids; Rodríguez-Amaya, D., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015; ISBN 9781118864364. [Google Scholar]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, N.; Cejudo-Bastante, M.J.; Heredia, F.J.; Hurtado, N. Identification of New Betalains in Separated Betacyanin and Betaxanthin Fractions from Ulluco (Ullucus tuberosus Caldas) by HPLC-DAD-ESI-MS. Plant. Foods Hum. Nutr. 2020, 75, 434–440. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.A.; Cruz y Victoria, M.T.; Barragán-Huerta, B.E. Betaxanthins and antioxidant capacity in Stenocereus pruinosus: Stability and use in food. Food Res. Int. 2017, 91, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Subramoniam, A.; Asha, V.V.; Nair, S.A.; Sasidharan, S.P.; Sureshkumar, P.K.; Rajendran, K.N.; Karunagaran, D.; Ramalingam, K. Chlorophyll Revisited: Anti-inflammatory Activities of Chlorophyll a and Inhibition of Expression of TNF-α Gene by the Same. Inflammation 2012, 35, 959–966. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2021, 110, 106055. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Genisheva, Z.; Oliveira, H.; de Freitas, V.; Teixeira, J.A.; Vicente, A.A. Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT 2016, 74, 493–503. [Google Scholar] [CrossRef] [Green Version]
- El Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of Polyphenols from Red Grape Pomace Assisted by Pulsed Ohmic Heating. Food Bioprocess. Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Sakr, M.; Liu, S. A comprehensive review on applications of ohmic heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Brochier, B.; Mercali, G.D.; Marczak, L.D.F. Effect of moderate electric field on peroxidase activity, phenolic compounds and color during ohmic heating of sugarcane juice. J. Food Process. Preserv. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Saeed Al-Hilphy, A.R.; Jabeer AlRikabi, A.K.; Al-Salim, A.M. Extraction of phenolic compounds from wheat bran using ohmic heating. Food Sci. Qual. Manag. 2015, 43, 21–30. [Google Scholar]
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colourant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Jaeschke, D.P.; Tessaro, I.C.; Marczak, L.D.F. Effects of ohmic and conventional heating on anthocyanin degradation during the processing of blueberry pulp. LWT Food Sci. Technol. 2013, 51, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.N.; Souza, B.W.S.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of Electric Fields on Protein Unfolding and Aggregation: Influence on Edible Films Formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Machado, D.; Morales-Oyervides, L.; Contreras-Esquivel, J.C.; Aguilar, C.; Méndez-Zavala, A.; Raso, J.; Montañez, J. Effect of ohmic heating processing conditions on color stability of fungal pigments. Food Sci. Technol. Int. 2017, 23, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Pilwat, G.; Riemann, F. Dielectric Breakdown of Cell Membranes. In Membrane Transport in Plants; Zimmermann, U., Dainty, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1974; pp. 146–153. [Google Scholar]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess. Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- He, Y.; Wen, L.; Liu, J.; Li, Y.; Zheng, F.; Min, W.; Yue, H.; Pan, P. Optimisation of pulsed electric fields extraction of anthocyanin from Beibinghong Vitis Amurensis Rupr. Nat. Prod. Res. 2018, 32, 23–29. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Álvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Loginova, K.V.; Lebovka, N.I.; Vorobiev, E. Pulsed electric field assisted aqueous extraction of colorants from red beet. J. Food Eng. 2011, 106, 127–133. [Google Scholar] [CrossRef]
- Martínez, J.M.; Gojkovic, Z.; Ferro, L.; Maza, M.; Álvarez, I.; Raso, J.; Funk, C. Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 2019, 289, 121694. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Wang, M.-S. Effect of pulsed electric fields (PEFs) on the pigments extracted from spinach (Spinacia oleracea L.). Innov. Food Sci. Emerg. Technol. 2017, 43, 26–34. [Google Scholar] [CrossRef]
- Mandal, R.; Kant, R. High-pressure processing and its applications in the dairy industry. Food Sci. Technol. An. Int. J. 2017, 1, 33–45. [Google Scholar]
- Ghafoor, K.; Gavahian, M.; Marszałek, K.; Barba, F.J.; Xia, Q.; Denoya, G.I. An overview of the potential applications based on HPP mechanism. In Present and Future of High Pressure Processing; Barba, F., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–11. ISBN 9780128164051. [Google Scholar]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. Experimental Design, Modeling, and Optimization of High-Pressure-Assisted Extraction of Bioactive Compounds from Pomegranate Peel. Food Bioprocess. Technol. 2017, 10, 886–900. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned High Pressure Extraction of Anthocyanins from Elderberry (Sambucus nigra L.) Pomace. Food Bioprocess. Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Luna-Vital, D.; Cortez, R.; Ongkowijoyo, P.; Gonzalez de Mejia, E. Protection of color and chemical degradation of anthocyanin from purple corn (Zea mays L.) by zinc ions and alginate through chemical interaction in a beverage model. Food Res. Int. 2018, 105, 169–177. [Google Scholar] [CrossRef]
- Luna-Vital, D.; Luzardo-Ocampo, I.; Cuellar-Nuñez, L.; Loarca-Pina, G.; de Mejia, E.G. Maize extract rich in ferulic acid and anthocyanins prevents high-fat induced obesity in mice by modulating SIRT1, AMPK, and IL-6 associated metabolic and inflammatory pathways. J. Nutr. Biochem. 2020, 91, 108343. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar] [CrossRef]
- Haining, Z.; Yongkun, M. Optimisation of High Hydrostatic Pressure Assisted Extraction of Anthocyanins from Rabbiteye Blueberry Pomace. Czech. J. Food Sci. 2017, 35, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez, E.; Mendiola, J.A.; Castro-Puyana, M. Supercritical Fluid Extraction. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–233. ISBN 978-0-12-384953-3. [Google Scholar]
- Abhari, K.; Mousavi Khaneghah, A. Alternative extraction techniques to obtain, isolate and purify proteins and bioactive from aquaculture and by-products. In Aquaculture and By-Products: Challenges and Opportunities in the Use of Alternative Protein Sources and Bioactive Compounds; Lorenzo, J.M., Barba, F.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–52. ISBN 978-0-12-820216-6. [Google Scholar]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Sapkale, G.N.; Patil, S.M.; Surwase, U.S.; Bhatbhage, P.K. Supercritical fluid extraction. A review. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Jiao, G. Kermanshahi pour, A. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Idham, Z.; Zaini, A.S.; Putra, N.R.; Rusli, N.M.; Mahat, N.S.; Yian, L.N.; Che Yunus, M.A. Effect of flow rate, particle size and modifier ratio on the supercritical fluid extraction of anthocyanins from Hibiscus sabdariffa (L). IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012031. [Google Scholar] [CrossRef]
- Seremet (Ceclu), L.; Nistor, O.-V.; Andronoiu, D.G.; Mocanu, G.D.; Barbu, V.V.; Maidan, A.; Rudi, L.; Botez, E. Development of several hybrid drying methods used to obtain red beetroot powder. Food Chem. 2020, 310, 125637. [Google Scholar] [CrossRef]
- Abdel-Moemin, A.R. Effect of Roselle calyces extract on the chemical and sensory properties of functional cupcakes. Food Sci. Hum. Wellness 2016, 5, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Carballo, D.E.; Caro, I.; Andrés, S.; Giráldez, F.J.; Mateo, J. Assessment of the antioxidant effect of astaxanthin in fresh, frozen and cooked lamb patties. Food Res. Int. 2018, 111, 342–350. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef] [Green Version]
- Montoya-Rodríguez, A.; Milán-Carrillo, J.; Dia, V.P.; Reyes-Moreno, C.; González de Mejía, E. Pepsin-pancreatin protein hydrolysates from extruded amaranth inhibit markers of atherosclerosis in LPS-induced THP-1 macrophages-like human cells by reducing expression of proteins in LOX-1 signalling pathway. Proteome Sci. 2014, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Freitas-Sá, D.D.G.C.; de Souza, R.C.; de Araujo, M.C.P.; Borguini, R.G.; de Mattos, L.D.S.; Pacheco, S.; de Godoy, R.L.O. Effect of jabuticaba (Myrciaria jaboticaba (Vell) O. Berg) and jamelão (Syzygium cumini (L.) Skeels) peel powders as colorants on color-flavor congruence and acceptability of yogurts. LWT 2018, 96, 215–221. [Google Scholar] [CrossRef]
- López, C.J.; Caleja, C.; Prieto, M.A.; Sokovic, M.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Stability of a cyanidin-3-O-glucoside extract obtained from Arbutus unedo L. and incorporation into wafers for colouring purposes. Food Chem. 2019, 275, 426–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Segat, A.; Kelly, A.L.; Sheehan, J.J. Colorants in cheese manufacture: Production, chemistry, interactions, and regulation. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1220–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, B.R.; Pinela, J.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Anthocyanin-rich extract of jabuticaba epicarp as a natural colorant: Optimization of heat- and ultrasound-assisted extractions and application in a bakery product. Food Chem. 2020, 316, 126364. [Google Scholar] [CrossRef]
- Aziz, A.A.; Modh Padzil, A.; Muhamad, I.I. Effects of incorporating purple-flashed sweet potato in biscuit on antioxidant content, antioxidant capacity, and colour characteristics. Malaysian J. Anal. Sci. 2018, 22, 665–667. [Google Scholar] [CrossRef]
- Croitoru, C.; Mureșan, C.; Turturică, M.; Stănciuc, N.; Andronoiu, D.; Dumitrașcu, L.; Barbu, V.; Enachi (Ioniță), E.; Horincar (Parfene), G.; Râpeanu, G. Improvement of Quality Properties and Shelf Life Stability of New Formulated Muffins Based on Black Rice. Molecules 2018, 23, 3047. [Google Scholar] [CrossRef] [Green Version]
- Harnly, J. Antioxidant methods. J. Food Compos. Anal. 2017, 64, 145–146. [Google Scholar] [CrossRef]
- Vinha, A.F.; Rodrigues, F.; Nunes, M.A.; Oliveira, M.B.P.P. Natural pigments and colorants in foods and beverages. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 363–391. ISBN 978-0-12-813572-3. [Google Scholar]
- Quilaqueo, M.; Millao, S.; Luzardo-Ocampo, I.; Campos-Vega, R.; Acevedo, F.; Shene, C.; Rubilar, M. Inclusion of piperine in β-cyclodextrin complexes improves their bioaccessibility and in vitro antioxidant capacity. Food Hydrocoll. 2019, 91, 143–152. [Google Scholar] [CrossRef]
- Aguilera, Y.; Mojica, L.; Rebollo-Hernanz, M.; Berhow, M.; De Mejía, E.G.; Martín-Cabrejas, M.A. Black bean coats: New source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem. 2016, 212, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.A.T.; Silva, V.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcão, D.; de Lima Araújo, K.G. Inclusion complexes of yellow bell pepper pigments with β-cyclodextrin: Preparation, characterisation and application as food natural colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Carmona, J.C.; Fabry, A.M.; Sáenz, C. Coloring foods from yellow-orange cactus pear. Acta Hortic. 2019, 15–22. [Google Scholar] [CrossRef]
- Giménez, P.J.; Fernández-López, J.A.; Angosto, J.M.; Obón, J.M. Comparative Thermal Degradation Patterns of Natural Yellow Colorants Used in Foods. Plant. Foods Hum. Nutr. 2015, 70, 380–387. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Del Franco, F.; Esti, M. Heat and light stability of natural yellow colourants in model beverage systems. Food Addit. Contam. Part A 2020, 37, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Howard, J.E.; Juvik, J.A. A natural colorant system from corn: Flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem. 2020, 310, 125734. [Google Scholar] [CrossRef]
- De Gomes Rocha, J.C.; Coutinho Viana, K.W.; Corrêa Mendonç, A.A.; de Andrade Neves, N.; Fernandes de Carvalho, A.; Rodrigues Minim, V.P.; Ribeiro de Barros, F.A.; Stringheta, P.C. Protein beverages containing anthocyanins of jabuticaba. Food Sci. Technol. 2019, 39, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Tensiska, T.; Marta, H.; Cahyana, Y.; Amirah, N.S. Application of Encapsulated Anthocyanin Pigments from Purple Sweet Potato (Ipomoea Batatas L.) in Jelly Drink. KnE Life Sci. 2017, 2, 482. [Google Scholar] [CrossRef] [Green Version]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Petruzzi, L.; Campaniello, D.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Thermal Treatments for Fruit and Vegetable Juices and Beverages: A Literature Overview. Compr. Rev. Food Sci. Food Saf. 2017, 16, 668–691. [Google Scholar] [CrossRef]
- Suryanarayana, R.; Chandrappa, M.; Santhosh, R. Awareness of use of artificial colourants in sweets preparation and their harmful effects. Int. J. Community Med. Public Health 2017, 4, 3893. [Google Scholar] [CrossRef] [Green Version]
- Backes, E.; Leichtweis, M.G.; Pereira, C.; Carocho, M.; Barreira, J.C.M.; Kamal Genena, A.; José Baraldi, I.; Filomena Barreiro, M.; Barros, L.; Ferreira, I.C.F.R. Ficus carica L. and Prunus spinosa L. extracts as new anthocyanin-based food colorants: A thorough study in confectionery products. Food Chem. 2020, 333, 127457. [Google Scholar] [CrossRef]
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated betalains (Opuntia ficus-indica) as natural colorants. Case study: Gummy candies. LWT 2019, 103, 222–227. [Google Scholar] [CrossRef]
- Chranioti, C.; Nikoloudaki, A.; Tzia, C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: Incorporation in a chewing gum system. Carbohydr. Polym. 2015, 127, 252–263. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Vasco-Leal, J.F.; Cuellar-Nuñez, L.; Luzardo-Ocampo, I.; Castellanos-Galeano, F.; Álvarez-Barreto, C.; Bello-Pérez, L.A.; Cortés-Rodriguez, M. Novel OSA-Modified Starch from Gros Michel Banana for Encapsulation of Andean Blackberry Concentrate: Production and Storage Stability. Starch Stärke 2020, 2000180, in press. [Google Scholar] [CrossRef]
- Da Silva, L.B.; Queiroz, M.B.; Fadini, A.L.; da Fonseca, R.C.C.; Germer, S.P.M.; Efraim, P. Chewy candy as a model system to study the influence of polyols and fruit pulp (açai) on texture and sensorial properties. LWT Food Sci. Technol. 2016, 65, 268–274. [Google Scholar] [CrossRef]
- Hartel, R.W.; von Elbe, J.H.; Hofberger, R. Sugar and Sugar-Free Panned Confections. In Confectionery Science and Technology; Hartel, R.W., von Elbe, J.H., Hofberger, R., Eds.; Springer International Publishing: New York, NY, USA, 2018; pp. 361–391. ISBN 978-3-319-61740-4. [Google Scholar]
- Hubbermann, E.M. Coloring of Low-Moisture and Gelatinized Food Products. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 179–196. ISBN 978-0-08-100371-8. [Google Scholar]
- Avelar, M.H.M.; Silva, L.B.; Azevedo, F.B.; Efraim, P. A byproduct of uvaia (Eugenia pyriformis) processing as a natural source for coloring sugar hard-panning confections. J. Food Process. Eng. 2019, 42. [Google Scholar] [CrossRef]
- Małgorzata, D.; Aleksandra, P.; Monika, T.; Ireneusz, K. A New Black Elderberry Dye Enriched in Antioxidants Designed for Healthy Sweets Production. Antioxidants 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durmaz, Y.; Bandarra, N.M. Fatty acids and pigments content of Nannochloropsis oculata(Eustigmatophyceae) culture at bag systems using different nitrogen sources and concentration in medium. Fresenius Environ. Bull. 2017, 36, 5289–5294. [Google Scholar]
- Genc Polat, D.; Durmaz, Y.; Konar, N.; Toker, O.S.; Palabiyik, I.; Tasan, M. Using encapsulated Nannochloropsis oculata in white chocolate as coloring agent. J. Appl. Phycol. 2020, 32, 3077–3088. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Hernández-Navarro, F.; Ramírez-Jiménez, A.K.; Campos-Vega, R.; de la Reyes-Vega, M.L.; Loarca-Piña, G.; Morales-Sánchez, E.; Wall-Medrano, A.; Gaytán-Martínez, M. Mango-bagasse functional-confectionery: Vehicle for enhancing bioaccessibility and permeability of phenolic compounds. Food Funct. 2017, 8, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Gawai, K.M.; Mudgal, S.P.; Prajapati, J.B. Stabilizers, Colorants, and Exopolysaccharides in Yogurt. In Yogurt in Health and Disease Prevention; Shah, N.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 49–68. ISBN 978-0-12-805134-4. [Google Scholar]
- Caldas-Cueva, J.P.; Morales, P.; Ludeña, F.; Betalleluz-Pallardel, I.; Chirinos, R.; Noratto, G.; Campos, D. Stability of Betacyanin Pigments and Antioxidants in Ayrampo (Opuntia soehrensii Britton and Rose) Seed Extracts and as a Yogurt Natural Colorant. J. Food Process. Preserv. 2016, 40, 541–549. [Google Scholar] [CrossRef]
- Almeida, H.H.S.; Barros, L.; Barreira, J.C.M.; Calhelha, R.C.; Heleno, S.A.; Sayer, C.; Miranda, C.G.; Leimann, F.V.; Barreiro, M.F.; Ferreira, I.C.F.R. Bioactive evaluation and application of different formulations of the natural colorant curcumin (E100) in a hydrophilic matrix (yogurt). Food Chem. 2018, 261, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Barreira, J.C.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Incorporation of natural colorants obtained from edible flowers in yogurts. LWT 2018, 97, 668–675. [Google Scholar] [CrossRef] [Green Version]
- Benchikh, Y.; Aissaoui, A.; Allouch, R.; Mohellebi, N. Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J. Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Gonçalves, M.S.T. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods 2020, 9, 771. [Google Scholar] [CrossRef]
- Arab, M.; Razavi, S.H.; Hosseini, S.M.; Nayebzadeh, K.; Meybodi, N.M.; Khanniri, E.; Mardi, P.; Mortazavian, A.M. Production and characterization of functional flavored milk and flavored fermented milk using microencapsulated canthaxanthin. LWT 2019, 114, 108373. [Google Scholar] [CrossRef]
- Montibeller, M.J.; de Lima Monteiro, P.; Tupuna-Yerovi, D.S.; de Rios, A.O.; Manfroi, V. Stability assessment of anthocyanins obtained from skin grape applied in kefir and carbonated water as a natural colorant. J. Food Process. Preserv. 2018, 42, e13698. [Google Scholar] [CrossRef]
- Ilansuriyan, P.; Shanmugam, M. Rheological, physiochemical, and sensory properties of no fat to high fat ice cream samples using stabilizer/emulsifier blends created with liquid and powder polisorbate-80. Int. Food Res. J. 2018, 25, 2579–2584. [Google Scholar]
- Krahl, T.; Fuhrmann, H.; Dimassi, S. Ice Cream. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–207. ISBN 978-0-08-100392-3. [Google Scholar]
- Singo, T.; Beswa, D. Effect of roselle extracts on the selected quality characteristics of ice cream. Int. J. Food Prop. 2019, 22, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.; Choo, W.S. Betacyanins from Hylocereus polyrhizus: Pectinase-assisted extraction and application as a natural food colourant in ice cream. J. Food Sci. Technol. 2020, in press. [Google Scholar] [CrossRef]
- Carter, B.G.; Park, C.W.; Drake, M.A. Short communication: Sensitive detection of norbixin in dried dairy ingredients at concentrations of less than 1 part per billion. J. Dairy Sci. 2017, 100, 8754–8758. [Google Scholar] [CrossRef]
- Aktypis, A.; Christodoulou, E.D.; Manolopoulou, E.; Georgala, A.; Daferera, D.; Polysiou, M. Fresh ovine cheese supplemented with saffron (Crocus sativus L.): Impact on microbiological, physicochemical, antioxidant, color and sensory characteristics during storage. Small Rumin. Res. 2018, 167, 32–38. [Google Scholar] [CrossRef]
- Ghendov-Moşanu, A.; Sturza, R.; Opriş, O.; Lung, I.; Popescu, L.; Popovici, V.; Soran, M.-L.; Patraş, A. Effect of lipophilic sea buckthorn extract on cream cheese properties. J. Food Sci. Technol. 2020, 57, 628–637. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of Hibiscus Extract Encapsulated by Ionic Gelation Incorporated in Yogurt. Food Bioprocess. Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Pöhnl, H. Applications of Different Curing Approaches and Natural Colorants in Meat Products. In Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 209–225. ISBN 978-0-08-100371-8. [Google Scholar]
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Ksouda, G.; Benslima, A.; Nasri, R.; Rinaudo, M.; Nasri, M.; Hajji, M. Enhancing colour and oxidative stabilities of reduced-nitrite turkey meat sausages during refrigerated storage using fucoxanthin purified from the Tunisian seaweed Cystoseira barbata. Food Chem. Toxicol. 2017, 107, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Nasri, R.; Dridi, N.; Moussa, H.; Ashour, L.; Nasri, M. Improvement of the quality and the shelf life of reduced-nitrites turkey meat sausages incorporated with carotenoproteins from blue crabs shells. Food Control 2018, 91, 148–159. [Google Scholar] [CrossRef]
- Estévez, M. What’s New in Meat Oxidation? In New Aspects of Meat Quality; Purslow, P.P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 91–109. ISBN 978-0-08-100593-4. [Google Scholar]
- Cunha, L.C.M.; Monteiro, M.L.G.; Costa-Lima, B.R.C.; Guedes-Oliveira, J.M.; Alves, V.H.M.; Almeida, A.L.; Tonon, R.V.; Rosenthal, A.; Conte-Junior, C.A. Effect of microencapsulated extract of pitaya (Hylocereus costaricensis) peel on color, texture and oxidative stability of refrigerated ground pork patties submitted to high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 49, 136–145. [Google Scholar] [CrossRef]
- Porto Dalla Costa, A.; Cruz Silveira Thys, R.; De Oliveira Rios, A.; Hickmann Flôres, S. Carrot Flour from Minimally Processed Residue as Substitute of β-Carotene Commercial in Dry Pasta Prepared with Common Wheat (Triticum aestivum). J. Food Qual. 2016, 39, 590–598. [Google Scholar] [CrossRef]
- Armellini, R.; Peinado, I.; Pittia, P.; Scampicchio, M.; Heredia, A.; Andres, A. Effect of saffron (Crocus sativus L.) enrichment on antioxidant and sensorial properties of wheat flour pasta. Fssood Chem. 2018, 254, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayuti, K.; Azima, F.; Marisa, M. The adition of “senduduk” fruit (Melastoma malabathricum L.) extract as colorants and antioxidant on Jackfruit straw (Artocarpus heterophyllus L.) jam. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 396–402. [Google Scholar] [CrossRef]
- Cerezal Mezquita, P.; Morales, J.; Palma, J.; Ruiz, M.D.C.; Jáuregui, M. Stability of Lutein Obtained from Muriellopsis sp biomass and used as a natural colorant and antioxidant in a mayonnaise-like dressing sauce. CyTA J. Food 2019, 17, 517–526. [Google Scholar] [CrossRef] [Green Version]
| Extraction Technology | Main Outcomes | Reference |
|---|---|---|
| Ohmic heating (OH) | Aqueous extraction of phenolic compounds extracted from wheat bran. The best conditions were 20 V/min, 80 °C, 10 min holding time to obtain 3150 mg/kg of phenolics and 82% antioxidant capacity. | [44] |
| Aqueous extraction of ANC with a yield > 80% from blue potato. Maximum recovery at 15 V/90 °C/10 min. | [40] | |
| Dark purple ANC were extracted from black rice bran with a higher yield (20.63%) using OH compared with steam extraction. Conditions used at 30% and 40% moisture and 100–200 V/cm (105 °C, 1 min). | [45] | |
| Polyphenols extraction was accelerated with OH due to higher cell wall disruption. Higher yield (36%) was achieved with 400 V/cm with 30% ethanol-water. | [41] | |
| Ethanol-water polyphenolic extracts were obtained from vine pruning residue. At 840 V/cm, 80 °C and 60 min extraction, antioxidant, antimicrobial and anticancer activity were observed. | [48] | |
| ANC have a high rate of degradation after OH application in blueberry pulp. | [46] | |
| OH treatment was used on fungal red colorant in a beverage model system. Pigment degradation of 33% was observed with OH compared with 23% with a conventional method. | [49] | |
| OH, and microwave-assisted extraction | Development of several hybrid drying methods used to obtain red beetroot powder | [72] |
| Pulsed electric fields (PEF) | A response surface model was used to obtain the optimal values for ANC extraction using PEF. Optimal extraction (166 mg ANC) was found at 15.08 kV and four pulses. | [52] |
| PEF was applied as pretreatment induced cell permeabilization and higher ANC yield. Maximum recovery (65.8 mg/100 g ANC) was achieved at 3.4 kV/cm, 105 ms pulses, 40 °C, and 480 s processing time. | [53] | |
| PEF treatment allow a “cold” extraction at low temperature (30 °C) with 95% yield and 10% colorant degradation. The conditions used were: 0.375–1.500 kV/cm; 120 pulses (100 ms), 30–80 °C. | [54] | |
| PEF-assisted extraction of astaxanthin from Haematococcus pluvialis was performed at 0.2–1 kV/cm, 10–80 pulses of 5 ms for 6 h. Methanol and ethanol improved the extraction. A further aqueous incubation was necessary to recover the colorant. | [55] | |
| Cell permeabilization caused by PEF pretreatment, allows nearly 100% b-phycoerythrin extraction from the alga Porphyridium cruentum. For this experiment, 10–50 pulses of 3 μs at electric field 2–10 kV/cm, room temperature were used. | [56] | |
| PEF was used to increase the stability of chlorophyll previously extracted with ethanol from Spinacia oleracea L. The maximum recovery was observed at 26.7 kV/cm, 35 °C and 0.32 ms. | [57] |
| Product | Pigment Origin | Obtention Method and Experimental Procedure | Technological Applications | Ref. |
|---|---|---|---|---|
| Bakery Products | ||||
| Cupcakes | Roselle (Hibiscus sabdariffa L.) | ANC-rich extract (delphinidin-3-sambubioside, cyanidin-3-sambubioside, and delphinidin-3-glucoside). The extract was obtained by drying Roselle calyces (28 °C, 3 h), followed by ground (0.55 mm) and soaking in water (200 mL). The suspension was heated at 80 °C for 1 h. | Improved proximal composition (higher dietary fiber and ash than control cupcakes), pinkish crumb and crust color, preservation of several sensory parameters (color, appearance, texture, taste, volume, and aroma). | [73] |
| French macarons | Jabuticaba (Myrciaria jaboticaba (Vell) Berg) | ANC-rich jaboticaba epicarp extract was obtained after optimized heat- and ultrasound-assisted extraction (21.8 min, 47.1 °C, 9.1% ethanol v/v; 7.49 min, 421.82 Watts, 48.30% ethanol v/v, respectively). | Proximal composition and color stability up to 6 days was obtained. Formulated cupcakes presented high TAC (81 ± 2 mg/g), being C3G and D3G the most notorious ANC. | [81] |
| Cake and cookies | Tomato waste | Lycopene was extracted from tomato waste using several temperatures (20, 30, and 40 °C) and extraction times (15, 30, 45, and 60 min) using 25:75 acetone:n-hexane ratio. Once the solvent was removed by evaporation (50 °C), the resulting lycopene was used (81.75–93.59% recovery yield). | Improved antioxidant capacity (measured by DPPH). Lycopene-added cakes and cookies showed higher volume and increased L*, a*, and b*, but there was no impact on the overall acceptability. | [26] |
| Water biscuits | Red beetroot (Beta vulgaris L., cv. “Bicor”) | Beetroot pomace was separated by vacuum filtration of the juice. Solvent extraction was then conducted for the pomace (83.3:16.7 ethanol:0.5% acetic acid proportion). After ultrasound treatment (30 min, 24–25 °C, water bath), centrifugation (9000 rm, 10 min), the solution was vacuum-filtered and vacuum-concentrated (35 °C), yielding 6.87 g dry matter/g. | Red beetroot-added biscuits showed increased betanin and isobetanin contents (up to ~55 mg/kg DM), TPC (up to ~2300 mg GAE/kg DM), and antioxidant capacity (FRAP and ABTS) compared to untreated biscuits. | [9] |
| Wafers | Arbutus unedo fruit | A C3G-rich A. unedo extract was prepared using heat-assisted extraction. Briefly, 600 mg sample was mixed with acidified ethanol (80% ethanol acidified with 0.05% HCl), stirred (500 rpm, 5 min, 90 °C), and filtered (Whatman n°. 4 paper). A residual extract yielding 60% of the total fruit dry weight and 500 μg/mL ANC was obtained. | Extract added wafers only showed a significant a* changes (p < 0.05) after 3- and 6-day storage. Compared to control wafers, higher sucrose, fatty acids contents, and antioxidant capacity. | [79] |
| Donuts | Blackberry (Rubus ulmifolius Schott) | Optimized blackberry ANC-rich extract was obtained using heat-assisted extraction and a RSM analysis. One gram of the fruit was mixed with 20 mL ethanol acidified with citric acid. The solid to liquid ratio was maintained at 50 g/L. The samples were then centrifuged (6000 rpm, 20 min, 10 °C), and filtered (Whatman paper filter n° 4). | Compared to control donuts, L* and b* were lower, but a* was higher. Free sugars (fructose, glucose, sucrose, and trehalose) decreased along storage time (3 days), and no differences in free fatty acids were obtained. | [76] |
| Beverages | ||||
| Alcoholic beverages (up to 30% alcohol) | Porphyridium sp. microalga | Fluorescent phycobiliproteins (240 kDa molecular weight, λ: 545–575 nm). Obtention after water or buffered solution extraction, centrifugation, microfiltration, and freeze-drying. | Yellow color, stable at pH 5.0–6.0 | [1] |
| No-heat treated carbonated beverages | Porphyridium aerugineum microalga | C-phycocyanin (λ: 620–642 nm). Color obtained after centrifugal separation of algae biomass, salt extraction, microfiltration, or co-precipitation of polysaccharides. | Color stability at pH 4.0–5.0 for at least 1 month at 25 °C, 40 min at 60 °C. The pigment was successfully assayed in Pepsi®® Blue. | [1] |
| Green tea model beverage | Purple carrot | ANC solution (0.05%) with 20 mM calcium hydroxide until reaching 0.02%, prepared at pH: 3.0 | Improvement of color stability from ANC (2.62–6.73 days), even better at higher temperatures (25–40 °C). | [13] |
| Sports beverage | Black bean (Phaseolus vulgaris L.) seed coat | Seed coats were subjected to an aqueous extraction (40 °C, 4 h), pH-adjusted with citric acid (2.0), centrifuged (27,200× g, 15 min), filtered, and stored at -20 °C (ANC-rich extracts). For their addition to a commercial sports beverage, extracts (0.1 mg/mL or 0.26 mg/mL) were added to 250 mL of a commercial glacier cherry-flavored sports drink. β-Cyclodextrin was then added to reach 2 g/100 mL concentration. | ANC extract-added beverages co-pigmented with β-cyclodextrin exhibited longer half-life, similar lightness, lower a*, and higher b* than commercial sports beverages. | [87] |
| Model commercial beverages | Pitaya (Stenocereus pruinosus). | Pitaya was collected, homogenized (1 g), mixed with 4 mL water, vortexed (3150 rpm, 1 min), and centrifuged (10,576× g, 20 min), and supernatants were recovered. | Yellow beverages displayed several yellow-orange shades. Juice-addition (5%) showed similarity with commercial beverages, retaining up to 75% of total betaxanthins. | [34] |
| Yellow bell pepper (Capsicum annuum L.) | Ripe yellow bell peppers were dried (55 °C, 15 h), powdered, and pigments were extracted after alcohol maceration with ethyl alcohol and water (90:10 v/v). Hexane partition was carried out, and the organic solvent was evaporated (40 °C, vacuum rotary evaporator). Inclusion complexes with β-cyclodextrin were prepared (1:2, 1:4, and 1:6 w/w) using ultrasound-freeze drying and molecular inclusion. | L* and a* parameters increased together with extract concentration, but b* decreased in the tested beverage models. | [88] | |
| Yellow-orange cactus (Opuntia ficus-indica) | Cactus pulp was vacuum-concentrated (30 °C, 17 mbar) up to 45 ºBrix. For the freeze-dried extract, maltodextrin was added (1:1 pulp:maltodextrin), homogenized, frozen (−50 °C, 48 h), and dried (−55 °C, 0–0.133 mbar). | Betaxanthin-rich extracts contained 256.53–264.76 mg indicaxanthin equivalents/kg. Soft-drink beverages displayed significant color changes after a 5 days-storage (4 °C). | [89] | |
| Annato from Bixa orellana L. seeds, gardenia yellow from Gardenia jasminoides Ellis, lutein from marigold flowers or Tagetes erecta L., curcumin from turmeric or Curcuma longa L., and safflower extract from Carthamus tinctorius L. | All colorants were acquired locally from commercial manufacturers. Beverages were formulated with McIlvaine buffer (pH: 3.5, 5.5, and 7.5; concentration: 0.001%, 0.005%, 0.01%, 0.02%, 0.03%, 0.05%, 0.10%, and 0.30%) with and without ethanol (15% v/v). | Gardenia, safflower, and curcumin displayed the highest color intensities and lowest turbidity levels. Safflower colorant was the most heat- (25–80 °C) and light-stable (550 Watts/m2, 30 °C). | [91] | |
| Purple corn (Zea mays L.) pericarp | ANC and flavones were extracted in a 1:2 ratio (w/v) from the corn seeds after aqueous incubation (80 °C, 1 h) under constant shaking. After cooling, extracts were filtered (Whatman n° 1 paper) and stored frozen at −80 °C. | Flavone addition increased the average half-life of cyanidin or pelargonidin-rich model beverages, but cyanidin beverages were the most stable ones. | [92] | |
| Protein beverage | Jabuticaba (Plinia cauliflora) | Jabuticaba skins (40 g) were ground, mixed with 70% v/v acidified ethanol with citric acid (pH: 2.0), and left to stand for 24 h (5 °C). The extract was vacuum-filtered (Whatman n° 1) and concentrated in a vacuum rotary evaporator (40 °C). The extract was added to whey (0.5%, 2.0%, 4.0%, and 6.0%)-based beverages, formulated with mineral water, sugar (15% w/v), strawberry pulp (10% w/v), gum arabic (0.45% w/v), potassium sorbate (0.03% w/v), and citric acid. | D3G and C3G were the main ANC from the extract. Formulated beverages showed whey concentration-dependent TPC (32.6–83.6 mg GAE/100 g) and antioxidant capacity (1.2–1.8 μM TEAC/g) values | [93] |
| Jelly drink | Purple sweet potato (Ipomoea batatas L.) cv. Ayamurasaki | ANC were extracted from purple sweet potato and encapsulated with 6% w/v maltodextrin. The jelly drinks contained 0.3% v/v jelly powder and 12% v/v sucrose dissolved at 75 °C for 5 min. Potassium citrate and sodium benzoate were added, and the product was cooled at 40 °C. | Beverages stored at 5 °C without light exposure presented the lowest ANC and b* decrease, and average shelf-life of 200 days. | [94] |
| Confectionery | ||||
| Gummies | Pitaya (Stenocereus pruinosus). | Pitaya was collected, homogenized (1 g), mixed with 4 mL water, vortexed (3150 rpm, 1 min), and centrifuged (10,576× g, 20 min), and supernatants were recovered. | Betaxanthins were reduced by half after 11 days of storage at 40 °C. Gummies showed high variations in yellow to orange color. | [34] |
| Cactus fruit (Opuntia ficus-indica) (purple pulp) | Betalains-rich extracts were obtained by crushing cactus fruit pulp and removing seeds by filtration. The product was then freeze-dried (1.9–2.3 g/100 g final moisture), and macerated with phosphate buffer (pH 5.5, 1:2 pulp:buffer ratio). The betalain-rich extract was mixed with sodium alginate (15 g/L, pH: 5.5), slowly added to calcium chloride solution (0.015 M) for 1 min, and washed with distilled water. The obtained beads were then dehydrated (30 °C, 24 h, forced-air circulating oven). | Gummies showed no significant (p > 0.05) variations in color during 30 days of storage at 4 °C. Vivid red-purple color gummies were obtained. | [99] | |
| Condensed milk-based confections and doughnut icing | Fig (Ficus carica) and blackthorn (Prunus spinosa L.) | Peel from F. carica and epicarp from P. spinosa were freeze-dried and milled (20 mesh size). Ultrasound-assisted extraction was conducted: 100 mL acidified ethanol (figs: 180 g/L, 21 min, 310 W) or 50:50 ethanol:water (blackthorns: 75 g/L, 5 min, 400 W). Samples were then centrifuged (6000 rpm, 20 min, 10 °C), filtered (Whatman n° 4), and supernatants were freeze-dried. Cyanidin-3-rutinoside-rich fig and cyanidin-3-rutinoside/peonidin-3-rutinoside-rich blackthorn extracts were obtained. | Formulated products mainly contained sucrose, palmitic acid, and mostly saturated fatty acids due to dairy ingredients. Blackthorn-added samples were the darkest one (purple color). | [98] |
| Gummy model | Saffron (Cocus sativus) and beetroot (Beta vulgaris) | Saffron (1 g) was extracted with water under constant shaking in a water bath (25 °C, 60 min, 30 kHz). Beetroots were washed, peeled, and extracted with water using a commercial juice extractor. Both extracts were microencapsulated using blends of gum arabic, modified starch, and chitosan, and mixtures were encapsulated by freeze-drying (0.017 mbar, −57 °C, and 48 h). | Storage temperature (25 °C and 40 °C) decreased luminosity, a*, and b* values for both extracts. The gum arabic and modified starch mixture exhibited the highest color stability: a* (for beetroot-added gums) and b* (for saffron-added gums). | [100] |
| Chewy candy | Açaí (Euterpe oleracea Mart.) pulp | Frozen Açaí pulp was thawed (25 °C), maltodextrin was added (60 g/100 g), and the mixture was homogenized (200 L/h, 10 HP). The powder was obtained by spray-drying (0.5 mm diameter nozzle and 6000 rpm atomizer, IAT: 170 °C, OAT: 80 °C, flow rate: 10–15 kg/h). This powder was added to candies prepared in an atmospheric batch system cooker. | The Açaí-added candies did not exhibit differences in the hardness or moisture content, presented higher color acceptance, and high purchase potential (from uncertain panelists), compared to non-added Açaí candies. | [102] |
| Hard-panning confections | Uvaia (Eugenia pyriformis) | The Uvaia by-product (peels and seeds) was thawed, centrifuged, and oven-dried (40 °C, 24 h). Seeds were removed, and peels were milled (particle size: 37 μm). The powder was added to hard-panning confections made after cooking gummy candies (110 °C), adding starch, and following sealing and panning stages. | Uvaia-added candies showed the highest a*, b*, hardness, the best appearance, and color sensory scores, but the lowest crispness, compared to fruit concentrate-added and artificial colorant-added candies. | [105] |
| Jelly gummy candies | Black Elderberry (Sambucus nigra) | S. nigra dyes obtained from fruits, flowers, and their mixture were freeze-dried (100 g of raw material mixed with 200 mL water, boiled for 10 min, frozen at −60 °C, and lyophilized). The obtained powder was dehydrated (48 h in heating shelves at 30 °C, 0.5 bar pressure). Jellies made from gelatin, Agar, and honey were used to add the powdered dyes. | Extract-added jelly gummy candies contained ANC such as cyanidin.3-O-sambubioside-5-glucoside, cyanidin-3,5-diglucoside, cyanidin-3-O-sambubioside, and cyanidin-3-O-rutinoside, and high antioxidant levels measured by FRAP and DPPH. | [106] |
| White chocolate | Nannochloropsis oculata microalga | Method 1: Algal biomass was dried in a spray-dryer (6 bar, 1.40 mL/min flow, and 65 mbar atomization pressure). IAT: 180 °C, OAT: 95 °C. 1:1. Method 2: Alga:maltodextrin proportion was mixed (10,000 rpm, 10 min). Encapsulation was carried out in a freeze-dryer. For adding the encapsulated products to white chocolate (6 h, 60 °C conching time), alga powders (0.125, 0.25, 0.50, and 0.75 g/100 g) were added on the last 15 min of the conching process. | The resulting alga-added chocolate exhibited higher a* and hue values than the control white chocolate samples. Chlorophyll a values ranged from 9.60 to 27.2 μg/g. No significant differences (p < 0.05) were shown for the sensory analysis of appearance, texture, and smell, despite being evaluated with lower values than the control chocolate samples. | [108] |
| Transparent lollipops made from sugar solutions Dry sugar-drop candies for cake decoration | Porphyridium sp. microalga | Fluorescent phycobiliproteins (240 kDa molecular weight, λ: 545–575 nm). Obtention after water or buffered solution extraction, centrifugation, microfiltration, and freeze-drying. | Pinkish-red color on confections, stable at 60 °C for 30 min, and long shelf-life (6 months) at pH 6.0–7.0 | [1] |
| Milk, dairy, and dairy-like products | ||||
| Yogurt | Ayrampo (Opuntia soehrensii Britton and Rose) seed | Betalains were obtained by soaking the seeds in distilled water (pH: 4.5, acidified with 0.25 N HCl, 1:3 w/v) for 24 h at 30 °C. Samples were then centrifuged (4000 rpm for 15 min), and supernatants were collected and filtered (Whatman paper n° 4). The purification was carried out by gel filtration chromatography (Bio-Gel P-2 columns) using a freeze-dried liquid-liquid extract with ethyl acetate (4:1 solvent:betalain extract) at pH: 4.5 (12 h). The fractions were eluted with distilled water (6.8 mL/h).Betacyanins were extracted by mixing the seeds’ extract with McIlvaine buffer (0.15 M, pH: 5.6) until obtention of absorbance between 0.2 and 0.8 (537 nm). | Betacyanin-added yogurts showed lower L* and higher ΔE than control yogurts, but the 5-week storage showed similar performance than the synthetic colorant Red no. 40 in color retention (>94%) and L* values. | [111] |
| Curcumin (Curcuma longa) | Commercially acquired curcumin (10 mg) was mixed with Tween 80 (10 mg) and stirred for 5 min. After sonication (15 min) under pulse conditions (30 s, 120 W, 25 °C), the solvent was evaporated (40 °C, 24 h), and the solid was ground with pistil and mortar (8.30% w/w curcumin was obtained). Different proportions of natural curcumin and encapsulated curcumin were added to commercial natural yogurts. | Formulated yogurts showed color ranges closer to orange (mango, peach, or papaya-like color). During 7-day storage, a* and b* values decreased compared to control yogurts, but the overall color was maintained a long time. | [112] | |
| Petals of Dahlia mignon, rose from Rosa damascena “Alexandria” and Rosa gallica “Francesca”; and flowers from Centaurea cyanus L. | Flowers were reduced to powder (20 mesh), and 1 g of the dry material was mixed with 50 mL of distilled water to be extracted by maceration (25 °C, 150 rpm, 1 h). Mixtures were filtered with Whatman Paper n 4, frozen, and freeze-dried. Commercial yogurts (3.8% fat) were supplemented with Dahlia (0.05% v/v), rose (0.15% v/v), or Centaurea (0.10% v/v) extracts. | Manufactured yogurts exhibited the same proximal composition and color parameters as artificially-colored yogurts (E163) but showed a higher monounsaturated fatty acids composition (oleic acid). | [113] | |
| Jabuticaba (Myrciaria jaboticaba (Vell) O. Berg) and jamelão (Syzygium cumini (L.) Skeels) | Fruits were washed, and peels were manually separated from the pulp, dried (60 °C, air speed: 1 m/s, 22 h). The dried product was ground and used to formulate yogurts (0.3 and 0.5% v/v). | Jabuticaba-colored yogurts displayed better appearance, flavor, and color scores than jamelão-colored yogurts (p < 0.05). No differences were found for the appearance between not-colored and jabuticaba-formulated yogurts. | [78] | |
| Strawberry (Fragaria ananassa) | ANC from Strawberries were extracted after mixing strawberry (0.5–2.0 g) with 85% distilled water and 15% HCl (0.1 M) (pH: 1.3) under agitation (400–800 rpm, 1–15 min), followed by centrifugation (2486× g), and filtration (13 μm). | ANC-addition produced yogurts with 10–40 mg/100 g TAC and a remaining red color at pH: 4.6 (yogurts’ pH) and 4 °C storage. | [114] | |
| Red beetroot (Beta vulgaris L.), opuntia (Opuntia stricta), Roselle (Hibiscus sabdariffa), and radish (Raphanus sativus L.) | Betalains-rich extracts (red beetroot and opuntia) were prepared using small hand-peeled raw materials pieces (5 g) and adding a water:ethanol:acetic acid (66.6:33:0.33 v/v/v) solution (25 °C) for 48 h (beetroot) or 20 min (opuntia). Mixtures were filtered and centrifugated (500 rpm, 16 min), and solvents were evaporated by rotary evaporation (40 °C). The ANC-rich extract (Roselle) 5 g of flowers were mixed with a water:ethanol:acetic acid (70:29.7:0.3 v/v/v) solution (4 °C, 72 h). The mixture was filtered, and the solvent was evaporated (40 °C, rotary evaporator). ANC-rich extract from red radish was obtained by making blends of radish (25 g) with water/acetic acid (95:5 v/v) (100 mL), and the solution was kept at 4 °C for 18 h. After filtration, the solvent was evaporated. All extracts were freeze-dried (−80 °C, 5 days), nanoencapsulated in liposomes, and applied to soy-based yogurt alternative. | Yogurts contained betacyanins, ANC, or betalains accordingly to the origin of their extracts. High color retention was observed after 21 days of storage, but Roselle and red radish-origin colorants were the most stable. | [115] | |
| Fermented flavored milk | Canthaxanthin from Dietzia natronolimnaea HS-1 | D. natronolimnaea HS-1 was transferred to a 100 mL liquid-pre-culture medium (10 g/L glucose, 5 g/L peptone, 5 g/L yeast extract, and 3 g/L malt extract). Then, the inoculum was transferred to another medium (10 g/L yeast and 40 g/L beetroot molasses) and incubated (28 °C, 180 rpm for 5 days). The biomass was removed by centrifugation (8000× g, 5 min), washed with physiological serum (9% NaCl), and extracted with ethanol by centrifugation (8000× g, 10 min). The pigment was microencapsulated using oil/water/oil multiple emulsion external gelation. Capsules were applied to pasteurized or flavored fermented milk samples (15 mg/L). | Colorant-added yogurts retained less than 50% of antioxidant capacity after 21-day storage. No differences in ΔE were shown between the formulations and a reference yogurt. | [116] |
| Kefir | Grape (Cabernet Sauvignon) | Grapes’ husks were manually separated and stored at -18 °C. Then, 25 mL of acetate buffer (pH: 4.0) was added to 5 g of frozen husks, heated at 40 °C, and stirred (150 rpm, 30 min). The resulting extracts were freeze-dried (−55 to 57 °C, 200 μHg, 4 days) to obtain ANC-concentrated extracts. Extracts were added to the prepared fermented product from kefir (400 mL ANC extract + 2 L kefir). | pH, L*, and a* decreased during 16-day storage, compared to initial values. High ANC retentions were obtained at the same time (77–88%). ANC-added kefir exhibited similar physical properties as natural kefir. | [117] |
| Ice cream | Roselle (Hybiscus sabdariffa) | Fresh Roselle calyces were washed and dried (50 °C, 36 h) in a hot-air oven dryer, powdered (0.8 mm particle size), and mixed with proper amounts of deionized water to achieve 5%, 10%, 15%, and 20% v/v. Mixtures were soaked in a water bath (75 °C, 1 h), filtered (Whatman paper n° 1), and residues were extracted with 300 mL water as described. | 5% Roselle-added ice creams displayed the best viscosity (242.3 cP), melting rates (1.3 g/min), and color attributes (L*: 72) among the formulations. Moreover, the lowest Roselle-added (5% and 10%) ice creams displayed no differences (p < 0.05) in the sweetness and gummy taste compared to commercial vanilla-flavored ice cream. | [120] |
| Microalga (Nannochloropsis oculata, Porphyridium cruentum, and Diacronema vilkianum) | Microalga was cultured in F/2 culture media prepared with seawater (350 g/L salinity, pH: 7.5, 25 °C, 2% CO2), and biomasses were harvested, concentrated, and dried in a spray-dryer (1.0 m nozzle diameter, AIT: 70 °C, OAT: 95 °C, 7–9 mL/min feed rate, residence chamber: 1.5 s). The spray-dried product was mixed with ice cream mix (0.1, 0.2, and 0.3 g/100 g ice cream) by centrifugation (1300 rpm, 3 min), followed by rapid colling at 4 °C. Samples were aged 24 h at 4 °C, whipped (0 °C, 10 min), and frozen at −18 °C for 24 h. | Formulated ice creams exhibited lower apparent viscosity and lower performance of melting behavior compared to control ice creams. P. cruentum provided a pinkish color, while the other two microalgae exhibited a greenish color. TPC were higher (p < 0.05) than the control ice creams, particularly for N. oculata alga (up to ~225 mg GAE/kg ice cream). No differences were shown between the P. cruentum-added ice creams and color, texture, taste, odor, resistance to melting, mouthfeel, or overall acceptability. | [121] | |
| Red pitahaya (Hylocereus polyrhizus) | Betacyanins were extracted from the pulp using distilled water, 50% ethanol, or 95% ethanol in a 1:1 or 1:2 fresh weight:solvent ratio (w/v). Pectinase (0, 0.5%, 1.0%, 1.5%, 2.0%, or 2.5%) was used to degrade the pectin. The pulp was then homogenized (2 h, 15,000× g, 15 min), and supernatants were placed on a vacuum oven for 24 h. Extracts (50 mg/mL) were added to fresh cow milk and pasteurized (63 °C, 30 min). After cooling (4 °C), a commercial powdered ice cream pre-mix (2% fat) was used, and the mixture was placed in an ice-cream maker. The resulting ice cream was frozen at −18 °C. | The betacyanin concentration and free radical scavenging activity increased during 21-day storage in the supplemented ice creams. No sensory evaluations were conducted. | [122] | |
| Cheese | Saffron (Crocus sativus L.) | Saffron flowers (0.5 g) were ground and added to 0.5 L of milk (1000 mg/L) at 42 °C under slow agitation for 45 min. The mixture was filtered (500 μm mesh) and used in the cheese trials. For the cheese, ovine milk (8 L) was pasteurized (68 °C, 10 min), the milk was cooled (30 °C), and inoculated with a starter culture (108 cfu/mL at 1% rate: 3.50 × 106 cfu/mL). Saffron extract (100, 150, and 200 mL of the extract), commercial rennet, and salt were added. Mixtures were incubated (25–28 °C, 12 h), mixtures were set in cheese-cloths, ripened (25 °C, 6 h), drained, and stored (4 °C). | The saffron addition did not affect moisture, total protein, salt, and fats, but these cheese showed the lowest pH (4.13–4.36) and the highest antioxidant capacity values (up to 25.97% RSA). Cheese with the lowest saffron concentration (50 mg/L) received the same sensory score as control cheeses. | [124] |
| Sea buckthorn (Hippophae rhamnoides L.) cv. “Elizaveta” fruits | Cylindrical fruits with a sweet-sour taste were powdered (particle size: 85 μm), mixed with deodorized refined sunflower oil (1 g extracted with 12 mL of oil), stirred, and sonicated at two different temperatures (20 °C and 45 °C) and three extraction times (0.5 h, 1.0 h, and 1.5 h). The extracts were centrifuged (7000 rpm, 10 min), decanted, and stored at 4 °C in dark glass bottles. The extracts (2.2% of cheese’s mass) were added to manufactured cream cheeses at 20 °C, homogenizing the samples for 5–10 min. | Manufactured cheeses incorporated chlorophylls, carotenoids, and TPC from the fruits’ extracts and received better sensory scores than tartrazine-supplemented cheeses. | [125] | |
| Meat and meat products | ||||
| Sausages | Jabuticaba (Myrciaria cauliflora) | Residues from Jabuticaba fruit (peels and seeds) were mixed with water (1:3 residue:water) under mechanical agitation (6 h). The fluid was filtered and concentrated to 1/3 of its original volume (rotary evaporation: 60 °C under vacuum). The extract was mixed with maltodextrin, stirred, and microencapsulated in a spray dryer (atomizing nozzle diameter: 1.5 mm, IAT: 150 °C, 40 L/min airflow, and 30 mL/min feed rate). Extracts (2% and 4% w/w) were added to manufactured sausages (pork shoulder and backfat, NaCl, condiments, and Na5P3O10). | No differences in the moisture, protein, lipids, or fat (p > 0.05) were found between all formulations. Jabuticaba-formulated sausages exhibited low TBARS formation (0.01–0.05 mg MDA/kg sample), L* (57.5–63.4), a* (5.7–9.1), and b* (4.8–11) changes during 15-days storage, compared to control sausages. Only 2% v/v manufactured sausage showed the same overall acceptance as control and carmine-added sausages. | [128] |
| Brown seaweed (Cystoseira barbata) | Brown seaweed was collected, water with seawater and tap water (25 °C), dried (20 days), milled (0.2 mm mesh size), and stored in amber glass bottles at 4 °C. Fucoxanthins were extracted by mixing the algal powder (100 g) with acetone:methanol (7:3 v/v, 24 h, 30 °C) under stirring (250 rpm). Extracts were concentrated and redissolved in 100 mL methanol, mixed with 300 mL water and 400 mL diethyl ether. The upper phase containing the pigment was collected, dried in a rotary evaporator, and dissolved in 5 mL of N-hexane. Silica gel column chromatography was used to purify the pigment. | Fucoxanthins-added sausages showed less L*, but higher a* and b* values than control sausages. The reddish color was improved compared to 150 ppm sodium nitrite and vitamin C references. Sausages containing fucoxanthin exhibited less TBARS formation compared to 80 ppm sodium nitrite formulated sausage. | [129] | |
| Blue crabs (Portunus segnis) | Blue crabs were obtained in fresh conditions. Shells were removed, washed, stored at -20 °C, macerated with solvent preparation (50:50 hexane:isopropanol) in a 30:1 solvent:raw material proportion under constant stirring (100 rpm, 120 h). Residual solvent was evaporated, and carotenoproteins were obtained with a petroleum ether:acetone:water (15:75:10 v/v/v) mixture (4 °C, 24 h). | The addition of carotenoproteins to sausages contributed to high inhibition zones of several gram negative (E. coli, K. pneumoniae, S. enterica, Enterobacter sp., and S. Typhimurium) and gram positive (S. aureus, B. cereus, M. luteus, and E. faecalis) bacteria, and fungi (A. niger, F. oxysporum, and A. flavus). Low TBARS (1.5–5.5 mg. MDA/kg sausages) and dienes formation; high heme iron (up to 6 μg/g sausages), and metmyoglobin contents (up to ~54%) were found in the manufactured sausages compared to control ones. | [130] | |
| Cooked lamb patties | Alga (Haematococcus pluvialis) | Astaxanthins from H. pluvialis were part of a commercial dietary supplement containing 1% astaxanthin and excipients such as maltodextrin, magnesium stearate, and silicon dioxide. Astaxanthins (20, 40, and 60 ppm) were added to ground patties prepared from lamb legs prepared at 5 °C. For the cooked patties, antioxidants were added at 4 °C and left for 5 days, and then the patties were cooked in a convection oven (150 °C, 15 min) until the core reached 70 °C. | Astaxanthin-added patties displayed no differences (p > 0.05) in the pH levels (5.58–5.68) with control patties, but TBARS values were significantly lower (p < 0.05, −21.55 to −41.44%). The developed patties exhibited the same L* values, but higher a* and b*. The lowest TBARS levels were shown for the astaxanthin-patties, and cooked patties with astaxanthin displayed lower 7-α-hydroxycholesterol; 7-β-hydroxycholesterol; 5,6-β-epoxycholesterol; cholestan-3,5-dien-7-one, and 7-ketocholesterol than control patties. | [75] |
| Ground pork patties | Pitaya (Hylocereus costaricensis) | Pitaya peels were removed, air-dried (25 °C), milled (125 μm sieve), and stored in amber flasks. A microwave-assisted extraction was conducted by mixing 0.5 g of the powder with 25 mL ethanol (400 W, 30 s), followed by centrifugation (1400× g, 15 min, 4 °C), and supernatant collection. The resulting extract was concentrated by rotary evaporation (50 rpm, 60 °C under vacuum), maltodextrin was added, and the mixture was spray-dried (feed flow: 1 kg/h, air pressure: 7 bar; IAT: 170 °C, OAT: 90 °C). The extract was then vacuum packed and frozen (−80 °C). Two concentrations (100 and 1000 ppm) were added to pork patties prepared from pork loin (Longissiums thoracis et lumborum, 82% lean and 18% fat). | Formulated patties showed the lowest pH values (~5.5 to 6.0), higher L* (11.79–13.61%), and lower b* (−4.56 to −7.75%) along storage time (9 days). During the same shelf-life analysis, cohesiveness and springiness were preserved in the patties, but hardness and chewiness increased. Overall low TBARS (<3.5 mg MDA/kg meat) were obtained. | [132] |
| Other food products | ||||
| Pasta | Carrot (Daucus carota sbsp. sativus) | Minimally processed carrot residues (peel, shavings, and peduncles) were cleaned (chlorine solution: 200 ppm, 15 min), ground (125 μm), and added to pasta formulations (10–20% w/w). | Carrot flour mainly contained lutein (320.98 g/100 g), zeaxanthin (109.12 g/100 g), cryptoxanthin (143.75 mg/g), α-carotene (4296.78 g/100 g), β-carotene (4429.77 g/100 g), and retinol (340.75 g/100 g). Formulated carrot pasta showed higher solid loss (7.55–11.71%) and weight increase (216.27–220.49%), and significantly higher (p < 0.05) DPPH inhibition (21.02% vs. 10.01%) than control pasta. | [133] |
| Saffron (Crocus sativus) | Saffron powder was commercially acquired and added (0.1, 0.2, and 0.4% w/w) to pasta (70% wheat flour and 30% water). Saffron dispersions were previously prepared with water and filtered (Whatman n° 40 paper). | Saffron-enriched pasta increased a* and b* values, decreased luminosity, and did not affect harness, cohesiveness, elasticity, nor chewiness, compared to control pasta. Saffron allowed high DPPH values (0.5–7.0-fold higher than control), and the formulated pasta was positively scored in terms of aspect, color, aroma, taste, and global acceptability. | [134] | |
| Fruit jam | “Senduduk” fruit (Melastoma malabathricum L.) | Chopped “Senduduk” (purplish-black color) was blended with water (1:3 water:fruit proportion) and filtered with a gauze. Jackfruit (45 g) was mixed with sugar, 0.5 g citric acid, 1 g pectin, and the blend was boiled and stirred. After cooling (40 °C), and senduku extracts were added (2–10%). The product was cooked at 50 °C for 5 min until jam was formed. | Senduku provided vitamin C (2.81–3.02 ppm), increased pH along with concentration (3.4–3.7), and decreased total acidity from jackfruit jam. Moreover, senduku delivered b-carotene, ANC, TPC, and antioxidant capacity (IC50: 83.89–102.01 ppm). | [135] |
| Mayonnaise-like dressing sauce | Muriellopsis sp. alga | Lutein oleoresin was prepared from the freeze-dried biomass of Muriellopsis sp. at a final concentration of 20% w/w, prepared using vegetable oil. The solution was ultrasound-homogenized (40 oscillations, 6 pulses/s, 10 min). | Formulated mayonnaises exhibited higher lutein and pigment stability than commercial mayonnaises. | [136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634. https://doi.org/10.3390/foods10030634
Luzardo-Ocampo I, Ramírez-Jiménez AK, Yañez J, Mojica L, Luna-Vital DA. Technological Applications of Natural Colorants in Food Systems: A Review. Foods. 2021; 10(3):634. https://doi.org/10.3390/foods10030634
Chicago/Turabian StyleLuzardo-Ocampo, Ivan, Aurea K. Ramírez-Jiménez, Jimena Yañez, Luis Mojica, and Diego A. Luna-Vital. 2021. "Technological Applications of Natural Colorants in Food Systems: A Review" Foods 10, no. 3: 634. https://doi.org/10.3390/foods10030634
APA StyleLuzardo-Ocampo, I., Ramírez-Jiménez, A. K., Yañez, J., Mojica, L., & Luna-Vital, D. A. (2021). Technological Applications of Natural Colorants in Food Systems: A Review. Foods, 10(3), 634. https://doi.org/10.3390/foods10030634










