Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedures
2.2. Extraction Yield
2.3. Chemical Characterisation
2.3.1. Total Protein Content
2.3.2. Total Carbohydrate Content
2.3.3. Chlorophyll Contents
2.3.4. Total Phenolic and Flavonoid Contents
2.3.5. Identification and Quantification of Individual Phenolic Compounds
2.4. Bioactivities
2.4.1. Antioxidant Activity
2.4.2. Antimicrobial Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Influence of Extraction Yield, Chemical Characteristics, and Antioxidant Activity on Extracts Differentiation
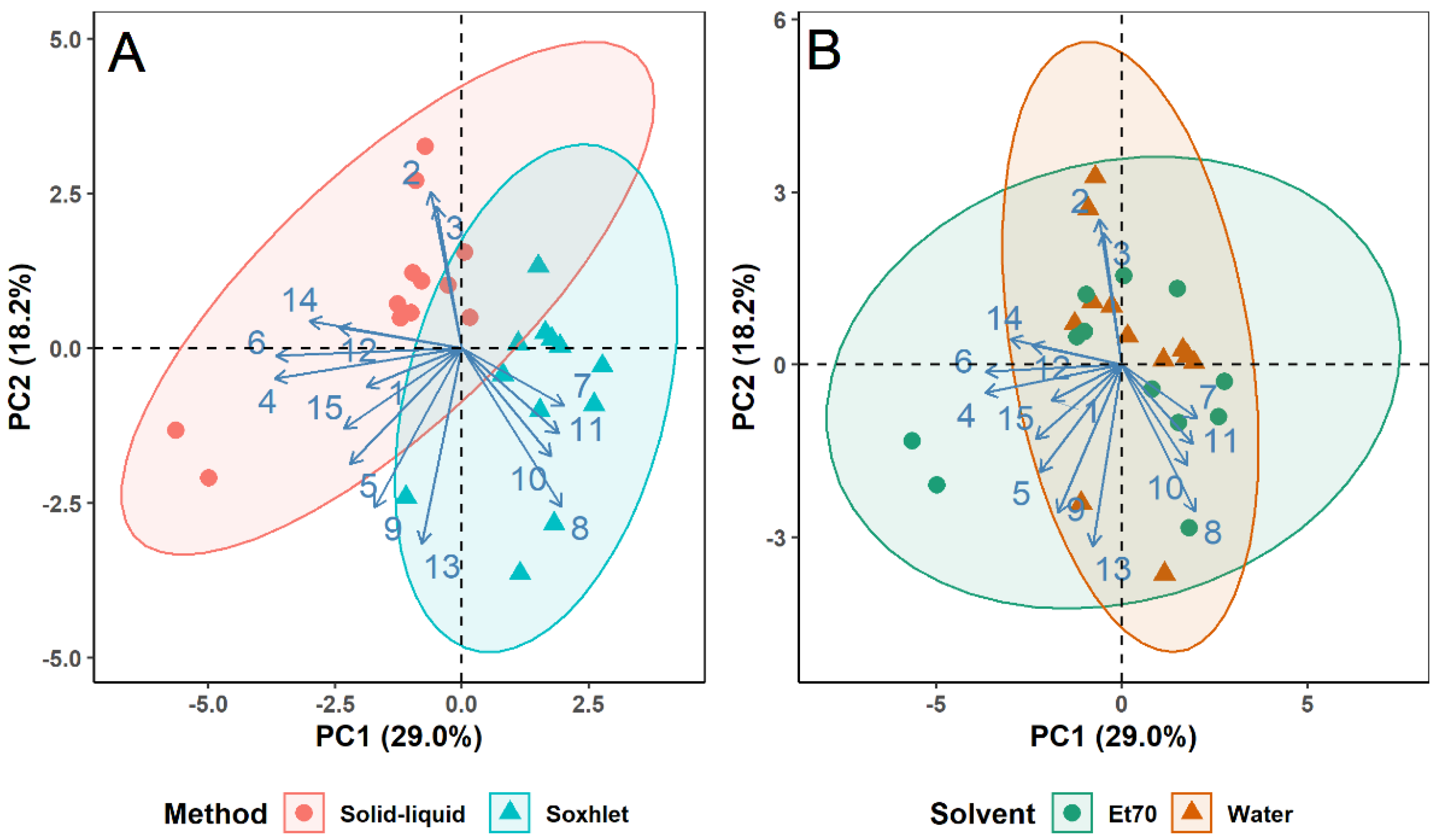
3.2. Influence of Extraction Method, Solvent, and Plant Type on Extracts Differentiation–Principal Component Analysis
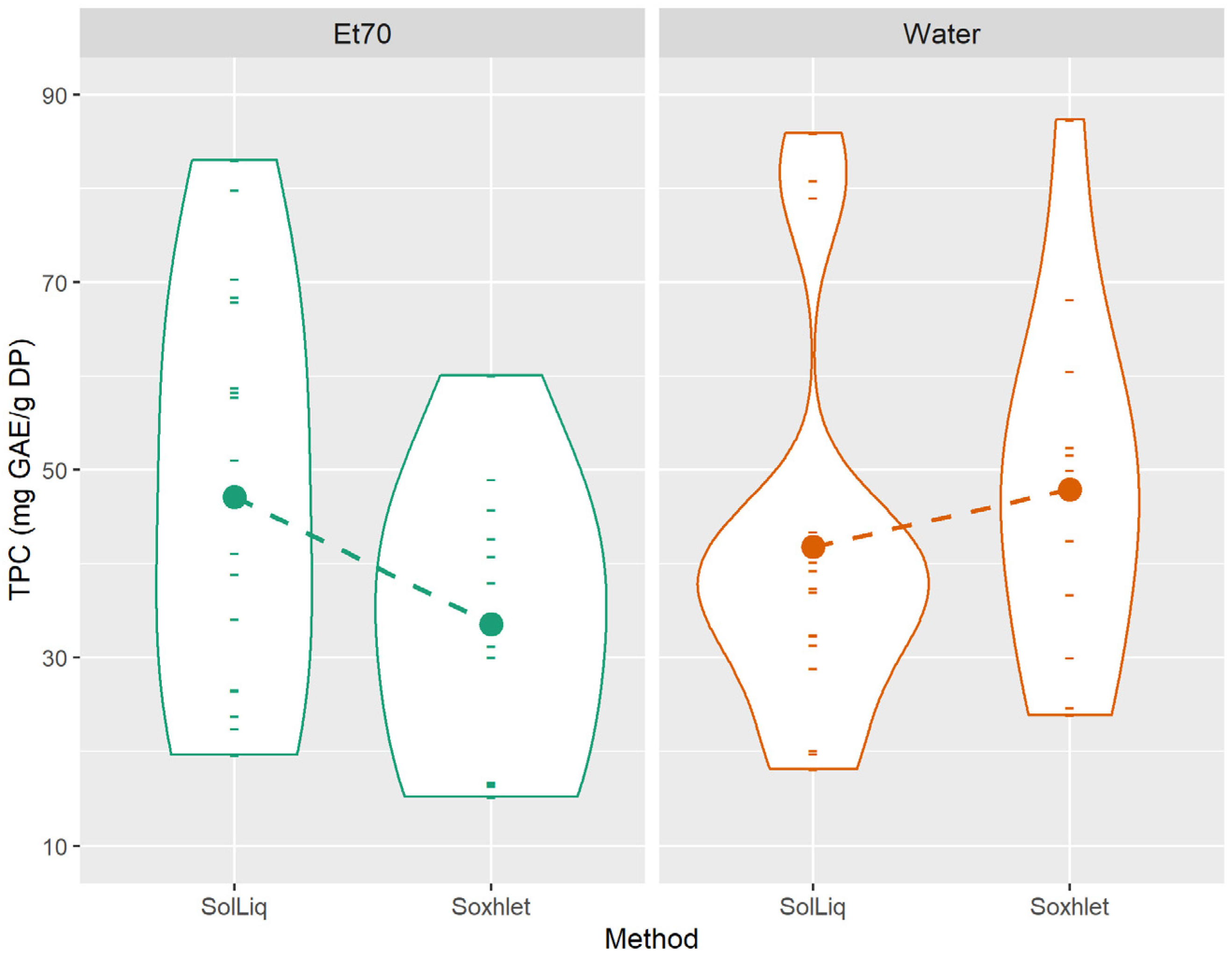
3.3. Influence of Extraction Method, Solvent, and Plant Type on Extracts Differentiation–Main Effects and Interactions
3.4. Influence of Extraction Method and Solvent on Phenolic Profile of Plant Extracts
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, B.G.; Bahna, S.L. Adverse reactions to food additives. Ann. Allergy Asthma Immunol. 2005, 95, 499–507. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural Preservatives to Improve Food Quality and Safety. J. Food Qual. 2017, 2017, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Antolak, H.; Kregiel, D. Food Preservatives from Plants. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; IntechOpen: London, UK, 2017; pp. 45–85. [Google Scholar]
- Regnier, T.; Combrinck, S.; Du Plooy, W. Essential Oils and Other Plant Extracts as Food Preservatives. In Progress in Food Preservation; Bhat, R., Karim Alias, A., Paliyath, G., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 539–579. [Google Scholar]
- Bouarab-Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Santos, J.S.; Salem, R.D.; Mortazavian, A.M.; Rocha, R.S.; Cruz, A.G. Effects of herbal extracts on quality traits of yogurts, cheeses, fermented milks, and ice creams: A technological perspective. Curr. Opin. Food Sci. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.; Leal, A.L.A.B.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Carocho, M.; Barreira, J.C.M.; Bento, A.; Fernández-Ruiz, V.; Morales, P.; Ferreira, I.C.F.R. Chestnut and lemon balm based ingredients as natural preserving agents of the nutritional profile in matured “Serra da Estrela” cheese. Food Chem. 2019, 204, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytoscience 2020, 6, 9. [Google Scholar]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Ereifej, K.; Al-Mahasneh, M.; Brewer, S.; Rawshdeh, M. Optimization extraction conditions for phenolic compounds, antioxidant and inhibitory activities of angiotensin-I converting enzyme (ACE), α-glucosidase and α-amylase from Mentha spicata. L. J. Food Biochem. 2015, 40, 335–344. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Effects of essential oils on Escherichia coli inactivation in cheese as described by meta-regression modelling. Foods 2020, 9, 716. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-Regression models describing the effects of essential oils and added lactic acid bacteria on pathogen inactivation in cheese. Microb. Risk Anal. 2020, in press. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Meawad, A.A.; Abou-Zeid, E.N.; Ali, M.S. Evaluation of volatile oil and its chemical constituents of some basil varieties in Egypt. Int. J. Plant Res. 2015, 1, 103–106. [Google Scholar]
- Pereira, R.P.; Boligon, A.A.; Appel, A.S.; Fachinetto, R.; Ceron, C.S.; Tanus-Santos, J.E.; Athayde, M.L.; Rocha, J.B.T. Chemical composition, antioxidant and anticholinesterase activity of Melissa officinalis. Ind. Crop. Prod. 2014, 53, 34–45. [Google Scholar] [CrossRef]
- Świąder, K.; Startek, K.; Wijaya, C.H. The therapeutic properties of Lemon balm (Melissa officinalis L.): Reviewing novel findings and medical indications. J. Appl. Bot. Food Qual. 2019, 92, 327–335. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum EMA/HMPC/150801/2015. 2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-salvia-officinalis-l-folium-salvia-officinalis-l-aetheroleum-revision-1_en.pdf (accessed on 12 December 2020).
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 2231–2606. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Ibarz, R.; Fernandes, J.-M.; Pinheiro, A.C.; Botelho, C.; Rocha, C.M.R.; Teixeira, J.A.; Martín-Belloso, O. Encapsulated pine bark polyphenolic extract during gastrointestinal digestion: Bioaccessibility, bioactivity and oxidative stress prevention. Foods 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Busaranon, K.; Suntornsuk, W.; Suntornsuk, L. Comparison of UV spectrophotometric method and high performance liquid chromatography for the analysis of flunarizine and its application for the dissolution test. J. Pharm. Biomed. Anal. 2006, 41, 158–164. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; CLSI standard M07 Clinical and Laboratory Standards Institute: Wayne, Pennsylvania, USA, 2012. [Google Scholar]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 February 2020).
- Højsgaard, S.; Halekoh, U. doBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities. R package version 4.6.9. 2021. Available online: https://CRAN.R-project.org/package=doBy (accessed on 5 January 2021).
- Kay, M.; Elkin, L.; Higgins, J.; Wobbrock, J. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs. R package version 0.11.0. 2021. Available online: https://cran.r-project.org/web/packages/ARTool/index.html (accessed on 7 January 2021).
- Length, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.4. 2021. Available online: https://CRAN.R-project.org/package=emmeans. (accessed on 15 February 2021).
- R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 8 October 2020).
- Mussatto, S.I. Generating Biomedical Polyphenolic Compounds from Spent Coffee or Silverskin. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Inc.: London, UK, 2015; pp. 93–106. [Google Scholar]
- Dunn, K. Process Improvement Using Data (version 73d667). 2020. Available online: https://learnche.org/pid/ (accessed on 15 February 2021).
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Turumtay, E.A.; Islamoǧlu, F.; Çavuş, D.; Şahin, H.; Turumtay, H.; Vanholme, B. Correlation between phenolic compounds and antioxidant activity of Anzer tea (Thymus praecox Opiz subsp. caucasicus var. caucasicus). Ind. Crops Prod. 2014, 52, 687–694. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Awad, M.A.; El-Dengawy, E.R.F.A.; Abdel-Mageed, H.M.; El-Badry, M.O.; Salah, H.A.; Abdel-Aty, A.M.; Fahmy, A.S. Total phenolic and flavonoid contents and antioxidant activities of sixteen commercial date cultivars grown in Saudi Arabia. RSC Adv. 2016, 6, 44814–44819. [Google Scholar] [CrossRef]
- Augusto, T.R.; Salinas, E.S.S.; Alencar, S.M.; D’arce, M.A.B.R.; de Camargo, A.C.; Vieira, T.M.F.S. Phenolic compounds and antioxidant activity of hydroalcoholic extracts of wild and cultivated murtilla (Ugni molinae Turcz.). Food Sci. Technol. 2014, 34, 667–679. [Google Scholar] [CrossRef] [Green Version]
- Teofilović, B.; Grujić-Letić, N.; Goločorbin-Kon, S.; Stojanović, S.; Vastag, G.; Gadžurić, S. Experimental and chemometric study of antioxidant capacity of basil (Ocimum basilicum) extracts. Ind. Crops Prod. 2017, 100, 176–182. [Google Scholar] [CrossRef]
- Martins, S.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Sep. Purif. Technol. 2012, 88, 163–167. [Google Scholar] [CrossRef] [Green Version]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Chemical basis of anti-listerial effects of rosemary herb during stomaching with fresh-cut vegetables. LWT Food Sci. Technol. 2014, 57, 16–21. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. High pressure solvent extraction of maritime pine bark: Study of fractionation, solvent flow rate and solvent composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Bai, X.-L.; Yue, T.-L.; Yuan, Y.-H.; Zhang, H.-W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate electric fields as a potential tool for sustainable recovery of phenolic compounds from Pinus pinaster bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef] [Green Version]
- Chin, F.-S.; Chong, K.-P.; Markus, A.; Wong, N.K. Tea polyphenols and alkaloids content using Soxhlet and direct extraction methods. World J. Agric. Sci. 2013, 9, 266–270. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Nunes, R.; Pasko, P.; Tyszka-Czochara, M.; Szewczyk, A.; Szlosarczyk, M.; Carvalho, I.S. Antibacterial, antioxidant and antiproliferative properties and zinc content of five south Portugal herbs. Pharm. Biol. 2017, 55, 114–123. [Google Scholar]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Brede, C. Flavonoids and other phenolics in herbs commonly used in Norwegian commercial kitchens. Food Chem. 2020, 309. [Google Scholar] [CrossRef] [PubMed]
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L.E.; Shokrpour, M.; Hadian, J. Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. Hortic. Environ. Biotechnol. 2017, 58, 414–422. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Bitssam, R.; Fatima, E.M.; Kamal, E.; Hassane, G.; Mohamed, N. Composition and antibacterial activity of hydro-alcohol and aqueous extracts obtained from the Lamiaceae family. Pharmacogn. J. 2018, 10, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Stanojević, D.; Čomić, L.; Stefanović, O. Synergy between Salvia officinalis L. and some preservatives. Cent. Eur. J. Biol. 2010, 5, 491–495. [Google Scholar] [CrossRef]
- Scherer, R.; Lemos, M.F.; Lemos, M.F.; Martinello, G.C.; Martins, J.D.L.; da Silva, A.G. Antioxidant and antibacterial activities and composition of Brazilian spearmint (Mentha spicata L.). Ind. Crops Prod. 2013, 50, 408–413. [Google Scholar] [CrossRef]
- Caleja, C.; Finimundy, T.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Smiljkovic, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951. [Google Scholar] [CrossRef] [Green Version]
- Ceyhan, N.; Keskin, D.; Uğur, A. Antimicrobial activities of different extracts of eight plant species from four different family against some pathogenic microorganisms. J. Food Agric. Environ. 2012, 10, 193–197. [Google Scholar]




| Yield (%) | Ch-a (μg/g DP) | Ch-b (μg/g DP) | TProtein (μg BSAE/g DP) | TFC (mg CE/g DP) | TPC (mg GAE/g DP) | Carbohydr. (μg GE/g DP) | DPPH (μmol TE/g DP) | ABTS (μmol TE/g DP) | FRAP (μmol Fe2+/g DP) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant | ||||||||||
| Tarragon | 23.1 ± 1.34 bc | 96.9 ± 28.7 a | 132 ± 33.0 bc | 4.19 ± 0.83 c | 8.05 ± 0.78 c | 20.0 ± 0.95 e | 17.6 ± 2.44 b | 61.8 ± 6.59 c | 107 ± 6.07 c | 191 ± 11.5 e |
| Spearmint | 21.5 ± 1.33 d | 92.6 ± 23.8 a | 149 ± 35.0 ab | 8.91 ± 0.81 b | 30.5 ± 1.23 b | 44.0 ± 3.16 c | 13.7 ± 1.39 cd | 259 ± 14.4 b | 361 ± 20.4 b | 722 ± 31.0 b |
| Lemon balm | 26.2 ± 1.24 a | 96.1 ± 14.8 a | 209 ± 33.1 a | 10.4 ± 0.77 a | 45.6 ± 4.86 a | 74.4 ± 3.90 a | 22.0 ± 1.76 a | 345 ± 11.0 a | 507 ± 28.6 a | 1013 ± 75.5 a |
| Basil | 22.2 ± 1.87 cd | 68.9 ± 16.8 b | 108 ± 22.1 d | 6.02 ± 0.94 c | 16.2 ± 1.07 c | 26.9 ± 2.07 d | 11.8 ± 1.21 d | 149 ± 12.0 c | 194 ± 12.6 c | 376 ± 28.0 d |
| French lavender | 25.2 ± 0.78 ab | 43.5 ± 6.60 c | 115 ± 9.18 cd | 10.7 ± 1.54 a | 32.2 ± 0.94 b | 43.1 ± 2.45 c | 21.6 ± 0.88 a | 241 ± 20.5 b | 326 ± 25.4 b | 614 ± 51.4 c |
| Sage | 22.4 ± 0.41 cd | 59.8 ± 12.4 bc | 99.9 ± 13.4 d | 8.98 ± 1.16 b | 30.9 ± 2.18 b | 49.5 ± 3.87 b | 16.5 ± 1.65 bc | 265 ± 13.8 b | 358 ± 23.1 b | 752 ± 44.5 b |
| Method | ||||||||||
| Solid-liquid | 24.8 ± 0.55 a | 80.8 ± 9.85 a | 163 ± 16.6 a | 6.15 ± 0.46 b | 26.3 ± 2.39 b | 44.5 ± 3.46 a | 18.0 ± 0.92 a | 216 ± 17.0 a | 310 ± 24.6 a | 579 ± 47.8 b |
| Soxhlet | 21.4 ± 0.91 b | 69.7 ± 12.7 b | 93.6 ± 8.46 b | 11.3 ± 0.71 a | 28.6 ± 2.83 a | 40.7 ± 3.68 b | 16.1 ± 1.44 b | 226 ± 20.5 a | 307 ± 28.7 a | 660 ± 65.7 a |
| Solvent | ||||||||||
| Water | 21.1 ± 0.73 a | 28.1 ± 4.41 b | 86.6 ± 12.2 b | 8.43 ± 0.70 a | 25.5 ± 2.23 b | 44.3 ± 3.54 a | 18.1 ± 1.29 a | 212 ± 16.1 a | 291 ± 23.2 b | 577 ± 49.4 b |
| EtOH 70% | 25.8 ± 0.50 b | 124 ± 8.06 a | 184 ± 14.5 a | 7.97 ± 0.75 b | 29.0 ± 2.88 a | 41.7 ± 3.67 b | 16.3 ± 0.95 b | 229 ± 20.6 b | 327 ± 29.0 a | 646 ± 60.2 a |
| Main effects | ||||||||||
| Plant | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Method | *** | *** | *** | *** | *** | ** | * | . | NS | *** |
| Solvent | *** | *** | *** | ** | *** | * | * | ** | *** | *** |
| Interactions | ||||||||||
| Plant × Method | ** | *** | *** | *** | *** | *** | *** | . | * | * |
| Plant × Solvent | *** | *** | *** | *** | *** | *** | ** | *** | *** | *** |
| Method × Solvent | *** | NS | *** | *** | *** | *** | NS | *** | *** | *** |
| Plant × Method × Solvent | * | ** | *** | *** | *** | ** | . | * | * | ** |
| Phenolic Compound (mg/L Extract) | Chlorogenic Acid | Vanillic Acid | Syringic Acid | Cinnamic Acid | p-Coumaric Acid + Epicatechin | o-Coumaric Acid | Rosmarinic Acid | Ellagic Acid | Naringin | Hesperidin | Kaempferol | Resveratrol | Ferulic Acid | Quercetin | 3,4HBA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soxhlet | H2O | Tarragon | 27.4 ± 0.79 | nd | nd | 9.61 ± 1.11 | 165 ± 10.0 | 62.6 ± 6.38 | 45.9 ± 2.60 | 645 ± 31.0 | 270 ± 18.9 | 99.4 ± 7.57 | nd | 15.5 ± 2.74 | 111 ± 2.83 | 3.31 ± 0.64 | 9.16 ± 0.56 |
| Spearmint | 8.77 ± 0.22 | nd | nd | nd | nd | nd | 324 ± 32.4 | 279 ± 21.9 | 55.7 ± 9.32 | 561 ± 45.9 | nd | 25.7 ± 2.12 | 55.1 ± 1.24 | 5.21 ± 0.26 | nd | ||
| Lemon balm | 12.2 ± 1.76 | nd | nd | nd | nd | nd | 448 ± 109 | 373 ± 179 | 105 ± 31.1 | 901 ± 232 | nd | 59.3 ± 21.8 | 18.2 ± 6.79 | 12.6 ± 5.26 | nd | ||
| Basil | nd | nd | nd | nd | 12.3 ± 1.72 | nd | 128 ± 2.73 | 420 ± 126 | 69.6 ± 3.30 | 206 ± 1.53 | nd | 5.94 ± 5.52 | 51.6 ± 2.89 | 4.13 ± 0.07 | nd | ||
| French lavender | nd | nd | nd | nd | 33.4 ± 12.1 | nd | 198 ± 0.75 | 75.9 ± 5.61 | 71.8 ± 10.8 | 85.0 ± 13.5 | 93.7 ± 6.69 | 103 ± 6.35 | 74.3 ± 12.1 | 10.3 ± 2.01 | nd | ||
| Sage | nd | nd | nd | nd | 184 ± 16.6 | nd | 435 ± 41.8 | 587 ± 423 | 523 ± 33.8 | 900 ± 71.4 | nd | 4.92 ± 0.62 | 161 ± 11.7 | 5.53 ± 0.04 | nd | ||
| EtOH 70% | Tarragon | nd | nd | nd | nd | 93.3 ± 0.75 | 2.45 ± 0.25 | 38.9 ± 1.44 | 472 ± 41.6 | 133 ± 8.66 | 61.4 ± 1.37 | nd | 16.3 ± 0.42 | 68.6 ± 2.55 | 5.79 ± 2.04 | 1.27 ± 0.14 | |
| Spearmint | nd | nd | nd | nd | nd | nd | 555 ± 30.7 | 416 ± 32.4 | 92.7 ± 9.53 | 1131 ± 63.2 | 63.2 ± 2.70 | 68.1 ± 5.23 | 49.1 ± 4.78 | 19.4 ± 1.87 | nd | ||
| Lemon balm | nd | nd | nd | nd | nd | nd | 679 ± 61.8 | 238 ± 0.48 | 93.5 ± 2.66 | 1369 ± 105 | 63 ± 3.37 | 97.9 ± 7.57 | 0.81 ± 0.06 | 8.60 ± 0.65 | nd | ||
| Basil | nd | nd | nd | nd | nd | nd | 143 ± 5.48 | 150 ± 5.07 | 34.3 ± 0.72 | 242 ± 10.4 | nd | 10.8 ± 1.21 | 9.00 ± 0.05 | 6.39 ± 2.55 | nd | ||
| French lavender | nd | nd | nd | nd | nd | nd | 244 ± 27.9 | 554 ± 69.2 | 116 ± 0.61 | 495 ± 45.5 | 77.1 ± 8.35 | 127 ± 7.71 | 88.8 ± 10.1 | 27.2 ± 6.52 | nd | ||
| Sage | nd | nd | nd | nd | 4.14 ± 0.84 | nd | 523 ± 3.85 | 249 ± 11.0 | 537 ± 8.05 | 996 ± 113 | 98.8 ± 4.43 | 44.5 ± 5.01 | 163 ± 3.85 | 33.0 ± 4.47 | nd | ||
| Solid-liquid | H2O | Tarragon | nd | nd | nd | 25.0 ± 3.32 | 57.6 ± 4.27 | 44.2 ± 5.08 | 341 ± 28.8 | 42.9 ± 5.33 | 267 ± 32.5 | 43.3 ± 6.59 | nd | 33.6 ± 1.21 | 34.9 ± 2.51 | 15.9 ± 1.01 | nd |
| Spearmint | nd | nd | nd | 150 ± 3.79 | 53.7 ± 6.40 | 20.5 ± 0.14 | 204 ± 53.2 | 28.3 ± 2.58 | 22.8 ± 0.53 | 110 ± 26.6 | nd | 75.2 ± 8.25 | 42.8 ± 1.28 | 71.4 ± 10.9 | nd | ||
| Lemon balm | nd | nd | nd | 56.3 ± 8.94 | nd | 99.9 ± 59.9 | 129 ± 11.9 | 82.1 ± 3.43 | 116 ± 5.43 | 31.6 ± 0.14 | nd | 90.9 ± 8.22 | 80.7 ± 1.71 | 43.1 ± 0.81 | nd | ||
| Basil | nd | 17.2 ± 0.30 | 10.5 ± 0.24 | 80.5 ± 1.32 | 103 ± 11.8 | 33.9 ± 1.35 | 274 ± 10.9 | 40.2 ± 3.54 | 287 ± 1.17 | 144 ± 9.77 | nd | 46.4 ± 0.95 | 39.0 ± 0.17 | 34.2 ± 0.62 | nd | ||
| French lavender | nd | nd | nd | 80.5 ± 6.93 | nd | 30.9 ± 6.91 | 120 ± 42.9 | 39.2 ± 0.32 | 63.5 ± 27.0 | 116 ± 5.51 | nd | 95.2 ± 2.01 | 64.5 ± 11.1 | 29.5 ± 0.39 | nd | ||
| Sage | nd | 12.4 ± 0.05 | 9.33 ± 0.63 | 53.3 ± 1.26 | nd | 36.7 ± 0.79 | 173 ± 101 | 43.3 ± 14.5 | 78.0 ± 12.0 | 279 ± 30.8 | nd | 93.9 ± 3.53 | 35.9 ± 3.55 | 54.6 ± 7.35 | nd | ||
| EtOH 70% | Tarragon | nd | 9.92 ± 0.03 | nd | 37.2 ± 4.66 | nd | 33.2 ± 4.99 | 355 ± 5.15 | 38.5 ± 7.58 | 123 ± 0.52 | 292 ± 5.89 | 2.73 ± 0.38 | 95.6 ± 2.38 | 47.0 ± 8.11 | 33.7 ± 0.29 | nd | |
| Spearmint | nd | nd | nd | 280 ± 10.6 | 69.0 ± 3.17 | 27.8 ± 0.61 | 333 ± 57.3 | 28.6 ± 0.40 | 62.9 ± 2.16 | 223 ± 12.7 | 1.13 ± 0.32 | 111 ± 7.50 | 60.7 ± 13.9 | 55.7 ± 2.67 | nd | ||
| Lemon balm | 71.2 ± 0.48 | nd | nd | 487 ± 15.8 | 123 ± 1.36 | 111 ± 92.4 | 185 ± 27.2 | 50.5 ± 4.25 | 894 ± 51.8 | 3.71 ± 3.34 | nd | 126 ± 9.33 | 108 ± 35.9 | 41.2 ± 0.11 | nd | ||
| Basil | 64.9 ± 1.25 | 12.9 ± 0.18 | nd | 79.9 ± 4.15 | 84.4 ± 10.7 | 25.5 ± 2.01 | 292 ± 4.31 | 31.0 ± 2.62 | 65.1 ± 7.86 | 188 ± 13.5 | nd | 85.4 ± 1.27 | 50.2 ± 7.47 | 18.1 ± 0.21 | nd | ||
| French lavender | 32.2 ± 0.22 | nd | nd | 121 ± 2.21 | 68.5 ± 0.85 | 31.6 ± 0.59 | 127 ± 18.4 | 49.9 ± 14.7 | 77.8 ± 2.31 | 123 ± 8.73 | nd | 96.2 ± 0.86 | 55.1 ± 0.56 | 27.3 ± 0.03 | nd | ||
| Sage | nd | nd | nd | 485 ± 66.3 | 119 ± 3.22 | 94.9 ± 4.13 | 170 ± 13.6 | 52.2 ± 9.61 | 279 ± 16.1 | 805 ± 40.0 | nd | 200 ± 13.0 | 78.5 ± 4.35 | 129 ± 4.76 | 16.0 ± 0.30 | ||
| Plant | L. monocytogenes | S. aureus | S. Typhimurium | E. coli |
|---|---|---|---|---|
| Sage | 2.5–5 | 0.625 | 10 | 1.25 |
| Spearmint | 2.5 | 1.25 | 20 | 1.25 |
| Lemon balm | 5 | 2.5 | 20 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.N.; Cadavez, V.; Ferreira-Santos, P.; Alves, M.J.; Ferreira, I.C.F.R.; Barros, L.; Teixeira, J.A.; Gonzales-Barron, U. Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal. Foods 2021, 10, 673. https://doi.org/10.3390/foods10030673
Silva BN, Cadavez V, Ferreira-Santos P, Alves MJ, Ferreira ICFR, Barros L, Teixeira JA, Gonzales-Barron U. Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal. Foods. 2021; 10(3):673. https://doi.org/10.3390/foods10030673
Chicago/Turabian StyleSilva, Beatriz Nunes, Vasco Cadavez, Pedro Ferreira-Santos, Maria José Alves, Isabel C. F. R. Ferreira, Lillian Barros, José António Teixeira, and Ursula Gonzales-Barron. 2021. "Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal" Foods 10, no. 3: 673. https://doi.org/10.3390/foods10030673
APA StyleSilva, B. N., Cadavez, V., Ferreira-Santos, P., Alves, M. J., Ferreira, I. C. F. R., Barros, L., Teixeira, J. A., & Gonzales-Barron, U. (2021). Chemical Profile and Bioactivities of Extracts from Edible Plants Readily Available in Portugal. Foods, 10(3), 673. https://doi.org/10.3390/foods10030673











