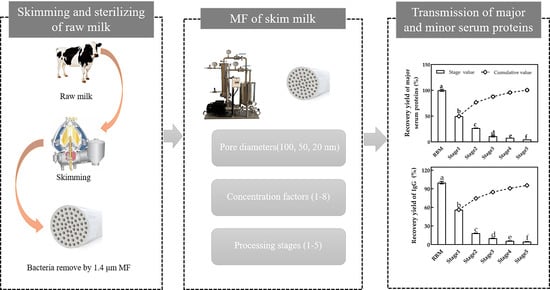
Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MF of Skim Milk with Different Pore Sizes, CF and Processing Stages
2.3. Major Serum Proteins Analysis
2.4. Minor Serum Proteins Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of Pore Diameters on Recovery Yields of Major and Minor Serum Proteins
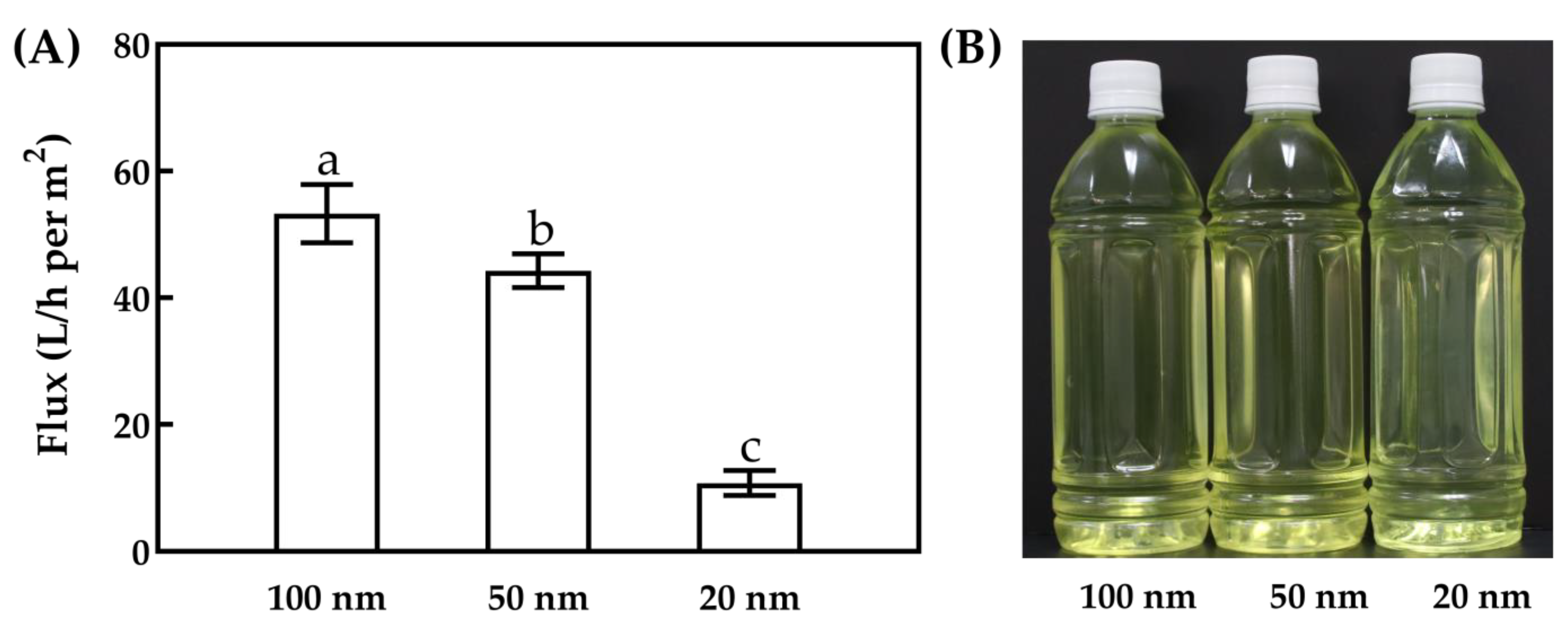
3.1.1. MF Flux
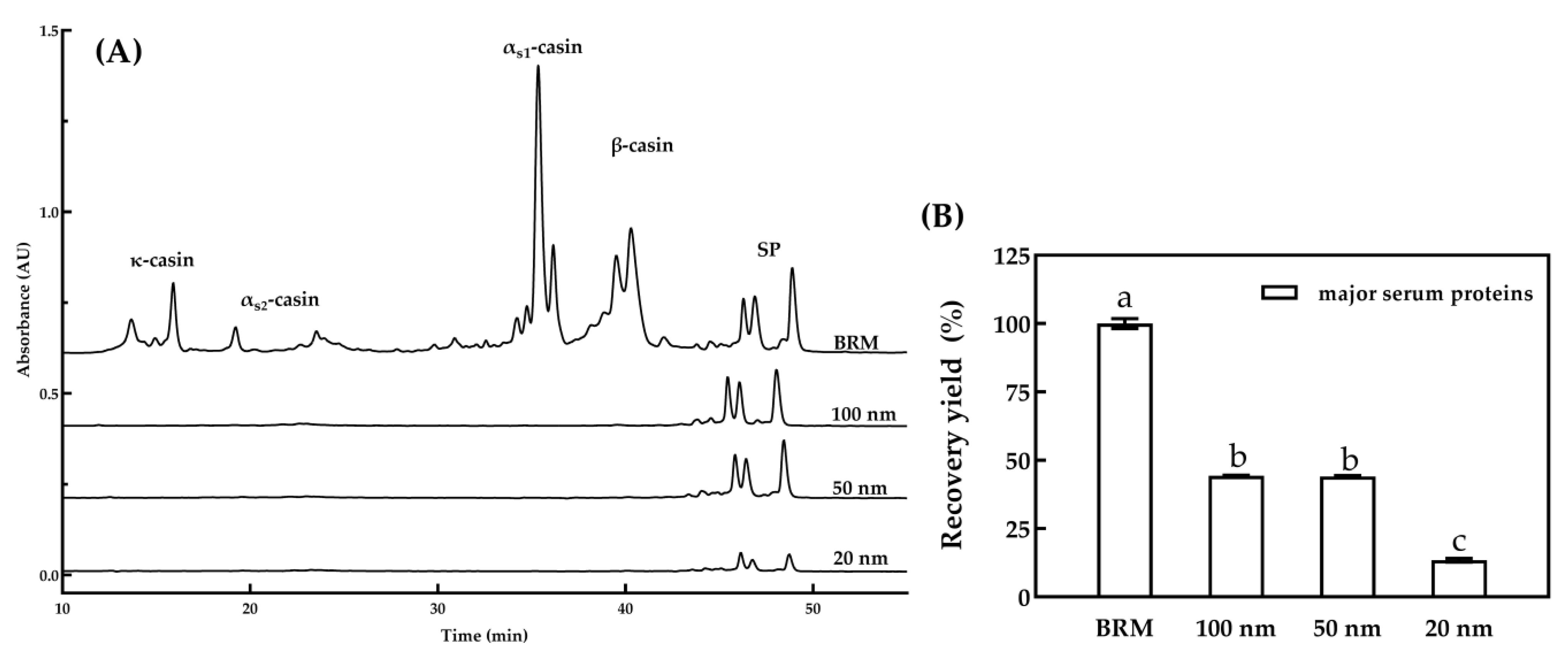
3.1.2. Major Serum Proteins
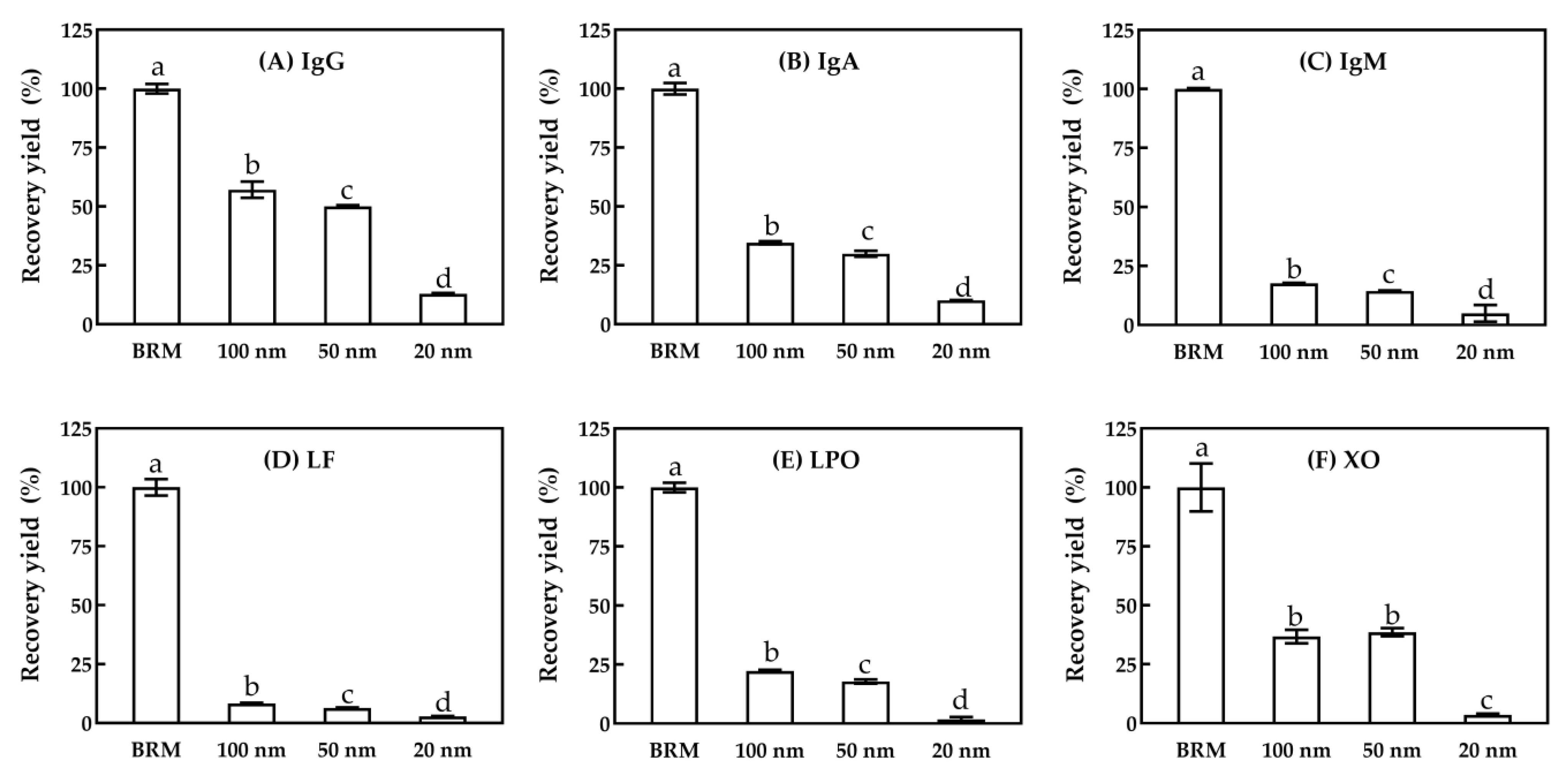
3.1.3. Minor Serum Proteins
3.2. Effects of CF on Transmission of Major and Minor Serum Proteins
3.2.1. MF Flux
3.2.2. Major Serum Proteins
3.2.3. Minor Serum Proteins
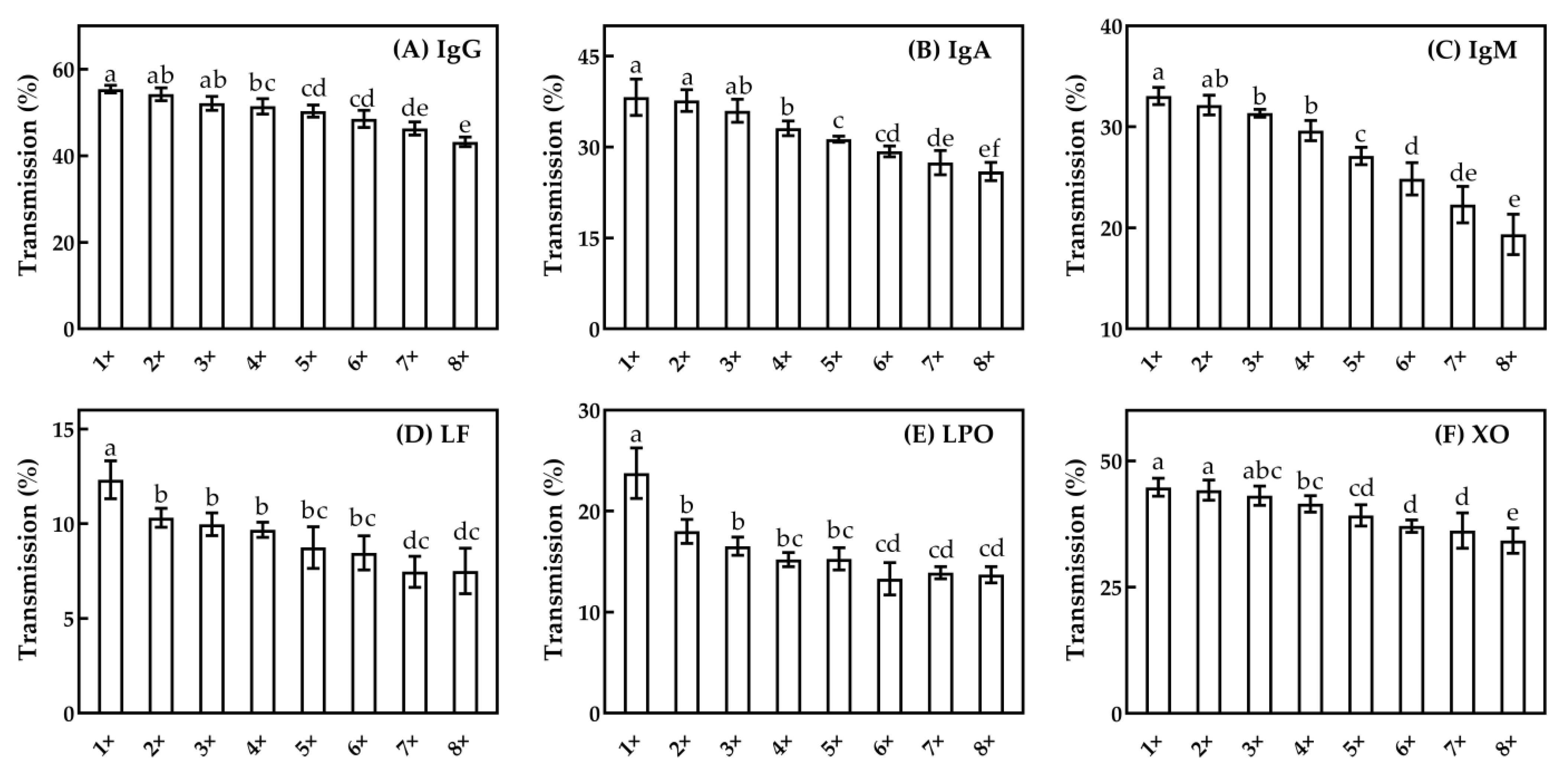
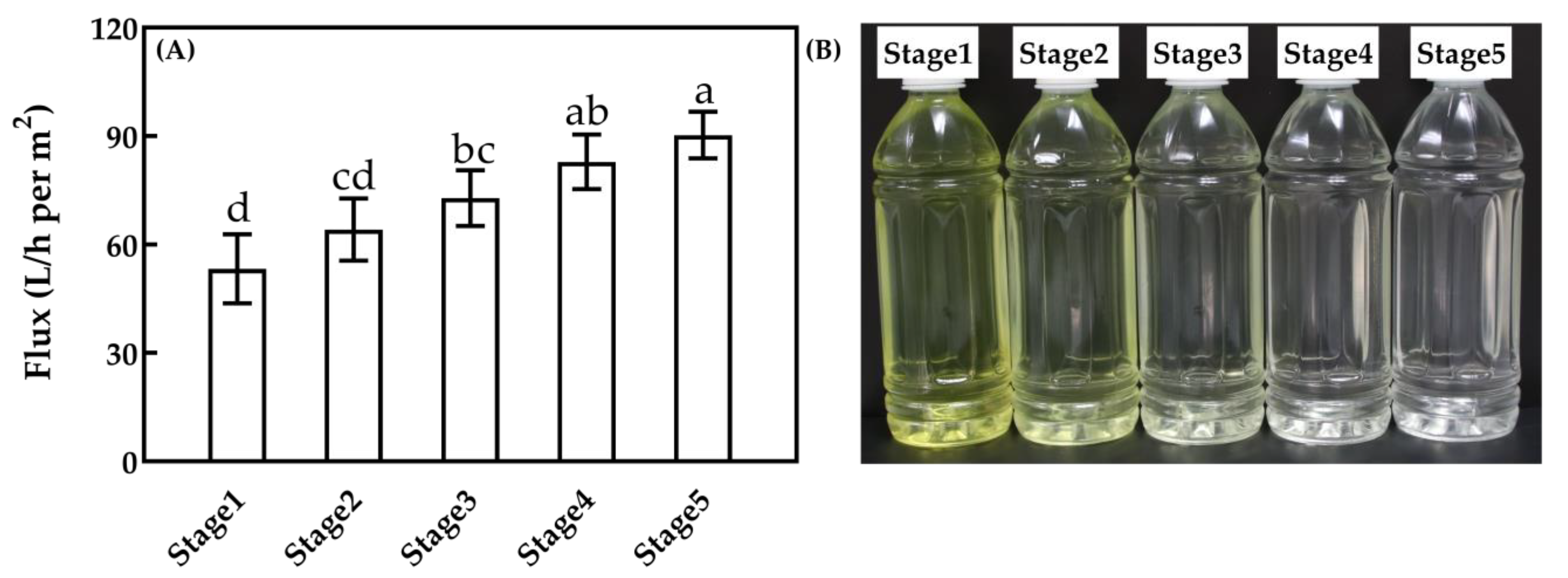
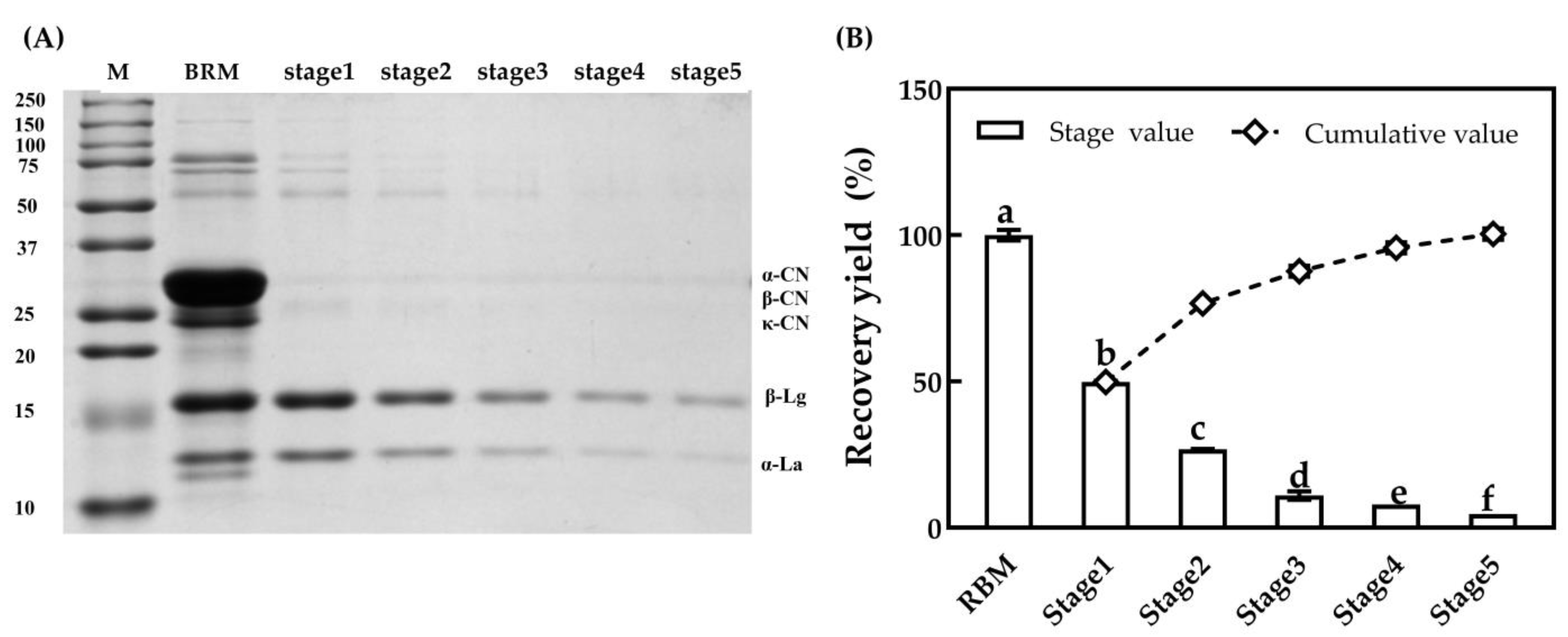
3.3. Effects of MF Stage on Recovery Yield of Major and Minor Serum Proteins
3.3.1. MF Flux
3.3.2. Major Serum Proteins
3.3.3. Minor Serum Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Production and characterization of infant milk formula powders: A review. Dry. Technol. 2020, 1–21. [Google Scholar] [CrossRef]
- Fenelon, M.A.; Hickey, R.M.; Buggy, A.; McCarthy, N.; Murphy, E.G. Chapter 12—Whey Proteins in Infant Formula. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 439–494. [Google Scholar]
- Korhonen, H.J.; Marnila, P. Milk bioactive proteins and peptides. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013; pp. 148–171. [Google Scholar]
- Claeys, W.L.; Verraes, C.; Cardoen, S.; De Block, J.; Huyghebaert, A.; Raes, K.; Dewettinck, K.; Herman, L. Consumption of raw or heated milk from different species: An evaluation of the nutritional and potential health benefits. Food Control 2014, 42, 188–201. [Google Scholar] [CrossRef]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef]
- Borad, S.G.; Singh, A.K.; Kapila, S.; Behare, P.; Arora, S.; Sabikhi, L. Influence of unit operations on immunoglobulins and thermal stability of colostrum fractions. Int. Dairy J. 2019, 93, 85–91. [Google Scholar] [CrossRef]
- Fernández-Menéndez, S.; Peixoto, R.R.A.; Fernández-Colomer, B.; Suarez-Rodríguez, M.; Sanz-Medel, A.; Fernández-Sánchez, M.L. Effect of holder pasteurisation on total concentrations and iron-binding profiles of holo -lactoferrin used as fortifier in donor human milk. Int. Dairy J. 2020, 100, 104564. [Google Scholar] [CrossRef]
- Xiong, L.; Li, C.; Boeren, S.; Vervoort, J.; Hettinga, K. Effect of heat treatment on bacteriostatic activity and protein profile of bovine whey proteins. Food Res. Int. 2020, 127, 108688. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Chase, H.A. Trends in whey protein fractionation. Biotechnol. Lett. 2011, 33, 1501–1511. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Fundamentals of Cheese Science; Springer: New York, NY, USA, 2017; pp. 11–25. [Google Scholar]
- Saboyainsta, L.V.; Maubois, J.L. Current developments of microfiltration technology in the dairy industry. Lait 2000, 80, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Glantz, M.; Devold, T.G.; Vegarud, G.E.; Lindmark Månsson, H.; Stålhammar, H.; Paulsson, M. Importance of casein micelle size and milk composition for milk gelation. J. Dairy Sci. 2010, 93, 1444–1451. [Google Scholar] [CrossRef] [Green Version]
- Heidebrecht, H.-J.; Kulozik, U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 2019, 93, 1–10. [Google Scholar] [CrossRef]
- Hurt, E.; Barbano, D.M. Processing factors that influence casein and serum protein separation by microfiltration1. J. Dairy Sci. 2010, 93, 4928–4941. [Google Scholar] [CrossRef]
- Jørgensen, C.E.; Abrahamsen, R.K.; Rukke, E.-O.; Johansen, A.-G.; Schüller, R.B.; Skeie, S.B. Optimization of protein fractionation by skim milk microfiltration: Choice of ceramic membrane pore size and filtration temperature. J. Dairy Sci. 2016, 99, 6164–6179. [Google Scholar] [CrossRef]
- Schiffer, S.; Matyssek, A.; Hartinger, M.; Bolduan, P.; Mund, P.; Kulozik, U. Effects of selective layer properties of ceramic multi-channel microfiltration membranes on the milk protein fractionation. Sep. Purif. Technol. 2021, 259, 118050. [Google Scholar] [CrossRef]
- Brans, G.; Schroën, C.G.P.H.; van der Sman, R.G.M.; Boom, R.M. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Luck, P.J.; Vardhanabhuti, B.; Yong, Y.H.; Laundon, T.; Barbano, D.M.; Foegeding, E.A. Comparison of functional properties of 34% and 80% whey protein and milk serum protein concentrates. J. Dairy Sci. 2013, 96, 5522–5531. [Google Scholar] [CrossRef] [Green Version]
- Maubois, J.L. Membrane microfiltration: A tool for a new approach in dairy technology. Aust. J. Dairy Technol. 2002, 57, 92–96. [Google Scholar]
- Nguyen, D.N.; Currie, A.J.; Ren, S.; Bering, S.B.; Sangild, P.T. Heat treatment and irradiation reduce anti-bacterial and immune-modulatory properties of bovine colostrum. J. Funct. Foods 2019, 57, 182–189. [Google Scholar] [CrossRef]
- Samuelsson, G.; Huisman, I.H.; Trägårdh, G.; Paulsson, M. Predicting limiting flux of skim milk in crossflow microfiltration. J. Membr. Sci. 1997, 129, 277–281. [Google Scholar] [CrossRef]
- Zulewska, J.; Barbano, D.M. The effect of linear velocity and flux on performance of ceramic graded permeability membranes when processing skim milk at 50°C. J. Dairy Sci. 2014, 97, 2619–2632. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, R.; Bandla, S.; Watson, D.G.; Haddock, J.; Abughazaleh, A.; Bhattacharya, B. Performance of coiled tube ultraviolet reactors to inactivate Escherichia coli W1485 and Bacillus cereus endospores in raw cow milk and commercially processed skimmed cow milk. J. Food Eng. 2011, 107, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Bacchin, P. A possible link between critical and limiting flux for colloidal systems: Consideration of critical deposit formation along a membrane. J. Membr. Sci. 2004, 228, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Hurt, E.; Zulewska, J.; Newbold, M.; Barbano, D.M. Micellar casein concentrate production with a 3X, 3-stage, uniform transmembrane pressure ceramic membrane process at 50 °C. J. Dairy Sci. 2010, 93, 5588–5600. [Google Scholar] [CrossRef]
- Visser, S.; Slangen, C.J.; Rollema, H.S. Phenotyping of bovine milk proteins by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1991, 548, 361–370. [Google Scholar] [CrossRef]
- Verdi, R.J.; Barbano, D.M.; Dellavalle, M.E.; Senyk, G.F. Variability in True Protein, Casein, Nonprotein Nitrogen, and Proteolysis in High and Low Somatic Cell Milks. J. Dairy Sci. 1987, 70, 230–242. [Google Scholar] [CrossRef]
- Zou, Z.; Bauland, J.; Hewavitharana, A.K.; Al-Shehri, S.S.; Bansal, N. A sensitive, high-throughput fluorescent method for the determination of lactoperoxidase activities in milk and comparison in human, bovine, goat and camel milk. Food Chem. 2021, 339, 128090. [Google Scholar] [CrossRef]
- Zou, Z.; Bouchereau-De Pury, C.; Hewavitharana, A.K.; Al-Shehri, S.S.; Duley, J.A.; Cowley, D.M.; Koorts, P.; Shaw, P.N.; Bansal, N. A sensitive and high-throughput fluorescent method for determination of oxidase activities in human, bovine, goat and camel milk. Food Chem. 2021, 336, 127689. [Google Scholar] [CrossRef]
- Adams, M.C.; Barbano, D.M. Serum protein removal from skim milk with a 3-stage, 3× ceramic Isoflux membrane process at 50°C1. J. Dairy Sci. 2013, 96, 2020–2034. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.; Pang, X.; Lu, J.; Wu, Z.; Yue, Y.; Wang, T.; Jiang, Z.; Lv, J. Separation of serum proteins and micellar casein from skim goat milk by pilot-scale 0.05-μm pore-sized ceramic membrane at 50 °C. J. Food Process Eng. 2020, 43, e13334. [Google Scholar] [CrossRef]
- Punidadas, P.; Rizvi, S.S.H. Separation of milk proteins into fractions rich in casein or whey proteins by cross flow filtration. Food Res. Int. 1998, 31, 265–272. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T.; Li, N.; Phelebon, L.; Garnier-Lambrouin, F.; Gésan-Guiziou, G. Interaction between lactoferrin and casein micelles in skimmed milk. Int. Dairy J. 2012, 27, 34–39. [Google Scholar] [CrossRef]
- Zydney, A.L. Protein Separations Using Membrane Filtration: New Opportunities for Whey Fractionation. Int. Dairy J. 1998, 8, 250. [Google Scholar] [CrossRef]
- Ng, K.S.Y.; Dunstan, D.E.; Martin, G.J.O. Influence of diafiltration on flux decline during skim milk ultrafiltration. Int. Dairy J. 2018, 87, 67–74. [Google Scholar] [CrossRef]
- Vadi, P.K.; Rizvi, S.S.H. Experimental evaluation of a uniform transmembrane pressure crossflow microfiltration unit for the concentration of micellar casein from skim milk. J. Membr. Sci. 2001, 189, 69–82. [Google Scholar] [CrossRef]
- Hu, K.; Dickson, J.M.; Kentish, S.E. Microfiltration for casein and serum protein separation. In Membrane Processing for Dairy Ingredient Separation; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2015; pp. 1–34. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, D.; Xu, S.; Zhang, W.; Zhou, P. Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages. Foods 2021, 10, 888. https://doi.org/10.3390/foods10040888
Li Z, Liu D, Xu S, Zhang W, Zhou P. Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages. Foods. 2021; 10(4):888. https://doi.org/10.3390/foods10040888
Chicago/Turabian StyleLi, Zhibin, Dasong Liu, Shu Xu, Wenjin Zhang, and Peng Zhou. 2021. "Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages" Foods 10, no. 4: 888. https://doi.org/10.3390/foods10040888
APA StyleLi, Z., Liu, D., Xu, S., Zhang, W., & Zhou, P. (2021). Transmission of Major and Minor Serum Proteins during Microfiltration of Skim Milk: Effects of Pore Diameters, Concentration Factors and Processing Stages. Foods, 10(4), 888. https://doi.org/10.3390/foods10040888