Optimization of High Hydrostatic Pressure Treatments on Soybean Protein Isolate to Improve Its Functionality and Evaluation of Its Application in Yogurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High Hydrostatic Pressure (HHP) Treatments
2.3. The Response Surface Methodology (RSM) Design
2.4. Determination of the Functional Properties of SPI
2.4.1. Solubility
2.4.2. Water Holding Capacity (WHC)
2.4.3. Emulsifying Properties
2.5. Determination of the Physicochemical Properties
2.5.1. Surface Hydrophobicity (H0)
2.5.2. Sulphydryl Group Content
2.5.3. Lipoxygenase (LOX) Activity
2.5.4. Circular Dichroism (CD) Spectra Analysis
2.6. Preparation of SPI Incorporated Yogurt
2.7. Determination of Physicochemical Properties of the SPI Incorporated Yogurt
2.8. Flavor Analysis of the SPI Incorporated Yogurt
2.9. Statistical Analysis
3. Results and Discussion
3.1. Optimization of HHP Parameters by RSM
3.2. Effect of HHP on Properties of SPI
3.2.1. Effect of HHP on Physicochemical Properties of SPI
3.2.2. Effect of HHP on Functional Properties of SPI
3.3. Yogurt Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirotani, N.; Hougaard, A.B.; Lametsch, R.; Petersen, M.A.; Rattray, F.P.; Ipsen, R. Proteolytic activity of selected commercial lactobacillus helveticus strains on soy protein isolates. Food Chem. 2021, 340, 128152. [Google Scholar] [CrossRef]
- He, M.; Wu, C.; Li, L.; Zheng, L.; Teng, F. Effects of cavitation jet treatment on the structure and emulsification properties of oxidized soy protein isolate. Foods 2020, 10, 2. [Google Scholar] [CrossRef]
- Huang, L.; Ding, X.; Dai, C.; Ma, H. Changes in the structure and dissociation of soybean protein isolate induced by ultrasound-assisted acid pretreatment. Food Chem. 2017, 232, 727–732. [Google Scholar] [CrossRef]
- Xia, W.; Pan, S.; Cheng, Z.; Tian, Y.; Huang, X. High-Intensity Ultrasound Treatment on Soy Protein after Selectively Proteolyzing Glycinin Component: Physical, Structural, and Aggregation Properties. Foods 2020, 9, 839. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and application of high pressure–based technologies in the food industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Winter, R.; Lopes, D.; Grudzielanek, S.; Vogtt, K. Towards an understanding of the temperature/pressure configurational and free-energy landscape of biomolecules. J. Non-Equilib. Thermodyn. 2007, 32, 41–97. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Aliabadi, S.; Hosseini, S. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Puppo, C.; Chapleau, N.; Speroni, F.; Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar] [CrossRef]
- Wang, X.; Tang, G.; Li, B.; Yang, X.; Li, L.; Ma, C. Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocoll. 2008, 22, 560–567. [Google Scholar] [CrossRef]
- Xi, J.; He, M. High hydrostatic pressure (HHP) effects on antigenicity and structural properties of soybean β-conglycinin. J. Food Sci. Technol. 2017, 55, 630–637. [Google Scholar] [CrossRef]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W. Effects of high hydrostatic pressure on some functional and nutritional properties of soy protein isolate for infant formula. J. Agric. Food Chem. 2011, 59, 12028–12036. [Google Scholar] [CrossRef] [PubMed]
- Manassero, C.A.; David-Briand, E.; Vaudagna, S.R.; Anton, M.; Speroni, F. Calcium Addition, pH, and High Hydrostatic Pressure Effects on Soybean Protein Isolates—Part 1: Colloidal Stability Improvement. Food Bioprocess Technol. 2018, 11, 1125–1138. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Liu, Z.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Effects of fat content on the textural and in vivo buccal breakdown properties of soy yogurt. Journal of Texture Studies. J. Texture Stud. 2021. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I.; Desjardins, M.P.; Gardner, N.; Fliss, I.; Champagne, C. Growth of probiotic bacteria and bifidobacteria in a soy yogurt formulation. Int. J. Food Microbiol. 2007, 116, 174–181. [Google Scholar] [CrossRef]
- Bernat, N.; Chafer, M.; Chiralt, A.; Gonzalez-Martineza, C. Vegetable milks and their fermented derivative products. Int. J. Food Stud. 2014, 3, 93–124. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef]
- Manassero, C.A.; Vaudagna, S.R.; Sancho, A.M.; Añón, M.C.; Speroni, F. Combined high hydrostatic pressure and thermal treatments fully inactivate trypsin inhibitors and lipoxygenase and improve protein solubility and physical stability of calcium-added soymilk. Innov. Food Sci. Emerg. Technol. 2016, 35, 86–95. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N. Amaranth protein films prepared with high-pressure treated proteins. J. Food Eng. 2015, 166, 38–44. [Google Scholar] [CrossRef]
- Ogunwolu, O.; Henshaw, F.; Mock, H.; Santros, A.; Awonorin, S. Functional properties of protein concentrates and isolates produced from cashew (Anacardium occidentale L.) nut. Food Chem. 2009, 115, 852–858. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Yang, H.; Yang, A.; Gao, J.; Chen, H. Characterization of Physicochemical Properties and IgE-Binding of Soybean Proteins Derived from the HHP-Treated Seeds. J. Food Sci. 2014, 79, C2157–C2163. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Mu, T.; Zhang, M.; Arogundade, L.A. The effects of pH and high hydrostatic pressure on the physicochemical properties of a sweet potato protein emulsion. Food Hydrocoll. 2014, 35, 209–216. [Google Scholar] [CrossRef]
- Beveridge, T.; Toma, S.; Nakai, S. Determination of SH-and SS-groups in some food proteins using Ellman’s reagent. J. Food Sci. 1974, 39, 49–51. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, Q.; Liu, X.H.; Chen, Z.X. Inactivation of soybean lipoxygenase in soymilk by pulsed electric fields. Food Chem. 2008, 109, 408–414. [Google Scholar] [CrossRef]
- Silva, J.V.C.; O’Mahony, J.A. Microparticulated whey protein addition modulates rheological and microstructural properties of high-protein acid milk gels. Int. Dairy J. 2018, 78, 145–151. [Google Scholar] [CrossRef]
- Sanz, T.; Salvador, A.; Jiménez, A.; Fiszman, S.M. Yogurt enrichment with functional asparagus fibre. Effect of fibre extraction method on rheological properties, colour, and sensory acceptance. Eur. Food Res. Technol. 2008, 227, 1515–1521. [Google Scholar] [CrossRef]
- Mei, J.; Feng, F.; Li, Y. Effective of different homogeneous methods on physicochemical, textural and sensory characteristics of soybean (Glycine max L.) yogurt. CyTA J. Food 2016, 15, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H. Volatile Flavor Compounds in Yogurt: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef]
- Molina, E.; Defaye, A.B.; Ledward, D.A. Soy protein pressure-induced gels. Food Hydrocoll. 2002, 16, 625–632. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7s and 11s globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; Silva, J. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, K.; Zhou, H.; Peng, W.; Guo, X. Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric. Immunol. 2016, 27, 1–20. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Hua, Y.; Kong, X.; Zhang, C.; Chen, Y.; Wang, S. The absence of lipoxygenase and 7S globulin of soybeans and heating temperatures on the properties of soymilks and soy yogurts. LWT Food Sci. Technol. 2019, 115, 108431. [Google Scholar] [CrossRef]
- Li, X.; Mao, L.; He, X.; Ma, P.; Yuan, F. Characterization of β-lactoglobulin gels induced by high pressure processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 335–345. [Google Scholar] [CrossRef]
- Zhu, S.M.; Lin, S.L.; Ramaswamy, H.S.; Yu, Y.; Zhang, Q.T. Enhancement of functional properties of rice bran proteins by high pressure treatment and their correlation with surface hydrophobicity. Food Bioprocess Technol. 2017, 10, 317–327. [Google Scholar] [CrossRef]
- Puppo, M.C.; Speroni, F.; Chapleau, N.; Lamballerie, M.; Añón, M.C.; Anton, M. Effect of high-pressure treatment on emulsifying properties of soybean proteins. Food Hydrocoll. 2005, 19, 289–296. [Google Scholar] [CrossRef]
- Mohammadi, R.; Yousefi, M.; Zahra, S.; Shah, N.P.; Mortazavian, A.M.; Sadeghi, E.; Khajavi, M.Z. Influence of commercial culture composition and cow milk to soy milk ratio on the biochemical, microbiological, and sensory characteristics of a probiotic fermented composite drink. Food Sci. Biotechnol. 2017, 26, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Cruz, N.; Capellas, M.; Jaramillo, D.P.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Soymilk treated by ultra high-pressure homogenization: Acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll. 2009, 23, 490–496. [Google Scholar] [CrossRef]
- Cruz, N.; Capellas, M.; Hernández, M.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Ultra high pressure homogenization of soymilk: Microbiological, physicochemical and microstructural characteristics. Food Res. Int. 2007, 40, 725–732. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- İçier, F.; Gündüz, G.T.; Yılmaz, B.; Memeli, Z. Changes on some quality characteristics of fermented soy milk beverage with added apple juice. LWT Food Sci. Technol. 2015, 63, 57–64. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, R.; Luo, T.; Che, X.; Bansal, N.; Liu, X. Physiochemical properties of modified starch under yogurt manufacturing conditions and its relation to the properties of yogurt. J. Food Eng. 2019, 245, 11–17. [Google Scholar] [CrossRef]
- Penna, A.L.B.; Sivieri, K.; Oliveira, M.N. Relation between quality and rheological properties of lactic beverages. J. Food Eng. 2001, 49, 7–13. [Google Scholar] [CrossRef]
- Blagden, T.D.; Gilliland, S.E. Reduction of levels of volatile components associated with the “beany” flavor in soymilk by lactobacilli and streptococci. J. Food Sci. 2005, 70, M186–M189. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Studies on the key aroma compounds in soy milk made from three different soybean cultivars. J. Agric. Food Chem. 2011, 59, 12204–12209. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, M.; Qiu, C.; Sun, W. Effect of malondialdehyde modification on the binding of aroma compounds to soy protein isolates. Food Res. Int. 2018, 105, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Alhendi, A.; Yang, W.; Goodrich-Schneider, R.; Sims, C.; Marshall, S.; Sarnoski, P.J. Sensory evaluation and flavour analysis of soymilk produced from lipoxygenase-free soya beans after modified processes and pulsed light treatment. Int. J. Food Sci. Technol. 2018, 53, 1434–1441. [Google Scholar] [CrossRef]
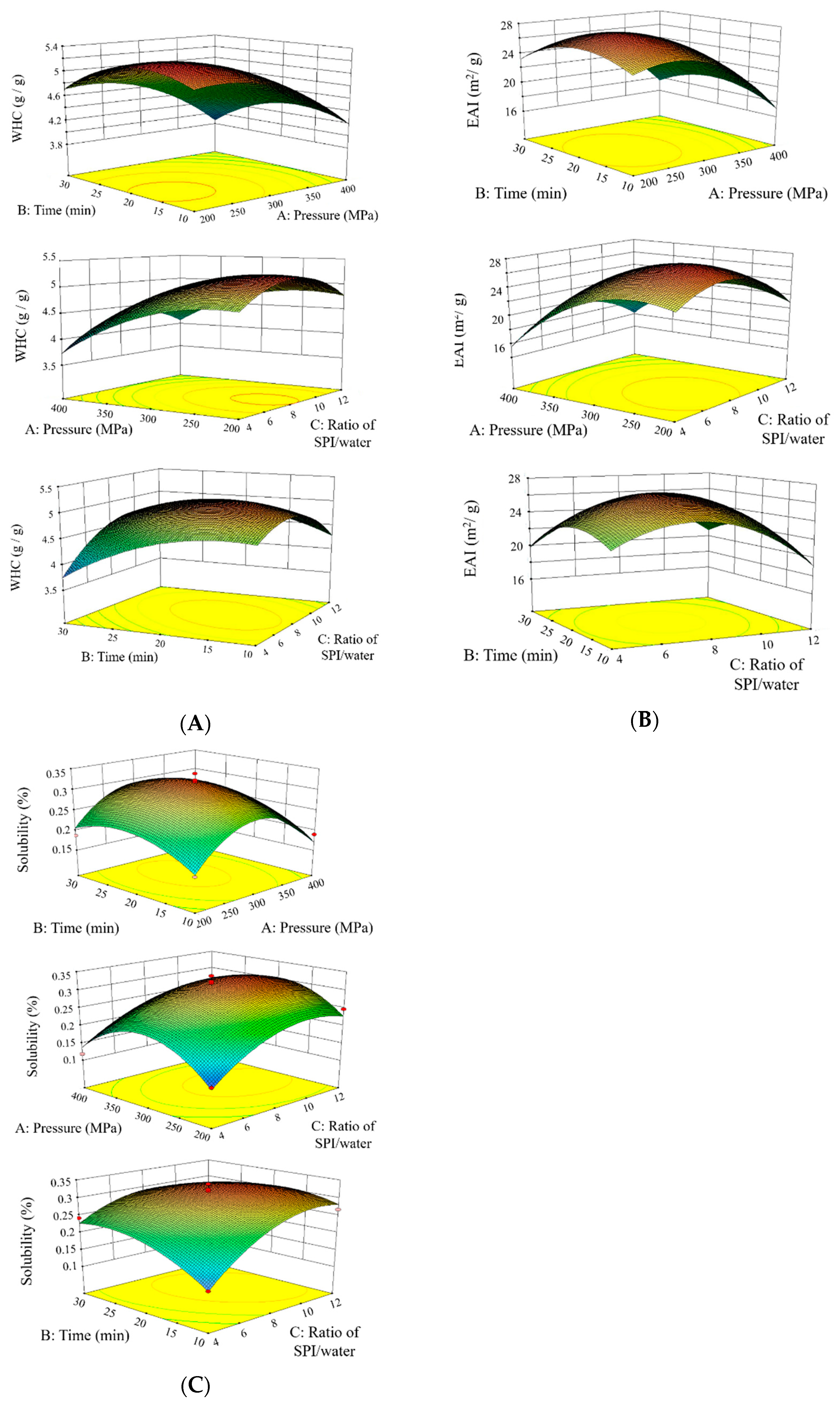




| Order | Pressure (MPa) | Time (min) | SPI/Water Ratio | Water Holding Capacity (g/g) | Emulsifying Activity Index (m2/g) | Solubility (%) |
|---|---|---|---|---|---|---|
| 1 | 200 | 20 | 1:4 | 4.63 ± 0.19 | 24.10 ± 0.52 | 12.6 ± 1.2 |
| 2 | 400 | 30 | 1:8 | 3.91 ± 0.05 | 18.40 ± 0.59 | 20.1 ± 1.7 |
| 3 | 400 | 20 | 1:4 | 3.77 ± 0.15 | 13.86 ± 1.24 | 11.8 ± 0.4 |
| 4 | 300 | 30 | 1:12 | 4.43 ± 0.29 | 20.33 ± 1.49 | 24.5 ± 0.8 |
| 5 | 300 | 20 | 1:8 | 5.04 ± 0.05 | 27.34 ± 0.93 | 33.8 ± 4.1 |
| 6 | 300 | 20 | 1:8 | 5.26 ± 0.31 | 27.13 ± 1.21 | 30.6 ± 2.3 |
| 7 | 200 | 30 | 1:8 | 4.88 ± 0.65 | 22.02 ± 1.24 | 18.7 ± 1.9 |
| 8 | 200 | 10 | 1:8 | 4.98 ± 0.15 | 23.49 ± 1.01 | 16.7 ± 3.3 |
| 9 | 300 | 10 | 1:12 | 4.37 ± 0.32 | 18.26 ± 0.31 | 26.7 ± 2.7 |
| 10 | 300 | 10 | 1:4 | 4.80 ± 0.47 | 22.64 ± 0.39 | 13.1 ± 1.6 |
| 11 | 300 | 20 | 1:8 | 5.02 ± 0.48 | 25.40 ± 0.12 | 29.6 ± 4.5 |
| 12 | 300 | 30 | 1:4 | 3.67 ± 0.11 | 20.75 ± 0.11 | 24.2 ± 2.6 |
| 13 | 300 | 20 | 1:8 | 5.11 ± 0.26 | 25.70 ± 0.87 | 31.9 ± 2.8 |
| 14 | 400 | 10 | 1:8 | 4.03 ± 0.03 | 18.37 ± 1.24 | 19.0 ± 1.2 |
| 15 | 300 | 20 | 1:8 | 5.09 ± 0.28 | 27.85 ± 1.39 | 32.3 ± 2.6 |
| 16 | 200 | 20 | 1:12 | 4.73 ± 0.75 | 22.65 ± 1.12 | 24.6 ± 3.4 |
| 17 | 400 | 20 | 1:12 | 4.76 ± 0.49 | 15.94 ± 0.78 | 19.3 ± 3.7 |
| Control | SPI Yogurt | HHP-SPI Yogurt | ||
|---|---|---|---|---|
| Final pH | 4.15 ± 0.02 a | 4.56 ± 0.05 b | 4.53 ± 0.03 b | |
| titration acidity (°T) | 67.2 ± 0.4 b | 63.6 ± 0.6 a | 64.1 ± 0.3 a | |
| WHC (%) | 56.37 ± 1.71 a | 83.42 ± 0.96 b | 90.22 ± 2.83 c | |
| color | L* 1 | 80.70 ± 0.02 c | 74.02 ± 0.01 a | 74.64 ± 0 b |
| A* 2 | −1.89 ± 0.01 a | 0.33 ± 0 c | −0.18 ± 0.01 b | |
| B* 3 | 10.30 ± 0.02 a | 15.21 ± 0.01 c | 13.66 ± 0.01 b | |
| - | 8.58 ± 0.01 b | 7.13 ± 0.01 a | ||
| Samples | Shear Rate Rise | Shear Rate Drop | ||||
|---|---|---|---|---|---|---|
| n | k(Pa*sn) | R2 | n | k(Pa*sn) | R2 | |
| control | 0.39 ± 0.01 c | 32.35 ± 0.13 a | 0.98 | 2.35 ± 0.01 c | 15.83 ± 0.02 c | 0.98 |
| SPI | 0.23 ± 0.01 a | 41.87 ± 3.08 b | 0.99 | 0.80 ± 0.01 b | 3.84 ± 1.41a | 0.99 |
| HHP-SPI | 0.31 ± 0.01 b | 58.85 ± 6.11 c | 0.98 | 0.77 ± 0.01 a | 9.53 ± 4.88 h | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yin, H.; Zhao, Y.; Zheng, Y.; Xu, X.; Yue, J. Optimization of High Hydrostatic Pressure Treatments on Soybean Protein Isolate to Improve Its Functionality and Evaluation of Its Application in Yogurt. Foods 2021, 10, 667. https://doi.org/10.3390/foods10030667
Wang C, Yin H, Zhao Y, Zheng Y, Xu X, Yue J. Optimization of High Hydrostatic Pressure Treatments on Soybean Protein Isolate to Improve Its Functionality and Evaluation of Its Application in Yogurt. Foods. 2021; 10(3):667. https://doi.org/10.3390/foods10030667
Chicago/Turabian StyleWang, Chenxiao, Hao Yin, Yanyun Zhao, Yan Zheng, Xuebing Xu, and Jin Yue. 2021. "Optimization of High Hydrostatic Pressure Treatments on Soybean Protein Isolate to Improve Its Functionality and Evaluation of Its Application in Yogurt" Foods 10, no. 3: 667. https://doi.org/10.3390/foods10030667







