Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
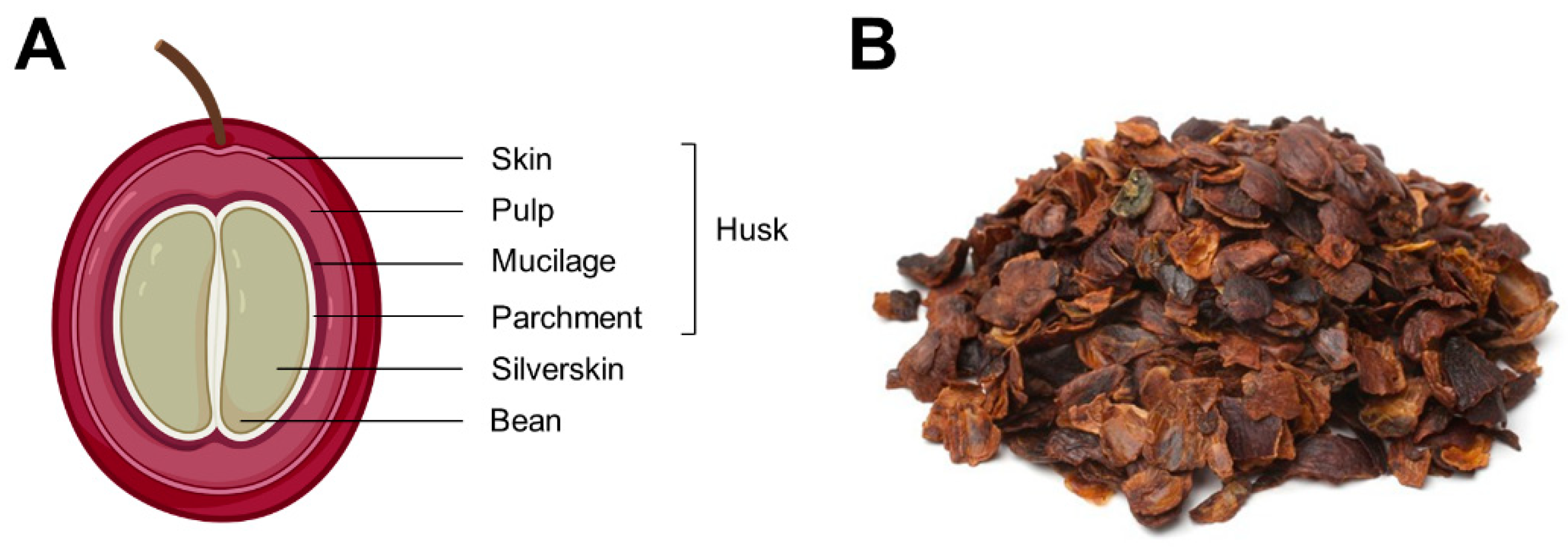
2.1. Material and Sample Preparation
2.2. Experimental Design
2.2.1. Response Surface Methodology (RSM)
2.2.2. Artificial Neural Networks (ANNs)
2.3. Comparison of the Prediction Ability of RSM and ANN
2.4. Validation of the Model
2.5. Heat-Assisted Extraction (HAE)
2.6. Organic Solvent Extraction of Free and Bound Phenolic Compound Fractions
2.6.1. Extraction of Free Phenolic Compounds
2.6.2. Extraction of Bound Phenolic Compounds
2.7. Determination of Phenolic Compounds
2.7.1. Total Phenolic Compounds (TPC)
2.7.2. Total Flavonoids (TF)
2.7.3. Total Flavanols (TFL)
2.7.4. Total Proanthocyanidins (PAC)
2.7.5. Total Phenolic Acids (TPA)
2.7.6. Total ortho-Diphenols (TOD)
2.7.7. Assessment of In Vitro Antioxidant Capacity (AC)
2.8. UPLC-ESI-MS/MS Analysis of Phenolic Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fitting of the RSM and ANN Models
3.2. Effect of HAE Parameters on the Different Response Variables
3.3. Evaluation and Experimental Validation of Optimal Conditions
3.4. UPLC-ESI-MS/MS Phenolic Compound Profile and Chemometric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Sustainable Consumption and Production. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 17 February 2021).
- FAO. Sustainable Food Systems Concept and Framework. What Is a Sustainable Food System? Why Take a Food Systems Approach? Changing Food Systems; FAO: Rome, Italy, 2018; pp. 1–8. [Google Scholar]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- de Sousa e Silva, J.; Moreli, A.P.; Donzeles, S.M.L.; Soares, S.F.; Vitor, D.G. Harvesting, Drying and Storage of Coffee. In Food Engineering Series; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–64. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; Rajcic de Rezende, T.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Swapna, T.S.; Sabu, A. Coffee Husk: A Potential Agro-Industrial Residue for Bioprocess. In Waste to Wealth. Energy, Environment, and Sustainability; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, 2018; pp. 97–109. [Google Scholar] [CrossRef]
- Heeger, A.; Kosińska-Cagnazzo, A.; Cantergiani, E.; Andlauer, W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017, 221, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.C.S.; Naozuka, J.; Da Luz, J.M.R.; De Assunão, L.S.; Oliveira, P.V.; Vanetti, M.C.D.; Bazzolli, D.M.S.; Kasuya, M.C.M. Enrichment of Pleurotus ostreatus mushrooms with selenium in coffee husks. Food Chem. 2012, 131, 558–563. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho Oliveira, F.; Srinivas, K.; Helms, G.L.; Isern, N.G.; Cort, J.R.; Gonçalves, A.R.; Ahring, B.K. Characterization of coffee (Coffea arabica) husk lignin and degradation products obtained after oxygen and alkali addition. Bioresour. Technol. 2018, 257, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sarrouh, B.; de Souza, R.O.A.; da Silva Florindo, R.H.; Lofrano, R.C.Z.; de Oliveira, A.M. Extraction and Identification of Biomolecules from Lignin Alkaline Hydrolysate from Coffee Husk. Waste Biomass Valorization 2021, 12, 787–794. [Google Scholar] [CrossRef]
- Morales-Martínez, J.L.; Aguilar-Uscanga, M.G.; Bolaños-Reynoso, E.; López-Zamora, L. Optimization of Chemical Pretreatments Using Response Surface Methodology for Second-Generation Ethanol Production from Coffee Husk Waste. Bioenergy Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Alhogbi, B.G. Potential of coffee husk biomass waste for the adsorption of Pb(II) ion from aqueous solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Dadi, D.; Daba, G.; Beyene, A.; Luis, P.; Van der Bruggen, B. Composting and co-composting of coffee husk and pulp with source-separated municipal solid waste: A breakthrough in valorization of coffee waste. Int. J. Recycl. Org. Waste Agric. 2019, 8, 263–277. [Google Scholar] [CrossRef] [Green Version]
- del Castillo, M.D.; Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Martín-Cabrejas, M.A.; Farah, A. Coffee Antioxidants in Chronic Diseases. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 20–56. [Google Scholar] [CrossRef]
- Silva, M.d.O.; Honfoga, J.N.B.; de Medeiros, L.L.; Madruga, M.S.; Bezerra, T.K.A. Obtaining Bioactive Compounds from the Coffee Husk (Coffea arabica L.) Using Different Extraction Methods. Molecules 2020, 26, 46. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, Y.; Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Martín-Cabrejas, M.A. Response surface methodology to optimise the heat-assisted aqueous extraction of phenolic compounds from coffee parchment and their comprehensive analysis. Food Funct. 2019, 10, 4739–4750. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Khadir, M.T. Artificial neural networks for food processes: A survey. In Artificial Neural Networks in Food Processing; De Gruyter: Berlin, Germany, 2021; pp. 27–50. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, I.; Iglesias-Otero, M.A.; Esteki, M.; Moldes, O.A.; Mejuto, J.C.; Simal-Gandara, J. A critical review on the use of artificial neural networks in olive oil production, characterization and authentication. Crit. Rev. Food Sci. Nutr. 2019, 59, 1913–1926. [Google Scholar] [CrossRef] [PubMed]
- Kass, R.E.; Dennis, J.E.; Schnabel, R.B. Numerical Methods for Unconstrained Optimization and Nonlinear Equations; SIAM: Philadelphia, PA, USA, 1985; Volume 80, ISBN 0898713641. [Google Scholar] [CrossRef]
- Priyadarshini, R. Functional Analysis of Artificial Neural Network for Dataset Classification. Spec. Issue IJCCT 2010, 1, 3–5. [Google Scholar] [CrossRef]
- Vogl, T.P.; Mangis, J.K.; Rigler, A.K.; Zink, W.T.; Alkon, D.L. Accelerating the convergence of the back-propagation method. Biol. Cybern. 1988, 59, 257–263. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Aguilera, Y.; Herrera, T.; Cayuelas, L.T.; Dueñas, M.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Arribas, S.M.; Martín-Cabrejas, M.A. Bioavailability of melatonin from lentil sprouts and its role in the plasmatic antioxidant status in rats. Foods 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard reaction by phytochemicals composing an aqueous coffee silverskin extract via a mixed mechanism of action. Foods 2019, 8, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Ribéreau-Gayón, P.; Stonestreet, E. Dógase des tanins du vin rouges et détermination du leur structure. Chem. Anal. 1966, 48, 188–196. [Google Scholar]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Obradovic, A.; Kacaniova, M.M.; Markovic, S.; Popović, S.; Baskić, D. Phytochemical analysis, antioxidant, antibacterial and cytotoxic activity of different plant organs of Eryngium serbicum L. Ind. Crops Prod. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Granato, D.; Margraf, T.; Brotzakis, I.; Capuano, E.; van Ruth, S.M. Characterization of Conventional, Biodynamic, and Organic Purple Grape Juices by Chemical Markers, Antioxidant Capacity, and Instrumental Taste Profile. J. Food Sci. 2015, 80, C55–C65. [Google Scholar] [CrossRef]
- Benítez, V.; Rebollo-Hernanz, M.; Aguilera, Y.; Bejerano, S.; Cañas, S.; Martín-Cabrejas, M.A. Extruded coffee parchment shows enhanced antioxidant, hypoglycaemic, and hypolipidemic properties by releasing phenolic compounds from the fibre matrix. Food Funct. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolomé, B. Determination of Microbial Phenolic Acids in Human Faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef]
- Shanmuganathan, S. Artificial neural network modelling: An introduction. In Studies in Computational Intelligence; Springer: New York, NY, USA, 2016; Volume 628, pp. 1–14. [Google Scholar] [CrossRef]
- Dahmoune, F.; Remini, H.; Dairi, S.; Aoun, O.; Moussi, K.; Bouaoudia-Madi, N.; Adjeroud, N.; Kadri, N.; Lefsih, K.; Boughani, L.; et al. Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crops Prod. 2015, 77, 251–261. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of temperature, solvent and pH on the selective extraction of phenolic compounds from tiger nuts by-products: Triple-TOF-LC-MS-MS characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [Green Version]
- Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Guyot, S.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B.; Contreras-Esquivel, J.C.; Aguilar, C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-HPLC-ESI-MS. Food Chem. 2021, 340, 127830. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Benitez, V.; Rebollo-Hernanz, M.; Hernanz, S.; Chantres, S.; Aguilera, Y.; Martin-Cabrejas, M.A. Coffee parchment as a new dietary fiber ingredient: Functional and physiological characterization. Food Res. Int. 2019, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cañas, S.; Rebollo-Hernanz, M.; Cano-Muñoz, P.; Aguilera, Y.; Benítez, V.; Braojos, C.; Gila-Díaz, A.; Rodríguez-Rodríguez, P.; Cobeta, I.M.; López De Pablo, Á.L.; et al. Critical Evaluation of Coffee Pulp as an Innovative Antioxidant Dietary Fiber Ingredient: Nutritional Value, Functional Properties and Acute and Sub-Chronic Toxicity. Proceedings 2021, 70, 7623. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Saura-Calixto, F. The Story of the Introduction of Non-extractable Polyphenols into Polyphenol Research: Origin, Development and Perspectives. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Royal Society of Chemistry: London, UK, 2018; Volume 2018, pp. 1–16. ISBN 9781782627081. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules extraction from coffee and cocoa by- and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Supramolecular solvent extraction of bioactives from coffee cherry pulp. J. Food Eng. 2020, 278, 109933. [Google Scholar] [CrossRef]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 2016, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Călinoiu, L.; Vodnar, D. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.S.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Bartolomé, B.; Aguilera, Y.; Martin-Cabrejas, M.A. Extraction of phenolic compounds from cocoa shell: Modeling using response surface methodology and artificial neural networks. Sep. Purif. Technol. 2021, 266. [Google Scholar]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Cocoa Shell Aqueous Phenolic Extract Preserves Mitochondrial Function and Insulin Sensitivity by Attenuating Inflammation between Macrophages and Adipocytes In Vitro. Mol. Nutr. Food Res. 2019, 63, 1801413. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; de Mejia, E.G. Relationship of the phytochemicals from coffee and cocoa by-products with their potential to modulate biomarkers of metabolic syndrome in vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, biof.1507. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martin-Cabrejas, M.A.; de Mejia, E.G. Regulation of lipid and glucose metabolism in hepatocytes by phytochemicals from coffee by-products and prevention of non-alcoholic fatty liver disease in vitro. Proceedings 2020, 61, 6996. [Google Scholar] [CrossRef]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic acid promotes development of brown adipocyte-like phenotype in 3T3-L1 adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]

 ) to the highest (
) to the highest (  ) value for each parameter) (D) showing the associations among the measured parameters and classifying phenolic extracts from coffee husk according to them, and the ten most significant coefficients from principal components regression, PCR (E) and principal least squares regression, PLS-R (F). Circles in different colors indicate minor phenolic or phenolic family, green (
) value for each parameter) (D) showing the associations among the measured parameters and classifying phenolic extracts from coffee husk according to them, and the ten most significant coefficients from principal components regression, PCR (E) and principal least squares regression, PLS-R (F). Circles in different colors indicate minor phenolic or phenolic family, green (  ), major phenolic, blue (
), major phenolic, blue (  ).
).
 ) to the highest (
) to the highest (  ) value for each parameter) (D) showing the associations among the measured parameters and classifying phenolic extracts from coffee husk according to them, and the ten most significant coefficients from principal components regression, PCR (E) and principal least squares regression, PLS-R (F). Circles in different colors indicate minor phenolic or phenolic family, green (
) value for each parameter) (D) showing the associations among the measured parameters and classifying phenolic extracts from coffee husk according to them, and the ten most significant coefficients from principal components regression, PCR (E) and principal least squares regression, PLS-R (F). Circles in different colors indicate minor phenolic or phenolic family, green (  ), major phenolic, blue (
), major phenolic, blue (  ).
).

| Run | Independent Variables | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (X1, °C) | Time (X2, Min) | Acidity (X3, %) | S/L Ratio (X4, g mL−1) | TPC (mg g−1) | TF (mg g−1) | TFL (mg g−1) | PAC (mg g−1) | TPA (mg g−1) | TOD (mg g−1) | AC (mg g−1) | |
| 1 | 30 (−1) | 5 (−1) | 1 (0) | 0.035 (0) | 4.19 ± 0.12 | 5.52 ± 0.27 | 0.74 ± 0.14 | 2.48 ± 0.25 | 1.28 ± 0.08 | 0.86 ± 0.11 | 11.95 ± 0.08 |
| 2 | 100 (1) | 5 (−1) | 1 (0) | 0.035 (0) | 5.45 ± 0.12 | 7.94 ± 0.25 | 0.84 ± 0.07 | 2.63 ± 0.22 | 2.29 ± 0.11 | 1.23 ± 0.06 | 15.61 ± 0.05 |
| 3 | 30 (−1) | 90 (1) | 1 (0) | 0.035 (0) | 4.41 ± 0.09 | 6.30 ± 0.24 | 0.73 ± 0.08 | 2.34 ± 0.09 | 1.69 ± 0.18 | 1.03 ± 0.10 | 11.90 ± 0.09 |
| 4 | 100 (1) | 90 (1) | 1 (0) | 0.035 (0) | 5.44 ± 0.10 | 7.34 ± 0.18 | 0.96 ± 0.08 | 2.74 ± 0.13 | 2.42 ± 0.06 | 1.28 ± 0.06 | 15.37 ± 0.07 |
| 5 | 65 (0) | 47.5 (0) | 0 (−1) | 0.02 (1) | 4.47 ± 0.13 | 8.60 ± 0.23 | 1.09 ± 0.12 | 2.99 ± 0.27 | 2.98 ± 0.16 | 1.56 ± 0.10 | 17.43 ± 0.05 |
| 6 | 65 (0) | 47.5 (0) | 2 (1) | 0.02 (1) | 4.56 ± 0.13 | 6.38 ± 0.34 | 0.71 ± 0.03 | 3.48 ± 0.11 | 1.55 ± 0.07 | 1.13 ± 0.05 | 10.32 ± 0.05 |
| 7 | 65 (0) | 47.5 (0) | 0 (−1) | 0.05 (−1) | 3.47 ± 0.15 | 6.99 ± 0.30 | 0.75 ± 0.05 | 2.23 ± 0.26 | 1.95 ± 0.07 | 1.10 ± 0.05 | 13.19 ± 0.05 |
| 8 | 65 (0) | 47.5 (0) | 2 (1) | 0.05 (−1) | 4.07 ± 0.10 | 5.97 ± 0.28 | 0.70 ± 0.05 | 2.45 ± 0.30 | 1.43 ± 0.06 | 1.00 ± 0.06 | 11.33 ± 0.09 |
| 9 | 65 (0) | 47.5 (0) | 1 (0) | 0.035 (0) | 3.70 ± 0.16 | 6.81 ± 0.30 | 0.65 ± 0.09 | 2.51 ± 0.18 | 1.77 ± 0.08 | 0.97 ± 0.05 | 12.17 ± 0.09 |
| 10 | 30 (−1) | 47.5 (0) | 1 (0) | 0.02 (1) | 3.94 ± 0.15 | 6.69 ± 0.34 | 0.67 ± 0.05 | 2.41 ± 0.15 | 1.37 ± 0.12 | 1.04 ± 0.06 | 11.09 ± 0.08 |
| 11 | 100 (1) | 47.5 (0) | 1 (0) | 0.02 (1) | 5.93 ± 0.15 | 10.07 ± 0.24 | 1.10 ± 0.10 | 3.04 ± 0.19 | 2.88 ± 0.10 | 1.56 ± 0.05 | 18.67 ± 0.08 |
| 12 | 30 (−1) | 47.5 (0) | 1 (0) | 0.05 (−1) | 3.64 ± 0.12 | 6.17 ± 0.25 | 0.66 ± 0.09 | 2.16 ± 0.09 | 1.69 ± 0.15 | 0.92 ± 0.08 | 11.28 ± 0.08 |
| 13 | 100 (1) | 47.5 (0) | 1 (0) | 0.05 (−1) | 4.07 ± 0.17 | 7.32 ± 0.28 | 0.78 ± 0.06 | 2.22 ± 0.27 | 2.19 ± 0.08 | 1.06 ± 0.05 | 13.47 ± 0.06 |
| 14 | 65 (0) | 5 (−1) | 0 (−1) | 0.035(0) | 4.30 ± 0.21 | 7.09 ± 0.34 | 0.76 ± 0.06 | 2.51 ± 0.20 | 2.14 ± 0.13 | 1.26 ± 0.04 | 16.20 ± 0.08 |
| 15 | 65 (0) | 90 (1) | 0 (−1) | 0.035 (0) | 4.27 ± 0.25 | 7.60 ± 0.27 | 0.97 ± 0.09 | 2.55 ± 0.13 | 2.74 ± 0.10 | 1.58 ± 0.07 | 17.33 ± 0.08 |
| 16 | 65 (0) | 5 (−1) | 2 (1) | 0.035 (0) | 4.50 ± 0.23 | 6.89 ± 0.35 | 0.63 ± 0.14 | 2.47 ± 0.16 | 1.54 ± 0.06 | 1.11 ± 0.06 | 12.52 ± 0.07 |
| 17 | 65 (0) | 90 (1) | 2 (1) | 0.035 (0) | 4.18 ± 0.10 | 5.55 ± 0.12 | 0.75 ± 0.09 | 2.67 ± 0.25 | 1.53 ± 0.18 | 1.06 ± 0.07 | 10.44 ± 0.06 |
| 18 | 65 (0) | 47.5 (0) | 1 (0) | 0.035 (0) | 3.68 ± 0.10 | 6.00 ± 0.21 | 0.69 ± 0.14 | 2.52 ± 0.16 | 1.74 ± 0.11 | 0.94 ± 0.06 | 12.04 ± 0.09 |
| 19 | 30 (−1) | 47.5 (0) | 0 (−1) | 0.035 (0) | 4.22 ± 0.10 | 7.06 ± 0.27 | 0.79 ± 0.10 | 2.49 ± 0.18 | 2.23 ± 0.06 | 1.26 ± 0.07 | 15.55 ± 0.04 |
| 20 | 100 (1) | 47.5 (0) | 0 (−1) | 0.035 (0) | 4.88 ± 0.14 | 10.10 ± 0.27 | 1.26 ± 0.09 | 2.89 ± 0.19 | 3.93 ± 0.15 | 1.85 ± 0.07 | 18.77 ± 0.09 |
| 21 | 30 (−1) | 47.5 (0) | 2 (1) | 0.035 (0) | 3.28 ± 0.12 | 6.54 ± 0.19 | 0.60 ± 0.06 | 2.22 ± 0.12 | 1.28 ± 0.08 | 0.95 ± 0.04 | 9.90 ± 0.06 |
| 22 | 100 (1) | 47.5 (0) | 2 (1) | 0.035 (0) | 5.00 ± 0.11 | 8.38 ± 0.21 | 0.85 ± 0.06 | 2.88 ± 0.29 | 2.53 ± 0.08 | 1.29 ± 0.07 | 14.12 ± 0.06 |
| 23 | 65 (0) | 5 (−1) | 1 (0) | 0.02 (1) | 4.40 ± 0.12 | 7.47 ± 0.18 | 0.76 ± 0.10 | 2.59 ± 0.22 | 2.09 ± 0.19 | 1.03 ± 0.04 | 13.99 ± 0.07 |
| 24 | 65 (0) | 90 (1) | 1 (0) | 0.02 (1) | 5.52 ± 0.08 | 8.15 ± 0.22 | 0.82 ± 0.09 | 2.66 ± 0.14 | 1.89 ± 0.05 | 1.18 ± 0.05 | 13.09 ± 0.07 |
| 25 | 65 (0) | 5 (−1) | 1 (0) | 0.05 (−1) | 3.89 ± 0.12 | 7.10 ± 0.18 | 0.65 ± 0.09 | 2.25 ± 0.13 | 1.58 ± 0.07 | 0.97 ± 0.04 | 12.76 ± 0.06 |
| 26 | 65 (0) | 90 (1) | 1 (0) | 0.05 (−1) | 3.58 ± 0.15 | 6.67 ± 0.20 | 0.51 ± 0.14 | 2.08 ± 0.35 | 1.19 ± 0.10 | 0.72 ± 0.04 | 10.00 ± 0.09 |
| 27 | 65 (0) | 47.5 (0) | 1 (0) | 0.035 (0) | 3.69 ± 0.07 | 6.79 ± 0.17 | 0.69 ± 0.11 | 2.59 ± 0.14 | 1.82 ± 0.07 | 0.94 ± 0.05 | 12.45 ± 0.08 |
| Response | Modeling Method | R2 | RMSE | AAD (%) |
|---|---|---|---|---|
| TPC | RSM | 0.9402 | 0.16 | 3.02 |
| RSM ST | 0.9110 | 0.20 | 3.58 | |
| ANN | 0.9802 | 0.09 | 1.48 | |
| TF | RSM | 0.8919 | 0.36 | 4.20 |
| RSM ST | 0.7101 | 0.63 | 6.95 | |
| ANN | 0.9950 | 0.08 | 0.57 | |
| TFL | RSM | 0.9222 | 0.05 | 0.50 |
| RSM ST | 0.8639 | 0.06 | 0.65 | |
| ANN | 0.9882 | 0.02 | 0.19 | |
| PAC | RSM | 0.9413 | 0.06 | 1.97 |
| RSM ST | 0.8747 | 0.09 | 2.92 | |
| ANN | 0.9879 | 0.03 | 0.78 | |
| TPA | RSM | 0.9355 | 0.16 | 6.61 |
| RSM ST | 0.8901 | 0.20 | 8.40 | |
| ANN | 0.9860 | 0.07 | 2.79 | |
| TOD | RSM | 0.9627 | 0.05 | 3.47 |
| RSM ST | 0.9489 | 0.06 | 4.19 | |
| ANN | 0.9882 | 0.03 | 1.59 | |
| AC | RSM | 0.9744 | 0.41 | 2.47 |
| RSM ST | 0.9597 | 0.51 | 3.16 | |
| ANN | 0.9912 | 0.24 | 1.52 |
| TPC | TF | TFL | PAC | TPA | TOD | AC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | Contrib. (%) | β | Contrib. (%) | β | Contrib. (%) | β | Contrib. (%) | β | Contrib. (%) | β | Contrib. (%) | β | Contrib. (%) | |
| Constant (X0) | 3.689 *** | 6.769 *** | 0.676 *** | 2.541 *** | 1.775 *** | 0.950 *** | 12.221 *** | |||||||
| Linear | 61.3 *** | 69.7 *** | 65.6 *** | 69.5 *** | 73.1 *** | 61.3 *** | 78.6 *** | |||||||
| X1 | 0.592 *** | 35.1 *** | 1.073 *** | 41.8 *** | 0.133 *** | 29.2 *** | 0.191 *** | 27.4 *** | 0.558 *** | 36.4 *** | 0.185 *** | 24.5 *** | 2.028 *** | 28.0 *** |
| X2 | 0.056 | 0.3 | −0.034 | 0.0 | 0.030 | 1.5 | 0.010 | 0.1 | 0.045 | 0.2 | 0.032 | 0.7 | −0.408 * | 1.1 * |
| X3 | −0.001 | 0.0 | −0.645 ** | 15.1 ** | −0.114 *** | 21.5 *** | −0.042 | 1.3 | −0.509 *** | 30.4 *** | −0.172 *** | 21.2 *** | −2.487 *** | 42.1 *** |
| X4 | −0.508 *** | 25.9 *** | −0.595 *** | 12.9 *** | −0.090 *** | 13.4 *** | −0.232 *** | 40.7 *** | −0.229 ** | 6.1 ** | −0.144 *** | 14.9 *** | −1.045 ** | 7.4 *** |
| Quadratic | 28.6 *** | 8.1 * | 17.3 * | 5.1 | 11.5 ** | 27.2 *** | 9.6 *** | |||||||
| X12 | 0.559 *** | 13.9 *** | 0.550 * | 4.9 * | 0.114 ** | 9.7 ** | 0.025 | 0.2 | 0.320 ** | 5.3 ** | 0.149 *** | 7.1 *** | 1.255 *** | 4.7 *** |
| X22 | 0.517 *** | 11.9 *** | −0.179 | 0.5 | 0.007 | 0.0 | −0.041 | 0.6 | −0.105 | 0.6 | 0.020 | 0.1 | 0.439 | 0.6 |
| X32 | 0.153 | 1.0 | 0.263 | 1.1 | 0.100 ** | 7.4 ** | 0.066 | 1.4 | 0.324 ** | 5.5 ** | 0.251 *** | 20.0 *** | 1.183* ** | 4.2 *** |
| X42 | 0.196 | 1.7 | 0.317 | 1.6 | 0.018 | 0.2 | −0.093 * | 2.9 * | −0.055 | 0.2 | 0.016 | 0.1 | −0.127 | 0.0 |
| Interaction | 12.5 ** | 10.9 | 11.2 * | 17.1 ** | 6.2 | 9.2 * | 10.1 ** | |||||||
| X12 | −0.060 | 0.1 | −0.344 | 1.4 | 0.032 | 0.6 | 0.066 | 1.1 | 0.070 | 0.2 | −0.033 | 0.3 | −0.048 | 0.0 |
| X13 | 0.267 * | 2.4 * | −0.298 | 1.1 | −0.057 | 1.8 | 0.065 | 1.1 | −0.113 | 0.5 | −0.063 | 1.0 | 0.249 | 0.1 |
| X14 | −0.390 ** | 5.1 ** | −0.559 | 3.8 | −0.079 * | 3.4 * | −0.145 ** | 5.3 ** | −0.254 * | 2.5 * | −0.094 * | 2.1 * | −1.345 *** | 4.1 *** |
| X23 | −0.072 | 0.2 | −0.460 | 2.6 | −0.021 | 0.2 | 0.042 | 0.5 | −0.153 | 0.9 | −0.089 * | 1.9 * | −0.799 * | 1.4 * |
| X24 | −0.357 * | 4.3 * | −0.280 | 1.0 | −0.051 | 1.4 | −0.060 | 0.9 | −0.047 | 0.1 | −0.099 * | 2.3 * | −0.465 | 0.5 |
| X34 | 0.126 | 0.5 | 0.301 | 1.1 | 0.082* | 3.7 * | 0.182 ** | 8.3 ** | 0.228 | 2.0 | 0.082 * | 1.6 * | 1.312 ** | 3.9 ** |
| Model | 94.0 *** | 89.2 *** | 92.2 *** | 94.1 *** | 93.6 *** | 96.3 *** | 97.4 *** | |||||||
| R2 | 0.9402 | 0.8919 | 0.9222 | 0.9413 | 0.9355 | 0.9627 | 0.9744 | |||||||
| Adj. R2 | 0.8705 | 0.8296 | 0.8314 | 0.8728 | 0.8603 | 0.9192 | 0.9446 | |||||||
| F value (model) | 13.48 *** | 7.07 *** | 10.16 *** | 13.75 *** | 12.43 *** | 22.13 *** | 32.67 *** | |||||||
| Non-Coded Equation | R2 | F-Value | p-Value |
|---|---|---|---|
| YTPC = 4.0 − 1.5 × 10−2 x1 − 1.7 × 10−3x2 − 5.0 × 10−1x3 + 4.1 × 10+1x4 + 3.8 × 10−4x12 + 2.4 × 10−4x22 + 7.6 × 10−3x13 − 7.4 × 10−1x14 − 5.6 × 10−1x24 | 0.9110 | 19.33 | <0.0001 |
| YTF = 8.6 − 1.9 × 10−2x1 − 6.4× 10−1 x3 − 4.0× 10+1 x4 + 3.8× 10−4 x12 | 0.7414 | 15.77 | <0.0001 |
| YTFL = 1.1 − 2.4 × 10−3x1 − 4.9 × 10−1x3 − 1.7x4 + 8.8 × 10−5x12 + 9.4 × 10−2x32 − 1.5 × 10−1x14 + 5.4x34 | 0.8639 | 17.23 | <0.0001 |
| YPAC = 2.0 + 1.5 × 10−2x1 − 4.7 × 10−1x3 + 2.2 × 10+1x4 − 4.6 × 10+2x42 − 2.8 × 10−1x14 + 1.2 × 10+1x34 | 0.8747 | 23.27 | <0.0001 |
| YTPA = 2.2 − 5.3 × 10−3x1 − 1.2x3 + 1.6 × 10+1x4 + 2.9 × 10−4x12 + 3.6 × 10−1x32 − 4.8 × 10−1x14 | 0.8901 | 26.99 | <0.0001 |
| YTOD = 1.3 − 3.3 × 10−3x1 + 8.3 × 10−3x2 − 7.5 × 10−1x3 + 4.0x4 + 1.1 × 10−4x12 + 2.4 × 10−1x32 − 1.8 × 10−1x14 − 2.1 × 10−3x23 − 1.6 × 10−1x24 + 5.4x34 | 0.9489 | 29.73 | <0.0001 |
| YAC = 1.6 × 10+1 + 2.3 × 10−2x1 + 9.2 × 10−3x2 − 6.9x3 + 9.4x4 + 9.6 × 10−4x12 + 1.1x32 − 2.6x14 − 1.9 × 10−2x23 + 8.7 × 10+1x34 | 0.9597 | 44.96 | <0.0001 |
| Response | Optimal Conditions Aqueous Extraction | Organic Solvent Extraction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 °C, 90 min, 0% acid, 0.02 g mL−1 | 100 °C, 5 min, 0% acid, 0.02 g mL−1 | MeOH:H2O | NaOH-AcEt | Ʃ | |||||||
| Predicted (CV, %) | Experimental | Predicted (CV, %) | Experimental | Free Phenolics | Bound Phenolics | Total Phenolics | |||||
| RSM | ANN | Milled | Raw | RSM | ANN | Milled | Raw | ||||
| TPC (mg g−1) | 6.56 (3.5) | 5.83 (11.8) | 6.89 ± 0.13 d | 6.31 ± 0.46 d | 5.70 (1.4) | 4.97 (8.2) | 5.59 ± 0.06 c | 3.94 ± 0.13 b | 15.99 ± 1.16 e | 2.39 ± 0.29 a | 18.38 |
| TF (mg g−1) | 11.29 (1.2) | 9.82 (11.0) | 11.48 ± 0.05 d | 11.01 ± 0.41 d | 10.56 (0.8) | 9.43 (7.2) | 10.44 ± 0.08 c | 7.08 ± 0.05 b | 26.82 ± 2.65 e | 4.72 ± 0.47 a | 31.55 |
| TFL (mg g−1) | 1.60 (5.5) | 1.61 (5.1) | 1.73 ± 0.07 d | 1.67 ± 0.06 d | 1.33 (1.6) | 1.18 (9.8) | 1.36 ± 0.04 c | 0.78 ± 0.01 b | 1.63 ± 0.30 d | 0.20 ± 0.06 a | 1.83 |
| PAC (mg g−1) | 3.42 (0.0) | 3.14 (5.9) | 3.42 ± 0.05 d | 3.24 ± 0.09 c | 3.23 (0.2) | 3.06 (3.7) | 3.22 ± 0.12 c | 2.68 ± 0.03 b | 3.91 ± 0.37 e | 1.32 ± 0.08 a | 5.23 |
| TPA (mg g−1) | 4.87 (0.5) | 5.01 (2.5) | 4.84 ± 0.48 d | 4.12 ± 0.29 c | 3.90 (0.8) | 3.76 (3.4) | 3.94 ± 0.18 c | 23.64 ± 0.08 b | 6.88 ± 0.23 e | 0.98 ± 0.06 a | 7.86 |
| TOD (mg g−1) | 2.25 (1.6) | 1.97 (7.9) | 2.20 ± 0.06 e | 2.04 ± 0.03 d | 1.87 (0.0) | 1.88 (0.2) | 1.87 ± 0.08 c | 1.28 ± 0.06 b | 5.56 ± 0.30 f | 0.91 ± 0.11 a | 6.47 |
| AC (mg g−1) | 23.64 (0.7) | 22.69 (2.3) | 23.42 ± 0.15 e | 22.36 ± 0.24 d | 21.12 (0.8) | 19.35 (7.0) | 21.36 ± 0.66 d | 14.99 ± 0.18 b | 22.79 ± 1.54 d e | 5.31 ± 0.42 a | 28.10 |
| Compound (µg g−1) | Rt (min) | Mass Spectral Data | Optimal Conditions Aqueous Extraction | Organic Solvent Extraction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [M − H]− (m/z) | MS2 (m/z) | 100 °C, 0% acid, 0.02 g mL−1 | MeOH:H2O | NaOH-AcEt | Ʃ | |||||
| 90 Min | 5 Min | Free Phenolics | Bound Phenolics | Total Phenolics | ||||||
| Milled | Raw | Milled | Raw | |||||||
| Hydroxybenzoic acids | ||||||||||
| Gallic acid | 1.73 | 169 | 125 | 22.79 ± 1.45 d | 10.94 ± 0.81 b | 12.11 ± 0.89 c | 8.51 ± 0.10 a | 23.15 ± 0.30 d | - | 23.15 |
| Protocatechuic acid | 3.34 | 153 | 109 | 127.96 ± 6.86 f | 83.53 ± 5.37 d | 66.20 ± 8.73 c | 55.08 ± 2.11 b | 99.94 ± 1.33 e | 42.82 ± 8.59 a | 142.76 |
| 4-hydroxybenzoic acid | 4.43 | 137 | 93 | 3.51 ± 0.34 c | 2.47 ± 0.19 b | 3.19 ± 0.36 c | 1.56 ± 0.07 a | 4.30 ± 0.07 d | 3.75 ± 0.51 c d | 8.06 |
| Vanillic acid | 5.43 | 167 | 152 | 6.00 ± 2.52 a | - | - | - | 9.55 ± 0.20 b | - | 9.55 |
| Syringic acid | 5.96 | 197 | 182 | - | - | 0.20 ± 0.03 a | - | 1.66 ± 0.10 b | 0.20 ± 0.01 a | 1.86 |
| Salicylic acid | 8.96 | 137 | 93 | 0.81 ± 0.03 b | 0.90 ± 0.08 b | 0.22 ± 0.04 a | - | 1.19 ± 0.02 c | 0.25 ± 0.03 a | 1.43 |
| Hydroxycinnamic acids | ||||||||||
| Chlorogenic acid | 5.38 | 353 | 191 | 905.67 ± 18.50 e | 747.17 ± 36.10 c | 840.04 ± 30.79 d | 669.54 ± 40.06 b | 1428.40 ± 25.80 f | 0.67 ± 0.08 a | 1429.07 |
| Caffeic acid | 5.48 | 179 | 135 | 15.16 ± 0.51 b | 10.51 ± 1.07 a | 14.00 ± 1.50 b | 9.35 ± 0.11 a | 26.21 ± 0.79 c | 89.93 ± 9.73 d | 116.14 |
| p-coumaric acid | 6.81 | 163 | 119 | 2.27 ± 0.04 b | 1.39 ± 0.11 a | 4.22 ± 0.14 d | 1.37 ± 0.06 a | 2.94 ± 0.04 c | 8.21 ± 1.31 e | 11.15 |
| Ferulic acid | 7.81 | 193 | 134 | - | - | 4.25 ± 0.36 b | - | 1.74 ± 0.08 a | 5.08 ± 0.67 c | 6.81 |
| Phenylacetic acids | ||||||||||
| 3,4-dihydroxyphenylacetic acid | 4.18 | 167 | 123 | 1.46 ± 0.51 b | 1.04 ± 0.04 a | - | - | - | - | - |
| Flavan-3-ols: monomers | ||||||||||
| (+)-catechin | 5.22 | 289 | 245 | 0.45 ± 0.05 b | - | 0.31 ± 0.03 a | - | 30.31 ± 0.28 d | 0.58 ± 0.08 c | 30.89 |
| (−)-epicatechin | 6.27 | 289 | 245 | 4.71 ± 0.51 c | 1.45 ± 0.06 b | - | - | 25.07 ± 0.23 d | 0.64 ± 0.05 a | 25.71 |
| Flavan-3-ols: dimers | ||||||||||
| Procyanidin B1 | 4.90 | 577 | 289 | 5.84 ± 0.69 | - | - | - | - | - | - |
| Procyanidin B2 | 5.93 | 577 | 289 | 3.03 ± 0.46 c | 0.93 ± 0.17 b | 0.76 ± 0.09 a | 0.77 ± 0.08 a | - | - | - |
| Flavonols | ||||||||||
| Quercetin-3-O-glucoside | 8.34 | 463 | 301 | 15.03 ± 0.96 d | 8.89 ± 1.17 b | 10.45 ± 0.73 c | 10.39 ± 0.44 c | 18.16 ± 0.42 e | 0.66 ± 0.03 a | 18.82 |
| Quercetin-3-O-galactoside | 8.65 | 463 | 301 | 14.33 ± 0.14 d | 8.79 ± 0.42 b | 10.20 ± 1.91 c | 10.25 ± 0.26 c | 18.17 ± 0.41 e | 0.67 ± 0.05 a | 18.84 |
| Kaempferol-3-O-galactoside | 9.46 | 447 | 284 | 32.12 ± 0.94 e | 18.27 ± 0.83 d | 11.50 ± 0.24 b | 14.13 ± 1.46 c | 40.32 ± 0.85 f | 1.05 ± 0.11 a | 41.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Benítez, V.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds. Foods 2021, 10, 653. https://doi.org/10.3390/foods10030653
Rebollo-Hernanz M, Cañas S, Taladrid D, Benítez V, Bartolomé B, Aguilera Y, Martín-Cabrejas MA. Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds. Foods. 2021; 10(3):653. https://doi.org/10.3390/foods10030653
Chicago/Turabian StyleRebollo-Hernanz, Miguel, Silvia Cañas, Diego Taladrid, Vanesa Benítez, Begoña Bartolomé, Yolanda Aguilera, and María A. Martín-Cabrejas. 2021. "Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds" Foods 10, no. 3: 653. https://doi.org/10.3390/foods10030653
APA StyleRebollo-Hernanz, M., Cañas, S., Taladrid, D., Benítez, V., Bartolomé, B., Aguilera, Y., & Martín-Cabrejas, M. A. (2021). Revalorization of Coffee Husk: Modeling and Optimizing the Green Sustainable Extraction of Phenolic Compounds. Foods, 10(3), 653. https://doi.org/10.3390/foods10030653












