Comparative Evaluation of Chemical Composition, Phenolic Compounds, and Antioxidant and Antimicrobial Activities of Tropical Black Bolete Mushroom Using Different Preservation Methods
Abstract
:1. Introduction
2. Materials and Methods
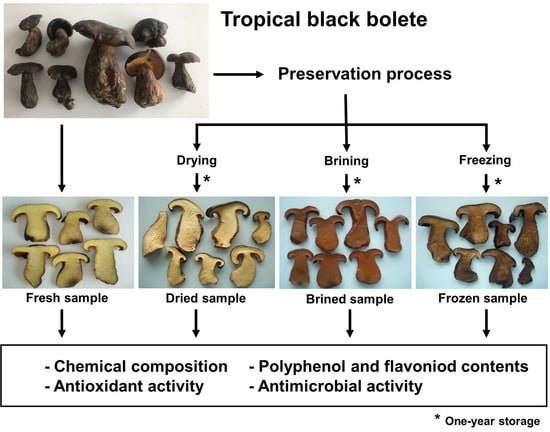
2.1. Source of Mushroom and Sample Preparation
2.2. Preservation Method
2.2.1. Drying
2.2.2. Brining
2.2.3. Freezing
2.3. Proximate Composition Analysis
2.4. Preparation of Mushroom Extracts
2.5. Determination of Phenolic Compounds
2.5.1. Total Polyphenol Content
2.5.2. Total Flavonoid Content
2.6. Antioxidant Assay
2.6.1. DPPH Scavenging Assay
2.6.2. ABTS Scavenging Assay
2.6.3. FRAP Assay
2.7. Antimicrobial Assay
2.7.1. Microorganisms
2.7.2. Determination of Antimicrobial Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Mushroom Samples
3.2. Proximate Composition
3.3. Determination of Phenolic Compounds
3.4. Antioxidant Assay
3.5. Antimicrobial Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, E.; Nair, N.G. Make Money by Growing Mushrooms; Rural Infrastructure and Agro-Industries Division Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Oei, P.; Nieuwenhuijzen, V.B. Small-scale Mushroom Cultivation: Oyster, Shiitake and Wood Ear Mushroom; Agrodok: Wageningen, The Netherlands, 2005. [Google Scholar]
- Chang, S.T.; Miles, P.G. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Reis, F.S.; Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Santos-Buelga, C.; Ferreia, I.C.C.R. Toward the antioxidant and chemical characterization of mycorrhizal mushrooms from Northeast Portugal. J. Food Sci. 2001, 76, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [Green Version]
- Yun, W.; Hall, I.R. Edible ectomycorrhizal mushrooms: Challenges and achievements. Can. J. Bot. 2004, 82, 1063–1073. [Google Scholar] [CrossRef]
- Hall, I.R.; Yun, W.; Amicucci, A. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 2003, 21, 433–438. [Google Scholar] [CrossRef]
- Sanmee, R.; Dell, B.; Lumyong, P.; Izumori, K.; Lumyong, S. Nutritive value of popular wild edible mushroom from Northen Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Kalmis, E.; Yildiz, H.; Ergönül, B.; Kalyoncu, F.; Solak, M.H. Chemical composition and nutritional value of a wild edible ectomycorrhizal mushroom, Tricholoma Anatolicum. Turk. J. Bot. 2011, 35, 627–633. [Google Scholar]
- Agahar-Murugkar, D.; Subbulakshmi, G. Nutritional value of edible wild mushrooms collected from the Khasi hills of Meghalaya. Food Chem. 2005, 89, 599–603. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushroom. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Saisamorn, L. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as possible antioxidant and antimicrobial agents. Iran J. Pharm. Res. 2012, 11, 1095–1102. [Google Scholar]
- Susanna, B. Medicinal aspects of edible ectomycorrhizal mushrooms. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G., Eds.; Springer Press: Berlin, Germany, 2012; pp. 317–334. [Google Scholar]
- Mortimer, P.E.; Karunarathna, S.C.; Li, Q.; Gui, H.; Yang, X.; Yang, X.; He, J.; Ye, L.; Li, H.; Sysouphanthong, P.; et al. Prized edible Asian mushrooms: Ecology, conservation and sustainability. Fungal Divers. 2012, 56, 31–47. [Google Scholar] [CrossRef]
- Zambonelli, A.; Bonito, G.M. Edible Ectomycorrhizal Mushroom; Springer Press: Berlin, Germany, 2012. [Google Scholar]
- Bernaś, E.; Jaworska, G.; Kmiecik, W. Storage and process of edible mushroom. ACTA Sci. Pol. Technol. Aliment. 2006, 52, 5–23. [Google Scholar]
- Singh, M.; Vijay, B.; Kamal, S.; Wakchure, G.C. Mushroom Cultivation, Marketing and Consumption; Indian Council of Agricultural Research: Chambaghat, India, 2011. [Google Scholar]
- Rai, R.D.; Arumuganathan, T. Post Harvest Technology of Mushroom; National Research Centre for Mushroom: Chambaghat, India, 2008. [Google Scholar]
- Apati, G.P.; Furlan, S.A.; Laurindo, J.B. Drying and rehydration of oyster mushroom. Braz. Arch. Biol. Technol. 2010, 53, 945–952. [Google Scholar] [CrossRef]
- Pilz, D.; Norvell, L.; Danell, E.; Molina, R. Ecology and Management of Commercially Harvested Chanterelle Mushrooms; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2003.
- Keefer, M.; Winder, R.; Hobby, T. Commercial development of non-timber forest resources: A case study of morels in the East Kootenay, British Columbia. BC J. Econ. Manag. 2010, 11, 39–51. [Google Scholar]
- Sitta, N.; Davoli., P. Edible ectomycorrhizal mushrooms: International markets and regulations. In Edible Ectomycorrhizal Mushrooms; Zambonelli, A., Bonito, G., Eds.; Springer Press: Berlin, Germany, 2012; pp. 355–380. [Google Scholar]
- Hyde, K.D.; Norphanphoun, C.; Chen, J.; Dissanayake, A.J.; Doilom, M.; Hongsanan, S.; Jayawardena, R.S.; Jeewon, R.; Perera, R.H.; Thongbai, B.; et al. Thailand’s amazing diversity–up to 96% of fungi in northern Thailand are novel. Fungal Divers. 2018, 93, 215–239. [Google Scholar] [CrossRef]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bioactivities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ruksawong, P.; Flegel, T.W. Thai Mushrooms and Other Fungi; National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency: Bangkok, Thailand, 2001. [Google Scholar]
- Kumla, J.; Hobbie, E.A.; Suwannarach, N.; Lumyong, L. The ectomycorrhizal status of a tropical black bolete, Phlebopus portentosus, assessed using mycorrhizal synthesis and isotopic analysis. Mycorrhiza 2016, 26, 333–343. [Google Scholar] [CrossRef]
- Kumla, J.; Bussaban, B.; Suwannarach, N.; Lumyong, S.; Danell, E. Basidiome formation od an edible wild, putatively ectomycorrhizal fungus, Phlebopus portentosus without host plant. Mycologia 2012, 104, 597–603. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Liu, J.; Xu, X.; Cao, Y.; Gao, F.; Fang, Y.; Wang, W.; Wang, Y. Brief introduction to a unique edible bolete–Phlebopus portentosus in southern China. J. Agric. Sci. Technol. B 2017, 7, 386–394. [Google Scholar]
- Bandala, V.M.; Montoya, L.; Jarvio, D. Two interesting records of boletes found in coffee plantations from Eastern Mexico. Persoonia 2004, 18, 365–380. [Google Scholar]
- Lumyong, S.; Sanmee, R.; Lumyong, P. Is large scale cultivation of boletes possible? Opera Mycol. 2007, 1, 34–37. [Google Scholar]
- Tolera, K.D.; Abera, S. Nutritional quality of oyster mushroom (Pleurotus Ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci. Nutr. 2017, 5, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.; Baston, O.; Pricop, E.M. The effect of brine preservation on mushroom. Ann. Food Sci. Technol. 2015, 16, 333–337. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1996. [Google Scholar]
- Thitilertdecha, N.; Teerawutgulrag, A.; Rakariyatham, N. Antioxidant and antimicrobial activities of Nephelium lappacium L. extracts. LWT Food Sci. Technol. 2008, 41, 2029–2035. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Niamsup, H.; Shank, L.; Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 2008, 41, 105–116. [Google Scholar]
- Gülçin, İ.; Oktay, M.; Kireçci, E.; Küfrevioğlu, İ.Ö. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003, 83, 371–382. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Farcas, A.C.; Tibulcu, D. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agonomy 2020, 10, 1972. [Google Scholar]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Gacia-Pascual, P.; Sanjuan, S.; Bon, J.; Carreres, J.E.; Mulet, A. Rehydration process of Boletus edulis mushroom: Characteristics and modeling. J. Sci. Food. Agric. 2005, 85, 1397–1404. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Tahir, M.; Muller, J. Effect of air temperature and pre-treatment on color changes and texture of dried Boletus edulis mushroom. Drying Technol. 2011, 29, 1890–1900. [Google Scholar] [CrossRef]
- Yuswa, M.H.; On, Y.Y.; Tan, Y.J.; Lai, W.; Zainal, Z.; Sz, J.X.; Daud, F. Nutritional and molecular analysis of wild edible Gelam mushroom (Boletus sp.) from Kelantan, Malaysia. J. Adv. Biol. Biotechnol. 2017, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liaotrakoon, W.; Liaotrakoon, V. Influence of drying process on total phenolics, antioxidative activity and selected physical properties of edible bolete (Phlebopus colossus (R. Heim) Singer) and changes during storage. Food Sci. Technol. 2018, 38, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Muyanja, C.; Kyambadde, D.; Namugumya, B. Effect of pretreatments and drying methods on chemical composition and sensory evaluation of oyster mushroom (Pluerotus oestreatus) powder and soup. J. Food Process. Preserv. 2014, 38, 457–465. [Google Scholar] [CrossRef]
- Adejumo, T.O.; Awosanya, O.B. Proximate and mineral composition of four edible mushrooms species from south western Nigeria. Afr. J. Biotechnol. 2005, 4, 1084–1088. [Google Scholar]
- Mo, A.; Basu, S.K.; Gyar, S.D.; Goyal, A.; Bhowmik, P.K.; Datta, B.S. Proximate composition and functional properties of mushroom flours from Ganoderma spp., Omphalotus olearius (DC.) Sing. and Hebeloma mesophaeum (Pers.) Quél. used in Nasarawa State, Nigeria. Malaysian J. Nut. 2009, 15, 233–241. [Google Scholar]
- Chittaragi, A.; Naika, R.; Vinayaka, K.S. Nutritive value of few wild mushrooms from the western Ghats of Shivamogga district, Karnataka, India. Asian J. Pharm. Clin. Res. 2014, 7, 50–53. [Google Scholar]
- Pagoń, K.; Gabor, A.; Jaworska, G.; Bernaś, E. Effect of traditional canning in acetic brine on the antioxidants and vitamins in Boletus edulis and Suillus luteus mushrooms. J. Food Process. Preserv. 2016, 41, e12826. [Google Scholar] [CrossRef]
- Jaworska, G.; Bernaś, E.; Mickowska, B. Effect of production process on the amino acid content of frozen and canned Pleurotus ostreatus mushrooms. Food Chem. 2011, 125, 936–943. [Google Scholar] [CrossRef]
- Bernaś, E.; Jaworska, G. Effect of preservation method on amino acid content in selected species of edible mushroom. LWT Food Sci. Technol. 2012, 48, 242–247. [Google Scholar] [CrossRef]
- Rameah, C.; Pattar, M.G. Antimicribial properties, antioxidant activity and bioactive compounds from six wild edible mushrooms of western ghats of Karnataka, India. Pharm. Res. 2010, 2, 107–112. [Google Scholar]
- Kosanić, M.; Rankovic, B.; Daašić, M. Antioxidant and antimicrobial activity of mushrooms. Bulg. J. Agric. Sci. 2013, 19, 1040–1046. [Google Scholar]
- Murcia, M.A.; Martinez-Tome, M.; Jimenez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P.J. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. J. Food Protect. 2002, 65, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Ganguli, A.; Ghosh, M.; Singh, S. Antioxidant activities and total phenolics of pickles produced from the edible mushroom, Agaricus bisporus. J. Culin. Sci Technol. 2006, 5, 131–142. [Google Scholar] [CrossRef]
- Jaworska, G.; Pagoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [Green Version]
- Smolskaitė, L.; Venskutonis, P.R.; Talou, T. Comprehensive evaluation of antioxidant and antimicrobial properties of different mushroom species. LWT Food Sci. Technol. 2015, 60, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Muruke, M.H. Evaluation of antioxidant and iron chelating activities of a wild edible oyster mushroom Pleurotus cystidiosus from Tanzania. Food Sci. Qual. Manag. 2014, 29, 18–28. [Google Scholar]
- Kapusta-Duch, J.; Leszczynska, T.; Borczak, B.; Florkiewicz, A.; Załubska, A. Impact of Different packaging systems on selected antioxidant properties of frozen-stored cauliflower (Brassica oleracea L. Var. botrytis). Polish J. Food Nutr. Sci. 2017, 67, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Miao, H.; Qian, H.; Yao, L.; Wang, B.; Wang, Q. E-ects of industrial pre-freezing processing and freezing handling on glucosinolates and antioxidant attributes in broccoli florets. Food Chem. 2016, 210, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebczynski, P. Content of selected antioxidative compounds in green asparagus depending on processing before freezing and on the period and conditions of storage. Polish J. Food Nutr. Sci. 2007, 57, 209–214. [Google Scholar]
- Neri, L.; Faieta, M.; Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Pittia, P. Antioxidant activity in frozen plant foods: Effect of cryoprotectectants, freezing process and frozen storage. Foods 2020, 9, 1886. [Google Scholar] [CrossRef]
- Shen, H.S.; Shao, S.; Chen, J.C.; Zhou, T. Antimicrobials from mushrooms for assuring food safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 316–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamantopoulou, P.A.; Philippoussis, A.N. Cultivated mushroom: Preservation and processing. In Handbook of Vegetable Preservation and Processing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 495–525. [Google Scholar]
- Matser, A.M.; Knott, E.R.; Teunissen, P.G.M.; Bartels, P.V. Effects of high isostatic pressure on mushrooms. J. Food. Eng. 2000, 45, 11–16. [Google Scholar] [CrossRef]
- Janeš, D.; Kreft, S.; Jurc, M.; Seme, K.; Štrukelj, B. Antibacterial activity in higher fungi (mushrooms) and endophytic fungi from Slovenia. Pharm. Biol. 2007, 45, 700–706. [Google Scholar] [CrossRef]


| Sample Type | % Dry Weight | |||||
|---|---|---|---|---|---|---|
| Moisture | Ash | Crude Protein | Fat | Fibre | Carbohydrate | |
| Fresh sample | 7.2 ± 0.1 a | 9.6 ± 0.2 a | 19.6 ± 0.4 a | 1.0 ± 0.1 a | 6.3 ± 0.1 a | 54.8 ± 0.7 a |
| Dried sample | 7.1 ± 0.2 a | 9.7 ± 0.2 a | 19.3 ± 0.4 a | 1.0 ± 0.2 a | 6.2 ± 0.1 a | 54.7 ± 0.6 a |
| Brined sample | 7.0 ± 0.1 a | 9.4 ± 0.1 a | 17.1 ± 0.8 b | 0.7 ± 0.1 b | 6.2 ± 0.2 a | 53.4 ± 0.5 a |
| Frozen sample | 7.1 ± 0.2 a | 9.6 ± 0.1 a | 19.4 ± 0.3 a | 0.9 ± 0.1 a | 6.3 ± 0.2 a | 54.6 ± 0.4 a |
| Mushroom Extract | Total Polyphenol Content (mg GAE/g dw) | Total Flavonoid Content (mg QE/g dw) |
|---|---|---|
| Fresh sample | 30.10 ± 1.04 a | 1.69 ± 0.17 a |
| Dried sample | 26.70 ± 0.63 b | 1.32 ± 0.26 b |
| Brined sample | 18.44 ± 0.54 c | 0.37 ± 0.31 c |
| Frozen sample | 29.06 ± 1.20 a | 1.63 ± 0.24 a |
| Mushroom Extract | DPPH Assay (IC50, mg/mL) | ABTS Assay (IC50, mg/mL) | FRAP Assay (mg GAE/g dw) |
|---|---|---|---|
| Fresh sample | 2.11 ± 0.80 b | 1.30 ± 0.20 c | 7.86 ± 0.24 a |
| Dried sample | 2.20 ± 0.60 b | 1.78 ± 0.27 b | 5.53 ± 0.62 b |
| Brined sample | 3.65 ± 0.48 a | 2.85 ± 0.31 a | 3.81 ± 0.46 c |
| Frozen sample | 2.14 ± 0.72 b | 1.72 ± 0.24 b | 7.52 ± 0.38 a |
| Microorganism | Diameter of Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Mushroom Extract (100 mg/mL) | Antimicrobial Compound (10 mg/mL) | ||||||
| Fresh Sample | Dried Sample | Brined Sample | Frozen Sample | Ampicillin | Streptomycin | Nystatin | |
| Gram-positive bacteria | |||||||
| Bacillus cereus | 11.2 ± 0.8 c | 10.5 ± 0.5 c | − | 10.2 ± 0.5 c | 28.3 ± 1.5 a | 24.5 ± 1.2 b | NT |
| Bacillus subtilis | 10.8 ± 0.7 c | − | − | 10.2 ± 0.6 c | 29.7 ± 1.2 b | 34.1 ± 0.8 a | NT |
| Enterococcus faecalis ATCC29212 | 11.5 ± 1.5 c | 11.1 ± 0.9 c | 9.8 ± 0.2 c | 11.1 ± 0.5 c | 40.8 ± 1.0 a | 22.2 ± 0.5 b | NT |
| Listeria monocytogenes | 11.0 ± 0.5 c | − | − | 10.5 ± 0.6 c | 34.0 ± 1.7 a | 22.7 ± 2.5 b | NT |
| Micrococcus luteus | 11.5 ± 0.9 c | 10.5 ± 0.8 cd | − | 9.6 ± 0.5 d | 17.5 ± 1.3 b | 20.8 ± 1.0 a | NT |
| Methicillin-resistant Staphylococcus aureus | 11.7 ± 0.6 c | 11.0 ± 1.0 cd | 9.9 ± 0.6 d | 11.2 ± 0.7 c | 16.8 ± 1.2 a | 14.2 ± 0.8 b | NT |
| Staphylococcus aureus ATCC29213 | 11.5 ± 0.5 c | 11.2 ± 0.8c | 10.5 ± 0.8 c | 11.0 ± 0.8 c | 17.6 ± 0.5 b | 26.5 ± 1.5 a | NT |
| Streptococcus pneumoniae ATCC49699 | 16.7 ± 1.2 c | 13.2 ± 1.3 d | 12.0 ± 1.0 d | 14.2 ± 1.3 d | 23.8 ± 1.3 b | 30.8 ± 1.0 a | NT |
| Gram-negative bacteria | |||||||
| Escherichia coli ATCC25922 | − | − | − | − | 18.0 ± 1.0 a | 11.2 ± 1.0 b | NT |
| Escherichia coli ATCC35218 | − | − | − | − | 17.6 ± 0.5 a | 11.8 ± 1.0 b | NT |
| Escherichia coli O157:H7 | − | − | − | − | 17.9 ± 0.2 a | 12.5 ± 0.8 b | NT |
| Klebsiella pneumoniae | − | − | − | − | 21.2 ± 1.0 a | 11.5 ± 0.5 b | NT |
| Proteus mirabilis | − | − | − | − | 34.2 ± 1.5 a | 21.6 ± 1.0 b | NT |
| Proteus vulgaris | − | − | − | − | 12.5 ± 0.7 b | 27.3 ± 1.0 a | NT |
| Pseudomonus fluorescens | − | − | − | − | 27.7 ± 2.0 a | 10.2 ± 0.5 b | NT |
| Pseudomonas aeruginosa ATCC27859 | 9.83 ± 0.8 b | − | − | 9.33 ± 0.6 b | 20.2 ± 1.0 a | 18.2 ± 1.0 a | NT |
| Salmonella typhi | − | − | − | − | 30.7 ± 1.1 b | 39.3 ± 1.1 a | NT |
| Salmonella sp. group D | − | − | − | − | 20.7 ± 1.2 a | 16.8 ± 0.8 b | NT |
| Yeasts | |||||||
| Candida albicans | − | − | − | − | NT | NT | 19.0 ± 1.0 a |
| Cryptococcus neoformans | − | − | − | − | NT | NT | 20.7 ± 1.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumla, J.; Suwannarach, N.; Tanruean, K.; Lumyong, S. Comparative Evaluation of Chemical Composition, Phenolic Compounds, and Antioxidant and Antimicrobial Activities of Tropical Black Bolete Mushroom Using Different Preservation Methods. Foods 2021, 10, 781. https://doi.org/10.3390/foods10040781
Kumla J, Suwannarach N, Tanruean K, Lumyong S. Comparative Evaluation of Chemical Composition, Phenolic Compounds, and Antioxidant and Antimicrobial Activities of Tropical Black Bolete Mushroom Using Different Preservation Methods. Foods. 2021; 10(4):781. https://doi.org/10.3390/foods10040781
Chicago/Turabian StyleKumla, Jaturong, Nakarin Suwannarach, Keerati Tanruean, and Saisamorn Lumyong. 2021. "Comparative Evaluation of Chemical Composition, Phenolic Compounds, and Antioxidant and Antimicrobial Activities of Tropical Black Bolete Mushroom Using Different Preservation Methods" Foods 10, no. 4: 781. https://doi.org/10.3390/foods10040781
APA StyleKumla, J., Suwannarach, N., Tanruean, K., & Lumyong, S. (2021). Comparative Evaluation of Chemical Composition, Phenolic Compounds, and Antioxidant and Antimicrobial Activities of Tropical Black Bolete Mushroom Using Different Preservation Methods. Foods, 10(4), 781. https://doi.org/10.3390/foods10040781