Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion and Oleogel (OG) Preparation
2.3. CB:OG Systems Preparation
2.4. Fatty Acids (FAs) Profile of CB:OG Systems
2.5. Rheological Measurements
2.6. Texture Measurements
2.7. Oil-Binding Capacity
2.8. Statistical Analysis
3. Results and Discussion
3.1. FA Composition of CB:OG Systems
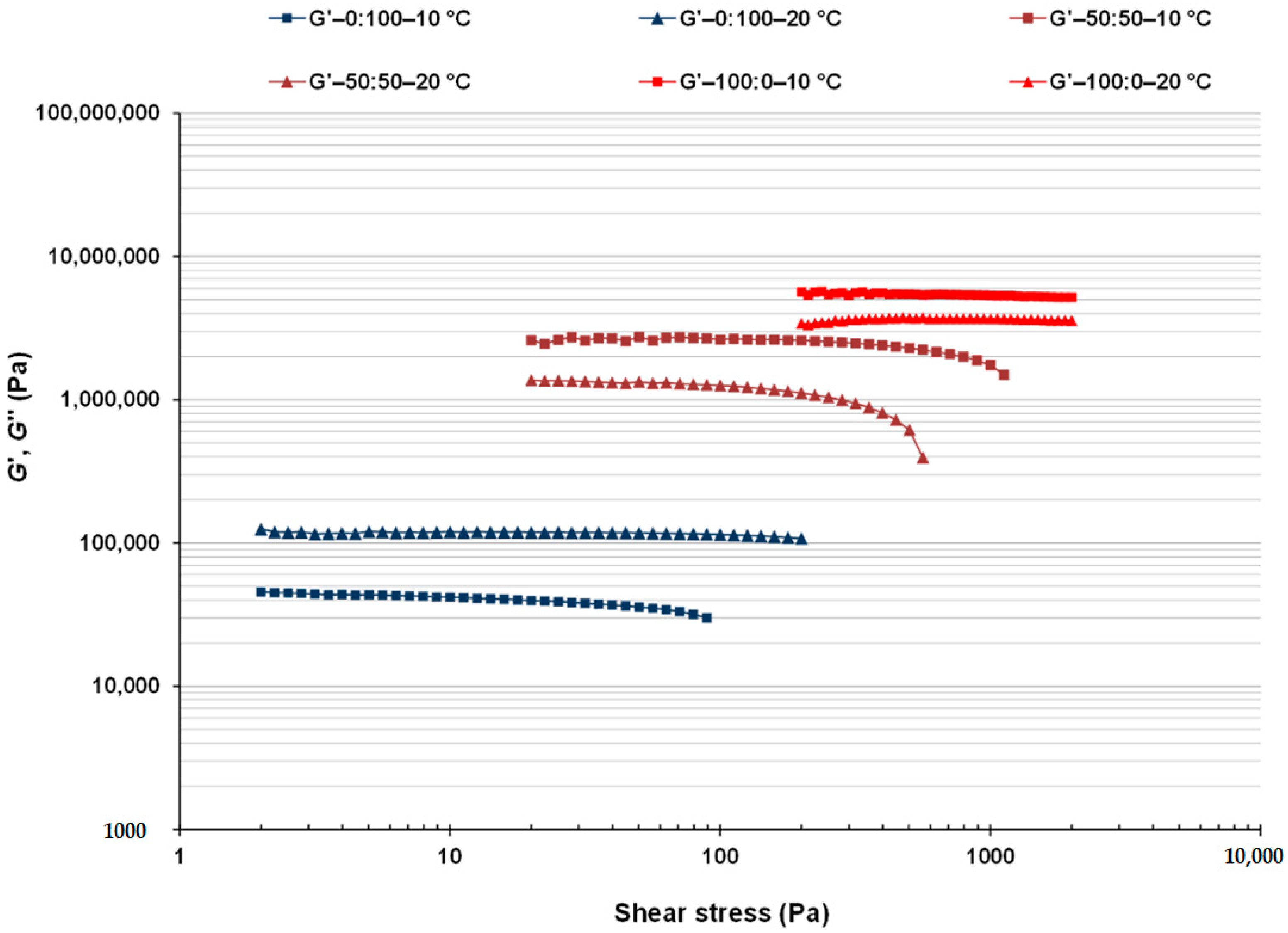
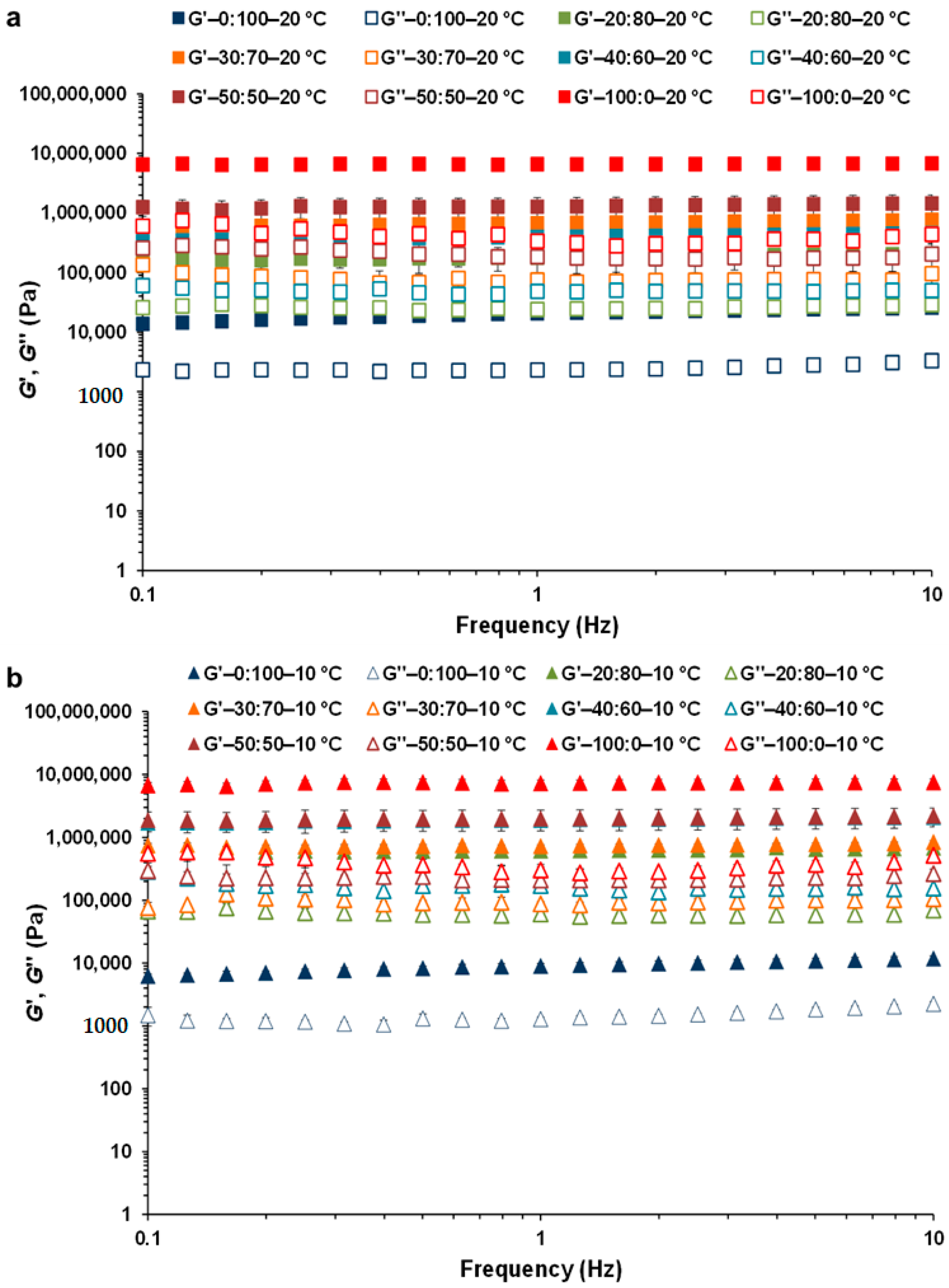
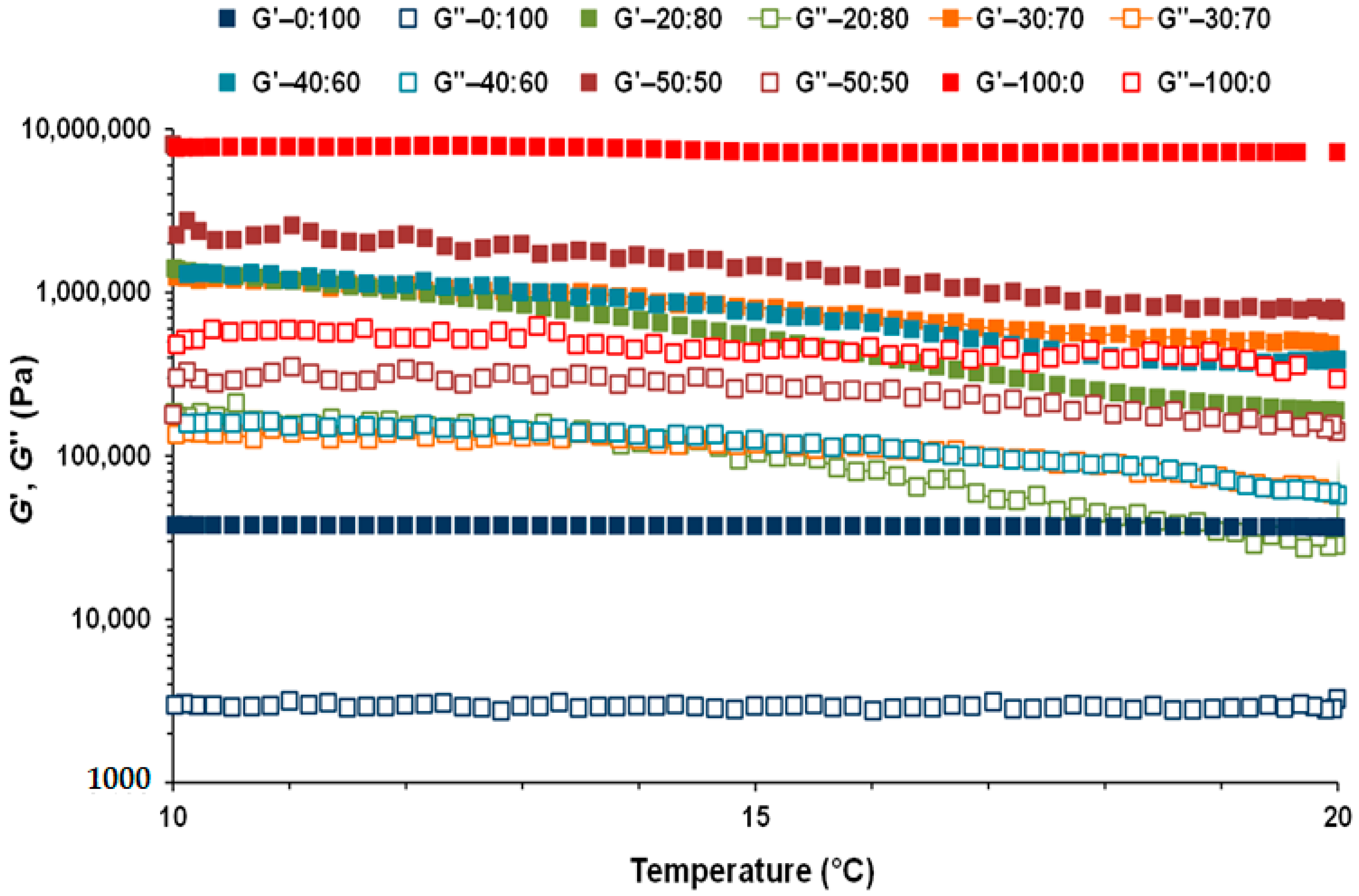
3.2. Linear Viscoelastic Properties of CB:OG Systems
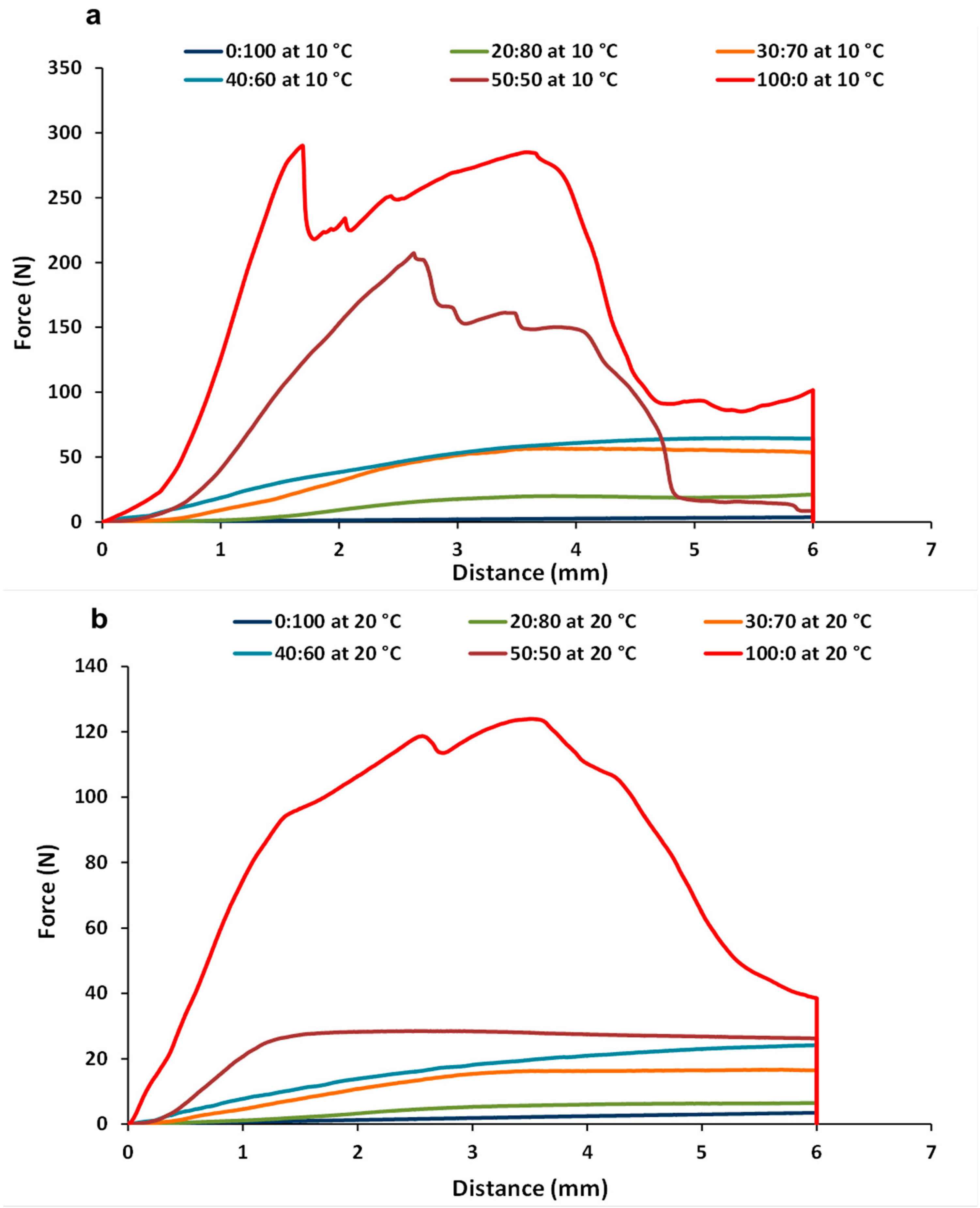
3.3. Texture Measurements of CB:OG Systems
3.4. Oil-Binding Capacity (OBC) of CB:OG Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirkesen, I.; Mert, B. Recent Developments of Oleogel Utilizations in Bakery Products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2460–2479. [Google Scholar] [CrossRef]
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, A Promising Structured Oil for Decreasing Saturated Fatty Acid Concentrations: Production and Food-Based Applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef]
- Tavernier, I.; Doan, C.D.; Van der Meeren, P.; Heyman, B.; Dewettinck, K. The Potential of Waxes to Alter the Microstructural Properties of Emulsion-Templated Oleogels. Eur. J. Lipid Sci. Technol. 2018, 120, 170039. [Google Scholar] [CrossRef]
- WHO. Diet Nutrition and the Prevention of Chronic Diseases; Technical Report Series 916; World Health Organization: Geneva, Switzerland, 2003.
- Espert, M.; Bresciani, A.; Salvador, A.; Sanz, T. Functionality of Low Digestibility Emulsions in Cocoa Creams. Structural Changes During in vitro Digestion and Sensory Perception. J. Funct. Foods 2019, 54, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Espert, M.; Wiking, L.; Salvador, A.; Sanz, T. Reduced-Fat Spreads Based on Anhydrous Milk Fat and Cellulose Ethers. Food Hydrocoll. 2020, 99, 105330. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Narine, S.S. Identifying Key Structural Indicators of Mechanical Strength in Networks of Fat Crystals. Food Res. Int. 2002, 35, 957–969. [Google Scholar] [CrossRef]
- Espert, M.; Salvador, A.; Sanz, T.; Hernandez, M.J. Cellulose Ether Emulsions as Fat Source in Cocoa Creams: Thermorheological Properties (Flow and Viscoelasticity). LWT Food Sci. Technol. 2020, 117, 108640. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Herrero, A.M.; Herranz, B.; Alvarez, M.D.; Jimenez-Colmenero, F.; Cofrades, S. Characterization of Ethyl Cellulose and Beeswax Oleogels and Their Suitability as Fat Replacers in Healthier Lipid Pâtés Development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Organogels: An Alternative Edible Oil-Structuring Method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar]
- Patel, A.R.; Rajarethinem, P.S.; Cludts, N.; Lewille, B.; De Vos, W.H.; Lesaffer, A.; Dewettinck, K. Biopolymer-Based Structuring of Liquid Oil Into Soft Solids and Oleogels Using Water-Continuous Emulsions as Templates. Langmuir 2015, 31, 2065–2073. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel Applications of Oil-Structuring Methods as a Strategy to Improve the Fat Content of Meat Products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Meng, Z.; Qi, K.Y.; Guo, Y.; Wang, Y.; Liu, Y.F. Effects of Thickening Agents on the Formation and Properties of Edible Oleogels Based on Hydroxypropyl Methyl Cellulose. Food Chem. 2018, 246, 137–149. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.Y.; Guo, Y.; Wang, Y.; Liu, Y.F. Macro-Micro Structure Characterization and Molecular Properties of Emulsion-Templated Polysaccharide Oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.Y.; Guo, Y.; Wang, Y.; Liu, Y.F. Physical Properties, Microstructure, Intermolecular Forces, and Oxidation Stability of Soybean Oil Oleogels Structured by Different Cellulose Ethers. Eur. J. Lipid Sci. Technol. 2018, 120, 1700287. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lewille, A.; Lesaffer, K.D. Polysaccharide-Based Oleogels Prepared with an Emulsion-Templated Approach. ChemPhysChem 2014, 15, 3435–3439. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Edible Oleogels Based on Water Soluble Food Polymers: Preparation, Characterization and Potential Application. Food Funct. 2014, 5, 2833–2841. [Google Scholar] [CrossRef] [Green Version]
- Espert, M.; Salvador, A.; Sanz, T. Cellulose Ether Oleogels Obtained by Emulsion-Templated Approach without Additional Thickeners. Food Hydrocoll. 2020, 109, 106085. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, Z.; Xie, Y.; Ma, A.; Zhang, H.; Rao, P.; Wang, Q. Synthesis, Physicochemical Properties, and Health Aspects of Structured Lipids: A. Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 759–800. [Google Scholar] [CrossRef]
- Patel, A.R. Structuring Edible Oils with Hydrocolloids: Where do we Stand? Food Biophys. 2018, 13, 113–115. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.L.; Wang, B.J.; Sui, X.F.; Zhong, Y.; Zhang, L.P.; Mao, Z.; Xu, H. Cellulose-Rich Oleogels Prepared with an Emulsion-Templated Approach. Food Hydrocoll. 2018, 77, 460–464. [Google Scholar] [CrossRef]
- Pizones Ruiz-Henestrosa, V.M.; Bellesi, F.A.; Camino, N.A.; Pilosof, A.M.R. The Impact of HPMC Structure in the Modulation of in Vitro Lipolysis: The Role of Bile Salts. Food Hydrocoll. 2017, 62, 251–261. [Google Scholar] [CrossRef]
- Espert, M.; Borriani, J.; Hernando, I.; Quiles, A.; Salvador, A.; Sanz, T. Relationship Between Cellulose Chemical Substitution, Structure and Fat Digestion in o/w Emulsions. Food Hydrocoll. 2017, 69, 76–85. [Google Scholar] [CrossRef]
- Bahari, A.; Akoh, C.C. Synthesis of a Cocoa Butter Equivalent by Enzymatic Interesterification of Illipe Butter and Palm Midfraction. J. Am. Oil Chem. Soc. 2018, 95, 547–555. [Google Scholar] [CrossRef]
- Kadivar, S.; De Clercq, N.; Danthine, S.; Dewettinck, K. Crystallization and Morphic Behavior of Enzymatically Produced Sunflower Oil Based Cocoa Butter Equivalents. Eur. J. Lipid Sci. Technol. 2016, 118, 1521–1538. [Google Scholar] [CrossRef]
- Biswas, N.; Cheow, Y.L.; Tan, C.P.; Kanagaratnam, S.; Siow, L.F. Cocoa Butter Substitute (CBS) Produced from Palm Mid-Fraction/Palm Kernel Oil/Palm Stearin for Confectionery Fillings. J. Am. Oil Chem. Soc. 2017, 94, 235–245. [Google Scholar] [CrossRef]
- Undurraga, D.; Markovits, A.; Erazo, S. Cocoa Butter Equivalent Through Enzymic Interesterification of Palm Oil Midfraction. Process. Biochem. 2001, 36, 933–939. [Google Scholar] [CrossRef]
- Bahari, A.; Akoh, C.C. Texture, Rheology and Fat Bloom Study of ‘Chocolates’ Made from Cocoa Butter Equivalent Synthesized from Illipe Butter and Palm Mid-Fraction. LWT Food Sci. Technol. 2018, 97, 349–354. [Google Scholar] [CrossRef]
- Kim, B.H.; Akoh, C.C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Huang, H.; Hallinan, R.; Maleky, F. Comparison of Different Oleogels in Processed Cheese Products Formulation. Int. J. Food Sci. Technol. 2018, 53, 2525–2534. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Mohd Omar, A.K. Cocoa Butter Fats and Possibilities of Substitution in Food Products Concerning Cocoa Varieties, Alternative Sources, Extraction Methods, Composition, and Characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Yu, S. Individual Fatty Acid Effects on Plasma Lipids and Lipoproteins: Human Studies. Am. J. Clin. Nutr. 1997, 65, 1628S–1644S. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanome, A.; Grundy, S.M. Effect of Dietary Stearic Acid on Plasma Cholesterol and Lipoprotein Levels. N. Engl. J. Med. 1988, 318, 1244–1248. [Google Scholar] [CrossRef]
- Kelly, F.; Sinclair, A.; Mann, N.; Turner, A.; Abedin, L.; Li, D. A Stearic Acid-Rich Diet Improves Thrombogenic and Atherogenic Risk Factor Profiles in Healthy Males. Eur. J. Clin. Nutr. 2001, 55, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red Meat Consumption: An Overview of the Risks and Benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of Fatty Acids on Meat Quality: A Review. Meat Sci. 2003, 66, 21–32. [Google Scholar] [CrossRef]
- Chai, X.; Meng, Z.; Liu, Y. Relationship Between Lipid Composition and Rheological Properties of Colloidal Fat Crystal Networks: A Comparative Study Using Chemometrics. LWT Food Sci. Technol. 2020, 118, 108814. [Google Scholar] [CrossRef]
- Herranz, H.; Tovar, C.A.; Solo-de-Zaldívar, B.; Borderias, J. Effect of Alkalis on Konjac Glucomannan Gels for Use as Potential Gelling Agents in Restructured Seafood Products. Food Hydrocoll. 2012, 27, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Nishinari, K. Some Thoughts on the Definition of a Gel. Prog. Colloid Polym. Sci. 2009, 136, 87–94. [Google Scholar]
- McGill, J.; Hartel, R.W. Investigation into the Microstructure, Texture and Rheological Properties of Chocolate Ganache. J. Food Sci. 2018, 83, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, H.; Hartel, R.W. Effects of Fat Content and Solid Fat Content on Caramel Texture Attributes. J. Am. Oil Chem. Soc. 2016, 93, 1191–1199. [Google Scholar] [CrossRef]

| mg Fatty Acid/g Sample | 100:0 | 50:50 | 40:60 | 30:70 | 20:80 | 0:100 | HORSO |
|---|---|---|---|---|---|---|---|
| Palmitic C16:0 | 238.83 ± 2.05 A | 143.43 ± 0.069 B | 119.86 ± 0.78 C | 95.59 ± 0.53 D | 100.37 ± 0.58 E | 39.89 ± 0.067 F | 40.44 ± 0.10 |
| Stearic C18:0 | 340.50 ± 3.34 A | 190.89 ± 0.19 B | 154.88 ± 1.33 C | 116.96 ± 0.94 D | 124.41 ± 1.00 E | 30.02 ± 0.015 F | 30.28 ± 0.074 |
| Arachidic C20:0 | 10.47 ± 0.14 A | 6.70 ± 0.046 B | 5.80 ± 0.048 C | 4.87 ± 0.029 D | 5.06 ± 0.033 E | 2.69 ± 0.014 F | 2.66 ± 0.019 |
| Behenic C22:0 | 1.71 ± 0.040 F | 5.85 ± 0.048 E | 6.52 ± 0.0011 D | 7.48 ± 0.0094 B | 7.29 ± 0.0077 C | 9.73 ± 0.029 A | 9.92 ± 0.029 |
| Other SFAs | 4.24 | 4.30 | 4.16 | 4.15 | 4.15 | 3.83 | 3.80 |
| ∑ SFAs | 595.74 A | 351.18 B | 290.17 C | 229.05 D | 241.27 E | 86.45 F | 87.11 |
| Palmitoleic C16:1n7 | 2.17 ± 0.016 A | 1.71 ± 0.014 B | 1.55 ± 0.0041 C | 1.43 ± 0.0018 D | 1.45 ± 0.0022 D | 1.14 ± 0.0037 E | 1.16 ± 0.024 |
| Vaccenic C18:1n7 | 3.32 ± 0.070 E | 5.39 ± 0.0029 D | 5.76 ± 0.072 C | 6.25 ± 0.072 B | 6.15 ± 0.072 B | 7.39 ± 0.073 A | 7.77 ± 0.0039 |
| Oleic C18:1n9 | 313.34 ± 2.94 F | 556.03 ± 0.037 E | 591.81 ± 0.91 D | 647.51 ± 0.51 B | 636.37 ± 0.59 C | 777.46 ± 0.43 A | 789.42 ± 2.15 |
| Eicosenoic C20:1n9 | 0.640 ± 0.0086 E | 1.60 ± 0.033 D | 1.76 ± 0.037 C | 1.99 ± 0.046 B | 1.95 ± 0.044 B | 2.52 ± 0.068 A | 2.73 ± 0.083 |
| ∑ MUFAs | 319.47 F | 564.73 E | 600.89 D | 657.18 B | 645.92 C | 788.51 A | 801.08 |
| Linoleic C18:2n6 | 28.26 ± 0.22 F | 62.4 ± 0.14 E | 67.92 ± 0.060 D | 75.85 ± 0.12 B | 74.26 ± 0.11 C | 94.35 ± 0.25 A | 97.30 ± 0.36 |
| Linolenic C18:3n3 | 1.73 ± 0.054 A | 1.12 ± 0.040 B | 1.04 ± 0.018 C | 0.90 ± 0.012 D | 0.92 ± 0.013 D | 0.57 ± 0.0048 E | 0.58 ± 0.032 |
| Other PUFAs | - | 0.30 | 0.23 | 0.28 | 0.27 | 0.39 | 0.38 |
| ∑ PUFAs | 29.99 F | 63.76 E | 69.18 D | 77.02 B | 75.5 C | 95.35 A | 98.24 |
| Nutritionally significant factors | |||||||
| PUFAs/SFAs | 0.050 F | 0.18 E | 0.24 D | 0.34 B | 0.31 C | 1.10 A | 1.12 |
| AI | 0.70 ± 0.00028 A | 0.23 ± 0.000073 B | 0.18 ± 0.00093 C | 0.13 ± 0.00064 E | 0.14 ± 0.00070 D | 0.047 ± 0.000071 F | - |
| TI | 3.24 ± 0.0040 A | 1.06 ± 0.0011 B | 0.81 ± 0.0053 C | 0.58 ± 0.0037 E | 0.62 ± 0.000 D | 0.16 ± 0.000085 F | - |
| CB:OG | 20 °C | 10 °C | ||||
|---|---|---|---|---|---|---|
| G′ (kPa) | G″ (kPa) | Tan δ | G′ (kPa) | G″ (kPa) | Tan δ | |
| 100:0 | 6630 ± 84 Aa | 339 ± 13 Aa | 0.051 ± 0.0013 Da | 7281 ± 848 Aa | 305 ± 69 Aa | 0.042 ± 0.011 Da |
| 50:50 | 1291 ± 20 Bb | 184 ± 16 Ba | 0.11 ± 0.0039 Ca | 1999 ± 25 Ba | 210 ± 13 Aa | 0.090 ± 0.015 Cb |
| 40:60 | 399 ± 35 Db | 48.5 ± 8.9 Db | 0.12 ± 0.012 B, Ca | 1900 ± 61 Ba | 172 ± 26 A, Ba | 0.11 ± 0.0079 B, Cb |
| 30:70 | 677 ± 2.3 Cb | 75.2 ± 2.6 Cb | 0.11 ± 0.0029 Ca | 732 ± 32 Ca | 87.9 ± 7.14 B, Ca | 0.12 ± 0.0048 A, Ba |
| 20:80 | 178 ± 15 Eb | 24.0 ± 3.0 D, Eb | 0.14 ± 0.0048 A, Ba | 619 ± 27 Ca | 60.9 ± 1.59 C, Da | 0.098 ± 0.0021 B, Cb |
| 0:100 | 20.8 ± 1.3 Fa | 2.33 ± 0.16 Ea | 0.14 ± 0.010 Aa | 9.04 ± 0.49 Cb | 1.29 ± 0.084 Db | 0.14 ± 0.0019 Aa |
| CB:OG | 10 °C | 20 °C | 30 °C | 40 °C | Temperature Cross-Over (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|
| G′ (kPa) | G″ (kPa) | G′ (kPa) | G″ (kPa) | G′ (kPa) | G″ (kPa) | G′ (kPa) | G″ (kPa) | ||
| 100:0 | 7245 Aa (143) | 459 Aa (122) | 5764 Ab (182) | 547 Aa (62) | 3.29 Bc (1.2) | 7.06 Ab (0.53) | 0.00012 Bc (0.000095) | 0.00027 Cb (0.0000074) | 29.0 A (0.10) |
| 50:50 | 3276 Ba (269) | 277 Ba (42) | 1405 Bb (121) | 272 Ba (20) | 0.00066 Bc (0.00014) | 0.0038 Cb (0.00052) | 0.00027 Bc (0.00011) | 0.0018 Cb (0.00029) | 27.3 B (0.47) |
| 40:60 | 2096 Ca (205) | 154 B, Ca (10) | 653 Cb (136) | 138 Ca (22) | 0.0016 Bc (0.00096) | 0.0072 Cb (0.0031) | 0.00035 Bc (0.000092) | 0.0022 Cb (0.00038) | 24.3 D (0.61) |
| 30:70 | 1803 Ca (109) | 129 C, Da (2.7) | 521 Cb (30) | 118 C, Da (11) | 0.00089 Bc (0.000054) | 0.062 Cb (0.0066) | 0.0014 Bc (0.00034) | 0.034 Cb (0.0030) | 25.5 C (0.31) |
| 20:80 | 1605 Ca (248) | 236 B, Ca (23) | 127 Db (16) | 40.3 D, Eb (9.3) | 1.18 Bb (0.0075) | 0.24 Cc (0.13) | 1.62 Bb (0.080) | 0.28 Bc (0.019) | - |
| 0:100 | 38.1 Da(1.8) | 3.04 Da, b (0.16) | 36.9 Da (3.5) | 2.94 Eb (0.097) | 34.6 Aa (3.8) | 3.18 Ba, b(0.13) | 33.4 Aa (4.03) | 3.34 Aa (0.14) | - |
| CB:OG | Maximum Force (N) | AUC (N × mm) | Maximum Force (N) | AUC (N × mm) |
|---|---|---|---|---|
| at 10 °C | at 10 °C | at 20 °C | at 20 °C | |
| 100:0 | 229 (18) Aa | 408 (14) Aa | 137 (1.4) Ab | 302 (15) Ab |
| 50:50 | 73.5 (8.5) Ba | 256 (36) Ba | 29.2 (0.89) Bb | 145 (2.4) Bb |
| 40:60 | 64.1 (0.96) Ba | 248 (16) Ba | 20.9 (2.4) Cb | 80.9 (13) Cb |
| 30:70 | 56.1 (0.46) Ba | 217 (9.5) Ba | 16.3 (0.39) Db | 72.8 (4.7) Cb |
| 20:80 | 22.1 (1.6) Ca | 83.0 (5.4) Ca | 5.01 (0.29) Eb | 17.8 (1.2) Db |
| 0:100 | 1.38 (0.016) Cb | 4.47 (0.095) Db | 3.49 (0.24) Ea | 10.6 (0.84) Da |
| CB:OG | OBC (%) after 2 h | OBC (%) after 24 h |
|---|---|---|
| 100:0 | 100 ± 0.000 Aa | 100 ± 0.000 Aa |
| 50:50 | 100 ± 0.015 Aa | 100 ± 0.01 Aa |
| 40:60 | 99.2 ± 0.35 A, Ba | 98.6 ± 0.20 Bb |
| 30:70 | 99.4 ± 0.61 Ba | 98.5 ± 0.78 Ba |
| 20:80 | 99.0 ± 0.19 B, Ca | 94.6 ± 0.35 Cb |
| 0:100 | 98.4 ± 0.048 Ca | 93.4 ± 0.28 Db |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, M.D.; Cofrades, S.; Espert, M.; Salvador, A.; Sanz, T. Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel. Foods 2021, 10, 793. https://doi.org/10.3390/foods10040793
Alvarez MD, Cofrades S, Espert M, Salvador A, Sanz T. Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel. Foods. 2021; 10(4):793. https://doi.org/10.3390/foods10040793
Chicago/Turabian StyleAlvarez, María Dolores, Susana Cofrades, María Espert, Ana Salvador, and Teresa Sanz. 2021. "Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel" Foods 10, no. 4: 793. https://doi.org/10.3390/foods10040793







