Characteristics of Nutraceutical Chewing Candy Formulations Based on Fermented Milk Permeate, Psyllium Husk, and Apple By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used for CCN Preparation
2.2. CCN Preparation
2.3. Evaluation of Overall Acceptability (OA) of CCN and Emotions (EMs) Induced in Consumers
2.4. Analysis of CCN Colour Characteristics and Texture
2.5. Determination of Viable LAB Count in CCN Formulations during Storage
2.6. Determination of the TPC Content and Antioxidant Activity of Prepared CCN
2.7. Statistical Analysis
3. Results and Discussion
3.1. Overall Acceptability (OA) and Emotions (EM) Induced in Consumers by the Prepared Fermented Milk Permeate (MP), Psyllium Husk (Ph), and Apple By-Product Combinations, and CCN
3.2. LAB Count in CCN Samples during Storage
3.3. CCN Colour Coordinates and Texture
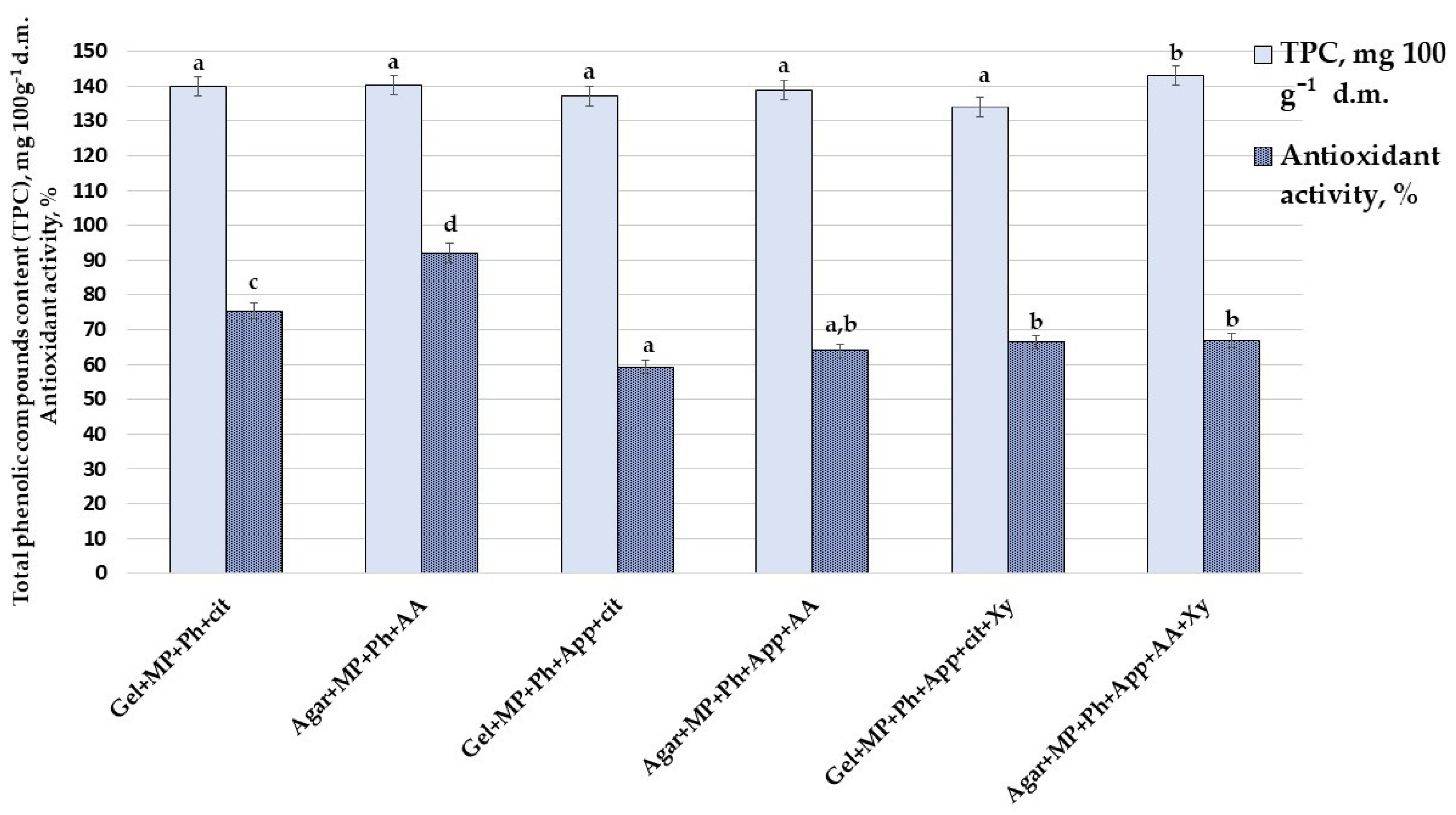
3.4. TPC Content and Antioxidant Activity of Prepared CCN
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple by-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides. Microorganisms 2020, 8, 1182. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological Activity of Phenolics Enriched Extracts from Industrial Apple Pomace. Ind. Crops Prod. 2021, 160, 113158. [Google Scholar] [CrossRef]
- Bhushan, S.; Gupta, M. Apple Pomace Source of Dietary Fibre and Antioxidant for Food Fortification. In Handbook of Food Fortification and Health: From Concepts to Public Health Applications; Preedy, V.R., Srirajaskanthan, R., Patel, V.B., Eds.; Nutrition and Health; Springer: New York, NY, USA, 2013; pp. 21–27. Volume 2, ISBN 978-1-4614-7110-3. [Google Scholar]
- Fernandes, P.A.R.; Silva, A.M.S.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The Hydrophobic Polysaccharides of Apple Pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the Antimicrobial Activity of Lactic Acid Bacteria in Combination with Berries/Fruits and Dairy Industry by-Products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Fradinho, P.; Soares, R.; Niccolai, A.; Sousa, I.; Raymundo, A. Psyllium Husk Gel to Reinforce Structure of Gluten-Free Pasta? LWT 2020, 131, 109787. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The Traditional Uses, Chemical Constituents and Biological Activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- CFR Code of Federal Regulations Title 21. 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.81 (accessed on 7 February 2021).
- Pollet, A.; van Craeyveld, V.; van de Wiele, T.; Verstraete, W.; Delcour, J.A.; Courtin, C.M. In Vitro Fermentation of Arabinoxylan Oligosaccharides and Low Molecular Mass Arabinoxylans with Different Structural Properties from Wheat (Triticum aestivum L.) Bran and Psyllium (Plantago ovata Forsk) Seed Husk. J. Agric. Food Chem. 2012, 60, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; de Wiele, T.V.; Verstraete, W.; Delcour, J.A. Prebiotic and Other Health-Related Effects of Cereal-Derived Arabinoxylans, Arabinoxylan-Oligosaccharides, and Xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr. Dev. Nutr. 2018, 2, nzy005. [Google Scholar] [CrossRef] [Green Version]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Dea, I.C.M. Xanthan-like ‘Weak Gel’ Rheology from Dispersions of Ispaghula Seed Husk. Carbohydr. Polym. 1993, 22, 223–232. [Google Scholar] [CrossRef]
- Belorio, M.; Sahagún, M.; Gómez, M. Influence of Flour Particle Size Distribution on the Quality of Maize Gluten-Free Cookies. Foods 2019, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. Recent Trends in Hydrogels Based on Psyllium Polysaccharide: A Review. J. Cleaner Prod. 2014, 82, 1–15. [Google Scholar] [CrossRef]
- Mehta, A.; Sharma, C.; Kanala, M.; Thakur, M.; Harrison, R.; Torrico, D.D. Self-Reported Emotions and Facial Expressions on Consumer Acceptability: A Study Using Energy Drinks. Foods 2021, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Sensory Analysis General Guidance for the Selection, Training and Monitoring of Assessor–Part 1: Selected Assessors. ISO 8586-1:1993. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/58/15875.html (accessed on 15 February 2021).
- Bartkiene, E.; Steibliene, V.; Adomaitiene, V.; Juodeikiene, G.; Cernauskas, D.; Lele, V.; Klupsaite, D.; Zadeike, D.; Jarutiene, L.; Guiné, R.P.F. Factors Affecting Consumer Food Preferences: Food Taste and Depression-Based Evoked Emotional Expressions with the Use of Face Reading Technology. BioMed Res. Int. 2019, 2019, 2097415. [Google Scholar] [CrossRef]
- Vaher, M.; Matso, K.; Levandi, T.; Helmja, K.; Kaljurand, M. Phenolic Compounds and the Antioxidant Activity of the Bran, flour and Whole Grain of Different wheat Varieties. Procedia Chem. 2010, 2, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.-X.; Lian, C.-X.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Antioxidant Activities and Total Phenolic Contents of Various Extracts from Defatted Wheat Germ. Food Chem. 2011, 126, 1122–1126. [Google Scholar] [CrossRef]
- Köster, E.P.; Mojet, J. From Mood to Food and from Food to Mood: A Psychological Perspective on the Measurement of Food-Related Emotions in Consumer Research. Food Res. Int. 2015, 76, 180–191. [Google Scholar] [CrossRef]
- Leigh Gibson, E. Emotional Influences on Food Choice: Sensory, Physiological and Psychological Pathways. Physiol. Behav. 2006, 89, 53–61. [Google Scholar] [CrossRef]
- Patel, K.A.; Schlundt, D.G. Impact of Moods and Social Context on Eating Behavior. Appetite 2001, 36, 111–118. [Google Scholar] [CrossRef]
- Danner, L.; Haindl, S.; Joechl, M.; Duerrschmid, K. Facial Expressions and Autonomous Nervous System Responses Elicited by Tasting Different Juices. Food Res. Int. 2014, 64, 81–90. [Google Scholar] [CrossRef]
- Triyanti, V.; Yassierli, Y.; Iridiastadi, H. Basic Emotion Recogniton Using Automatic Facial Expression Analysis Software. J. Optimasi Sist. Ind. 2019, 18, 55–64. [Google Scholar] [CrossRef]
- Miceli, M.; Castelfranchi, C. Contempt and Disgust: The Emotions of Disrespect. J. Theory Soc. Behav. 2018, 48, 205–229. [Google Scholar] [CrossRef]
- Alaoui-Ismaïli, O.; Vernet-Maury, E.; Dittmar, A.; Delhomme, G.; Chanel, J. Odor Hedonics: Connection with Emotional Response Estimated by Autonomic Parameters. Chem. Senses 1997, 22, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Zeinstra, G.G.; Koelen, M.A.; Colindres, D.; Kok, F.J.; de Graaf, C. Facial Expressions in School-Aged Children are a Good Indicator of ‘Dislikes’, but not of ‘Likes’. Food Qual. Prefer. 2009, 20, 620–624. [Google Scholar] [CrossRef]
- Desmet, P.M.A.; Schifferstein, H.N.J. Sources of Positive and Negative Emotions in Food Experience. Appetite 2008, 50, 290–301. [Google Scholar] [CrossRef]
- Kanjanakorn, A.; Lee, J. Examining Emotions and Comparing the EsSense Profile® and the Coffee Drinking Experience in Coffee Drinkers in the Natural Environment. Food Qual. Prefer. 2017, 56, 69–79. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V.; Hager, J.C. Facial Action Coding System (FACS); A Human Face: Salt Lake City, UT, USA, 2002. [Google Scholar]
- Weiss, J.; Ekman, P. Telling Lies: Clues to Deceit in the Marketplace, Politics, and Marriage; Norton: New York, NY, USA, 2011; Volume 53, pp. 287–288. [Google Scholar] [CrossRef]
- Woodzicka, J.A.; la France, M. Real Versus Imagined Gender Harassment. J. Soc. Issues 2001, 57, 15–30. [Google Scholar] [CrossRef]
- Gunnery, S.D.; Hall, J.A.; Ruben, M.A. The Deliberate Duchenne Smile: Individual Differences in Expressive Control. J. Nonverbal Behav. 2013, 37, 29–41. [Google Scholar] [CrossRef]
- Mojet, J.; Dürrschmid, K.; Danner, L.; Jöchl, M.; Heiniö, R.-L.; Holthuysen, N.; Köster, E. Are Implicit Emotion Measurements Evoked by Food Unrelated to Liking? Food Res. Int. 2015, 76, 224–232. [Google Scholar] [CrossRef]
- Nsogning-Dongmo, S.; Procopio, S.; Sacher, B.; Becker, T. Flavor of Lactic Acid Fermented Malt Based Beverages: Current Status and Perspectives. Trends Food Sci. Technol. 2016, 54, 37–51. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of Fermented Broth from Lactic acid Bacteria on Pathogenic Bacteria Proliferation. J. Dairy Sci. 2016, 99, 2654–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Jelen, P. Survival of Lactic Acid Bacteria and Their Lactases Under Acidic Conditions. J. Food Sci. 1990, 55, 506–509. [Google Scholar] [CrossRef]
- Nussinovitch, A.; Nussinovitch, M.; Shapira, R.; Gershon, Z. Influence of Immobilization of Bacteria, Yeasts and Fungal Spores on the Mechanical Properties of Agar and Alginate Gels. Food Hydrocoll. 1994, 8, 361–372. [Google Scholar] [CrossRef]
- Chen, B.; Lin, X.; Lin, X.; Li, W.; Zheng, B.; He, Z. Pectin-Microfibrillated Cellulose Microgel: Effects on Survival of Lactic Acid Bacteria in a Simulated Gastrointestinal Tract. Int. J. Biol. Macromol. 2020, 158, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, J.; Nazemiyeh, H.; Maghsoodi, M.; Lotfipour, F. Evaluation of the Effect of Psyllium on the Viability of Lactobacillus Acidophilus in Alginate-Polyl Lysine Beads. Adv. Pharm. Bull. 2016, 6, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasmi-Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health Benefits of Xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Rafeek, R.; Carrington, C.V.F.; Gomez, A.; Harkins, D.; Torralba, M.; Kuelbs, C.; Addae, J.; Moustafa, A.; Nelson, K.E. Xylitol and Sorbitol Effects on the Microbiome of Saliva and Plaque. J. Oral Microbiol. 2019, 11, 1536181. [Google Scholar] [CrossRef] [Green Version]
- Gregirchak, N.; Stabnikova, O.; Stabnikov, V. Application of Lactic Acid Bacteria for Coating of Wheat Bread to Protect it From Microbial Spoilage. Plant. Foods Hum. Nutr. 2020, 75, 223–229. [Google Scholar] [CrossRef]
- López de Lacey, A.M.; López-Caballero, M.E.; Gómez-Estaca, J.; Gómez-Guillén, M.C.; Montero, P. Functionality of Lactobacillus acidophilus and Bifidobacterium bifidum Incorporated to Edible Coatings and Films. Innov. Food Sci. Emerging Technol. 2012, 16, 277–282. [Google Scholar] [CrossRef]
- Cubeddu, A.; Fava, P.; Pulvirenti, A.; Haghighi, H.; Licciardello, F. Suitability Assessment of PLA Bottles for High-Pressure Processing of Apple Juice. Foods 2021, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Genovese, D.B. Structural and Sensory Analysis of Compositionally Optimized Apple Jellies Enriched with Dietary Fibre Compared to Commercial Apple Jams. J. Food Sci. Technol. 2020, 57, 1661–1670. [Google Scholar] [CrossRef]
- Šeremet, D.; Mandura, A.; Cebin, A.V.; Martinić, A.; Galić, K.; Komes, D. Challenges in Confectionery Industry: Development and Storage Stability of Innovative white Tea-Based Candies. J. Food Sci. 2020, 85, 2060–2068. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Moghaddam, T.; Firoozzare, A.; Kariminejad, M.; Sorahi, M.; Tavakoli, Z. Black Plum Peel as a Useful by-Product for the Production of New Foods: Chemical, Textural, and Sensory Characteristics of Halva Masghati. Int. J. Food Prop. 2020, 23, 2005–2019. [Google Scholar] [CrossRef]
- Altınok, E.; Palabiyik, I.; Gunes, R.; Toker, O.S.; Konar, N.; Kurultay, S. Valorisation of Grape by-Products as a Bulking Agent in Soft Candies: Effect of Particle Size. LWT 2020, 118, 108776. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Calín-Sánchez, Á.; Clemente-Villalba, J.; Hernández, F.; Carbonell-Barrachina, Á.A.; Sendra, E.; Wojdyło, A. Quality Parameters and Consumer Acceptance of Jelly Candies Based on Pomegranate Juice Mollar de Elche. Foods 2020, 9, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marghmaleki, S.N.; Mortazavi, S.M.H.; Saei, H.; Mostaan, A. The Effect of Alginate-Based Edible Coating Enriched with Citric Acid and Ascorbic Acid on Texture, Appearance and Eating Quality of Apple Fresh-Cut. Int. J. Fruit Sci. 2020, 0, 1–12. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Non-Covalent Dietary Fiber Polyphenol Interactions and their Influence on Polyphenol Bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]


| Nutraceutical Chewing Candy Formula | Milk Permeate | Psyllium Husk | Apple By-Products | Water | Gelatin | Agar | Citric Acid | Ascorbic Acid | Xylitol |
|---|---|---|---|---|---|---|---|---|---|
| mL | g | mL | g | ||||||
| Gel+MP+Ph+cit | 100 | 7 | - | 100 | 10 | - | 1 | - | - |
| Agar+MP+Ph+AA | - | - | 10 | - | 1 | - | |||
| Gel+MP+Ph+App+cit | 7 | 10 | - | 1 | - | - | |||
| Agar+MP+Ph+App+AA | - | 10 | - | 1 | - | ||||
| Gel+MP+Ph+App+cit+Xy | 10 | - | 1 | - | 4 | ||||
| Agar+MP+Ph+App+AA+Xy | - | 10 | - | 1 | 4 | ||||
| Ingredient Combination | OA | Emotions Induced in Consumers (from 0 to 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | Valence | ||
| MP+3gPh | 5.7± 0.3 a | 0.42 ± 0.03 b | 0.14 ± 0.02 b | 0.09 ± 0.01 a | 0.05 ± 0.01 b | 0.002 ± 0.0003 a | 0.003 ± 0.0005 b | 0.001 ± 0.0003 a | 0.01 ± 0.003 a | 0.104 ± 0.011 a |
| MP+5gPh | 7.7 ± 0.5 b | 0.44 ± 0.02 b | 0.17 ± 0.01 b | 0.17 ± 0.02c | 0.01 ± 0.001 a | 0.007 ± 0.001c | 0.001 ± 0.0004 a | 0.001 ± 0.0004 a | 0.03 ± 0.001 c | 0.107 ± 0.009 a |
| MP+7gPh | 9.0 ± 0.4c | 0.24 ± 0.01 a | 0.33 ± 0.02d | 0.07 ± 0.01 a | 0.02 ± 0.002 a | 0.001 ± 0.0002 a | 0.002 ± 0.0006 a, b | 0.001 ± 0.0003 a | 0.09 ± 0.004 f | 0.114 ± 0.008 a |
| MP+7gPh+2.5gApp | 5.7 ± 0.5 a | 0.49 ± 0.04 b | 0.13 ± 0.02 b | 0.11 ± 0.01 b | 0.04 ± 0.003 b | 0.010 ± 0.0002 d | 0.001 ± 0.0003 a | 0.002 ± 0.0006 b | 0.07 ± 0.002 e | 0.110 ± 0.009 a |
| MP+7gPh+5.0gApp | 6.8 ± 0.4 b | 0.65 ± 0.05c | 0.09 ± 0.01 a | 0.13 ± 0.02 b | 0.08 ± 0.004c | 0.003 ± 0.0004 a, b | 0.001 ± 0.0004 a | 0.001 ± 0.0003 a | 0.02 ± 0.001 b | 0.121 ± 0.010 a |
| MP+7gPh+7.0gApp | 8.3 ± 0.4c | 0.21 ± 0.02 a | 0.23 ± 0.03 c | 0.08 ± 0.01 a | 0.01 ± 0.003 a | 0.002 ± 0.0003 a | 0.001 ± 0.0003 a | 0.001 ± 0.0004 a | 0.04 ± 0.003 d | 0.113 ± 0.011 a |
| CCN | OA | Emotions Induced in Consumers (from 0 to 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | Valence | ||
| Gel+MP+Ph+cit | 5.4 ± 0.4 b | 0.33 ± 0.03 a | 0.12 ± 0.02 a | 0.20 ± 0.03 b | 0.002 ± 0.001 b | 0.005 ± 0.0002 c | 0.014 ± 0.002 c | 0.019 ± 0.004 d | 0.08 ± 0.01 b | 0.43 ± 0.05 a |
| Agar+MP+Ph+AA | 4.1 ± 0.3 a | 0.64 ± 0.05 d | 0.11 ± 0.02 a | 0.44 ± 0.04 c | 0.007 ± 0.001 e | 0.008 ± 0.001 d | 0.002 ± 0.0001 b | 0.004 ± 0.001 b | 0.23 ± 0.03 d | 0.54 ± 0.04 b |
| Gel+MP+Ph+App+cit | 7.3 ± 0.5 c | 0.52 ± 0.04 c | 0.31 ± 0.03 c | 0.20 ± 0.05 b | 0.001 ± 0.0003 a | 0.013 ± 0.002 e | 0.030 ± 0.005 d | 0.003 ± 0.001 b | 0.01 ± 0.002 a | 0.53 ± 0.03 b |
| Agar+MP+Ph+App+AA | 5.5 ± 0.4 b | 0.47 ± 0.05 b | 0.13 ± 0.02 a | 0.11 ± 0.02 a | 0.003 ± 0.0005 c | 0.009 ± 0.001 d | 0.001 ± 0.0005 a | 0.010 ± 0.002 c | 0.08 ± 0.01 b | 0.58 ± 0.04 b |
| Gel+MP+Ph+App+cit+Xy | 8.5 ± 0.6 d | 0.41 ± 0.04 b | 0.28 ± 0.03 c | 0.13 ± 0.03 a | 0.004 ± 0.0003 d | 0.003 ± 0.0005 b | 0.012 ± 0.002 c | 0.020 ± 0.005 d | 0.15 ± 0.03 c | 0.41 ± 0.03 a |
| Agar+MP+Ph+App+AA+Xy | 6.3 ± 0.2 c | 0.29 ± 0.03 a | 0.19 ± 0.02 b | 0.16 ± 0.04 a | 0.002 ± 0.0004 b | 0.001 ± 0.0005 a | 0.002 ± 0.0006 b | 0.001 ± 0.0003 a | 0.07 ± 0.01 b | 0.69 ± 0.05 c |
| CCN | Colour Coordinates, NBS | Texture, mJ | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| Gel+MP+Ph+cit | 54.4 ± 3.5 a | 0.12 ± 0.02 a | 18.2 ± 0.5 a | 2.3 ± 0.2c |
| Agar+MP+Ph+AA | 56.8 ± 2.9 a | 0.22 ± 0.02 b | 20.8 ± 0.6 b | 1.6 ± 0.1 b |
| Gel+MP+Ph+App+cit | 49.9 ± 3.4 a | 5.93 ± 0.21 e | 22.5 ± 0.4 c | 2.5 ± 0.2 c, d |
| Agar+MP+Ph+App+AA | 53.3 ± 4.0 a | 5.05 ± 0.14 d | 22.9 ± 0.5 c | 1.8 ± 0.1 b |
| Gel+MP+Ph+App+cit+Xy | 51.5 ± 2.9 a | 6.01 ± 0.27 f | 22.4 ± 0.6 c | 2.8 ± 0.3 d |
| Agar+MP+Ph+App+AA+Xy | 54.9 ± 3.5 a | 4.11 ± 0.23 c | 23.3 ± 0.4 d | 1.3 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zokaityte, E.; Siriakovaite, K.; Starkute, V.; Zavistanaviciute, P.; Lele, V.; Mozuriene, E.; Klupsaite, D.; Viskelis, P.; Ruibys, R.; Guiné, R.P.F.; et al. Characteristics of Nutraceutical Chewing Candy Formulations Based on Fermented Milk Permeate, Psyllium Husk, and Apple By-Products. Foods 2021, 10, 777. https://doi.org/10.3390/foods10040777
Zokaityte E, Siriakovaite K, Starkute V, Zavistanaviciute P, Lele V, Mozuriene E, Klupsaite D, Viskelis P, Ruibys R, Guiné RPF, et al. Characteristics of Nutraceutical Chewing Candy Formulations Based on Fermented Milk Permeate, Psyllium Husk, and Apple By-Products. Foods. 2021; 10(4):777. https://doi.org/10.3390/foods10040777
Chicago/Turabian StyleZokaityte, Egle, Karolina Siriakovaite, Vytaute Starkute, Paulina Zavistanaviciute, Vita Lele, Erika Mozuriene, Dovile Klupsaite, Pranas Viskelis, Romas Ruibys, Raquel P. F. Guiné, and et al. 2021. "Characteristics of Nutraceutical Chewing Candy Formulations Based on Fermented Milk Permeate, Psyllium Husk, and Apple By-Products" Foods 10, no. 4: 777. https://doi.org/10.3390/foods10040777
APA StyleZokaityte, E., Siriakovaite, K., Starkute, V., Zavistanaviciute, P., Lele, V., Mozuriene, E., Klupsaite, D., Viskelis, P., Ruibys, R., Guiné, R. P. F., & Bartkiene, E. (2021). Characteristics of Nutraceutical Chewing Candy Formulations Based on Fermented Milk Permeate, Psyllium Husk, and Apple By-Products. Foods, 10(4), 777. https://doi.org/10.3390/foods10040777











