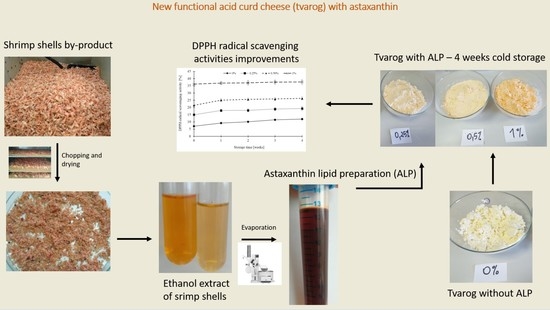
Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Astaxanthin of Lipid Preparation (ALP)
2.2. Acid Curd Cheese (ACC) with Astaxanthin
2.3. Moisture, Water Activity, PH and Titratable Acidity
2.4. Lipids, Astaxanthin Content and Thiobarbituric Acid Values
2.5. Determination of DPPH-Free Radical-Scavenging Activity
2.6. Colour Analysis
2.7. Hardness Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU 1331. Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008 Establishing A Common Authorization Procedure for Food Additives, Food Enzymes and Food Flavourings; Official Journal of the European Union: Brussels, Belgium, 2008; Volume 51, Available online: https://eur-lex.europa.eu/ (accessed on 7 January 2021).
- Deng, J.J.; Mao, H.H.; Fang, W.; Li, Z.Q.; Shi, D.; Li, Z.W.; Zhou, T.; Luo, X.C. Enzymatic conversion and recovery of protein, chitin, and astaxanthin from shrimp shell waste. J. Clean. Prod. 2020, 271, 122655. [Google Scholar] [CrossRef]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Sieradzka, M.; Kołodziejczyk-Czepas, J. Astaxanthin-carotenoid antioxidant with cardioprotective properties. Probl. Hyg. Epidem. 2016, 97, 197–206. [Google Scholar]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Sharma, V.; Chand, S. Astaxanthin: An antioxidant god. Int. J. Phytother. 2014, 4, 40–44. [Google Scholar]
- Stachowiak, B. Astaxanthin synthesis by Xanthophyllomyces dendrorhous DSM 5626 and its astaxanthin overproducing mutants on xylose media under different illumination. Acta Sci. Pol. Technol. Aliment. 2014, 13, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Ushakumari, U.N.; Ramanujan, R. Astaxanthin from shrimp shell waste. Int. J. Pharm. Chem. Res. 2012, 1, 1–6. [Google Scholar]
- Yamashita, E. Astaxanthin as a medical food. Funct. Food Health Dis. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Antioxidant and antimicrobial activities of astaxanthin from Penaeus monodon in comparison between chemical extraction and High-Pressure Processing (HPP). Int. Food Res. J. 2017, 4, S508–S513. [Google Scholar]
- Electronic Code of Federal Regulations. Title 21, Chapter I, Part 73, §73.35 Astaxanthin. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.35 (accessed on 7 January 2021).
- Sachindra, N.M.; Bhaskar, N.; Siddegowda, G.S.; Sathisha, A.D.; Suresh, P.V. Recovery of carotenoids from ensilaged shrimp waste. Bioresour. Technol. 2007, 98, 1642–1646. [Google Scholar] [CrossRef]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Krishna, C.R.; Mohan, C.M. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- EU 32. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the Approximation of the Laws of the Member States on Extraction Solvents Used in the Production of Foodstuffs and Food Ingredients; Official Journal of the European Union: Brussels, Belgium, 2009; Volume 52. [Google Scholar]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer and application in yogurt as evaluated by consumer sensory profile. LWT Food Sci. Technol. 2015, 62, 431–437. [Google Scholar] [CrossRef]
- Morton, R.A. Fat Soluble Vitamins, International Encyclopedia of Food and Nutrition; Pergamon Press: Oxford, UK, 1970; Volume 9, pp. 128–131. [Google Scholar]
- Lüddecke, E.; Auweter, H.; Schweikert, L. Use of Carotenoid Aggregates as Colorants. U.S. Patent 2004 6827941: B1, 7 December 2004. [Google Scholar]
- Mezguita, C.P.; Barragán Huerta, B.E.; Palma Ramirez, J.C.; Ortiz Hinojosa, C.P. Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. J. Food Sci. Technol. 2015, 52, 1634–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmytrów, I.; Mituniewicz-Malek, A.; Dmytrów, K. Physicochemical and sensory characteristics of acid curd cheese produced from goat’s milk and mixture of goat’s and cow’s milk. Food Sci. Technol. Qual. 2010, 2, 46–61. [Google Scholar]
- Dmytrów, I. Selected Technological Factors as Determinants of Sensory Quality and Storage Stability of Acid Curd Cheeses. Habilitation Thesis, West Pomeranian University in Szczecin, Szczecin, Poland, 2012. [Google Scholar]
- ISO 5534:2005. Determination of Total Dry Matter Content (Reference Method); Polish Committee for Standardization: Warsaw, Poland, 2005; Available online: https://sklep.pkn.pl/pn-en-iso-5534-2005p.html (accessed on 7 January 2021).
- Association of Official Agricultural Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Bhattarai, R.R.; Acharya, P.P. Preparation and quality evaluation of Mozzarella cheese from different milk sources. J. Food Sci. Technol. Nepal 2010, 6, 94–101. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Fenaille, F.; Mottier, P.; Turesky, R.J.; Ali, S.; Guy, P.A. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J. Chromatogr. A 2001, 921, 237–245. [Google Scholar] [CrossRef]
- Rhimou, B.; Hassane, R.; Nathalie, B. Antioxidant activity of Rhodophyceae extracts from atlantic and mediterranean coasts of morocco. Afr. J. Plant Sci. 2013, 7, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Kubo, M.T.K.; Maus, D.; Xavier, A.A.O.; Mercadante, A.Z.; Viotto, W.H. Transference of lutein during cheese making, color stability, and sensory acceptance of Prato cheese. Cienc. Tecnol. Alime. 2013, 33 (Suppl. 1), 81–88. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Rożnowski, J. Color assessment of food products. Laboratory 2006, 5, 36–44. [Google Scholar]
- ISO 11036:1999. Sensory Analysis. Methodology. Texture Profiling; Polish Committee for Standardization: Warsaw, Poland, 1999. [Google Scholar]
- ISO 22935-2:2013-07. Milk and Milk Products. Sensory Analysis. Part 2: Recommended Methods of Sensory Evaluation; Polish Committee for Standardization: Warsaw, Poland, 2013; Available online: https://sklep.pkn.pl/pn-iso-22935-2-2013-07p.html (accessed on 7 January 2021).
- ISO 22935-3:2013-07. Milk and Milk Products. Sensory Analysis. Part 3: Guidelines for Assessing the Conformity of Sensory Properties with Product Specifications Using the Scoring Method; Polish Committee for Standardization: Warsaw, Poland, 2013; Available online: https://sklep.pkn.pl/pn-iso-22935-3-2013-07p.html (accessed on 7 January 2021).
- Kowalczuk, I.; Jeżewska-Zychowicz, M.; Trafiałek, J. Conditions of honey consumption in selected regions of Poland. Acta Sci. Pol. Technol. Aliment. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Krochmal, A.; Jędrusyna, J.; Dopieralska, P.; Stobiecka, M.; Teter, A.; Kędzierska-Matysek, M. Quality of tvarog cheeses with different fat content. Environ. Threat. Food Saf. 2020, 1, 38–49. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Scientific opinion on the safety of astaxanthin-rich ingredients (AstaREAL A1010 and AstaREAL L10) as novel food ingredients. EFSA J. 2014, 12, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Calvo, M.M.; Gómez-Guillén, M.C. The effect of high-pressure treatment on functional components of shrimp (Litopenaeus vannamei) cephalothorax. IFSET 2016, 34, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Śmietana, Z.; Szpendowski, J.; Bohodziewicz, K. Characteristics of the traditional “Polish acid curd cheese” obtained according to its own modern technique and technology. Dairy Rev. 2003, 4, 126–129. [Google Scholar]
- Godula, K.; Dmytrów, I.; Mituniewicz-Małek, A.; Mulawka, E. Storage stability of traditional and lactose-free tvarog. Food Sci. Technol. Qual. 2018, 4, 140–149. [Google Scholar]
- Aljewicz, M.; Cichosz, G.; Kowalska, M. Water activity—A parameter influencing the quality of ripened cheeses. Adv. J. Food Sci. Technol. 2011, 2, 97–101. [Google Scholar]
- Bonczar, G.; Wszołek, M.; Walczycka, M.; Żebrowska, A.; Maciejowski, K. Influence of selected factors on water activity and microbiological quality of soft cheeses made of sheep’s milk. Food Sci. Technol. Qual. 2011, 3, 98–108. [Google Scholar]
- Alla El-Bialy, H.A.; Abd El-Khalek, H.H. A comparative study on astaxanthin recovery from shrimp wastes using lactic fermentation and green solvents: An applied model on minced Tilapia. J. Radiat. Res. Appl. Sci. 2020, 13, 594–605. [Google Scholar] [CrossRef]
- Dmytrów, I. Influence of probiotic lactic acid bacteria on the storage stability of acid curd cheese. Food Sci. Technol. Qual. 2015, 5, 49–60. [Google Scholar]
- Kariyawasam, K.M.G.M.M.; Jeewanthi, R.K.C.; Lee, N.K.; Paik, H.D. Characterization of cottage cheese using Weissella cibaria D30: Physicochemical, antioxidant, and antilisterial properties. J. Dairy Sci. 2019, 102, 3887–3893, S0022030219301857. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Chudy, S.; Gierałtowska, U.; Krzywdzińska-Bartkowiak, M.; Piątek, M. Color measurement of dairy products. In Contemporary Trends in Shaping Food Quality; Piasecka-Kwiatkowska, D., Cegielska-Radziejewska, R., Eds.; University of Life Sciences in Poznań: Poznań, Poland, 2016; pp. 85–95. [Google Scholar]
- Karczewska, D.; Pikul, J.; Płuszka, H.; Chudy, S. Changes in selected physicochemical properties of traditionally packed acid curd cheese depending on the type of packaging material used. Refrigeration 2005, 40, 46–51. [Google Scholar]






| Parameter | Factor | F | p | Test F |
|---|---|---|---|---|
| Lipids content | storage time | 0.229 | 0.921 | 2.606 |
| cheese variant | 1.867 | 0.151 | 2.839 | |
| interaction | 0.248 | 0.994 | 2.003 | |
| Moisture | storage time | 0.471 | 0.757 | 2.606 |
| cheese variant | 9.106 | ˂0.001 * | 2.839 | |
| interaction | 1.396 | 0.208 | 2.003 | |
| pH | storage time | 24.633 | ˂0.001 * | 2.606 |
| cheese variant | 4.626 | 0.007 * | 2.839 | |
| interaction | 0.772 | 0.675 | 2.003 | |
| Titratable acidity | storage time | 3.181 | 0.023 * | 2.606 |
| cheese variant | 17.166 | ˂0.001 * | 2.839 | |
| interaction | 0.297 | 0.986 | 2.003 | |
| Water activity | storage time | 1.840 | 0.140 | 2.606 |
| cheese variant | 42.347 | ˂0.001 * | 2.839 | |
| interaction | 1.436 | 0.190 | 2.003 | |
| Peroxide values | storage time | 70.490 | ˂0.001 * | 2.606 |
| cheese variant | 8.313 | ˂0.001 * | 2.839 | |
| interaction | 30.914 | ˂0.001 * | 2.003 | |
| Antioxidant activity | storage time | 14.581 | ˂0.001 * | 2.606 |
| cheese variant | 143.215 | ˂0.001 * | 2.839 | |
| interaction | 7.032 | ˂0.001 * | 2.003 | |
| Hardness | storage time | 169.915 | ˂0.001 * | 2.486 |
| cheese variant | 16.977 | ˂0.001 * | 2.718 | |
| interaction | 0.687 | 0.759 | 1.875 | |
| L* | storage time | 18.811 | ˂0.001 * | 2.486 |
| cheese variant | 14.251 | ˂0.001* | 2.719 | |
| interaction | 3.1874 | ˂0.001 * | 1.875 | |
| a* | storage time | 7.658 | ˂0.001 * | 2.486 |
| cheese variant | 406.139 | ˂0.001 * | 2.719 | |
| interaction | 1.510 | 0.138 | 1.875 | |
| b* | storage time | 4.258 | ˂0.001 * | 2.486 |
| cheese variant | 79.259 | ˂0.001 * | 2.719 | |
| interaction | 3.262 | ˂0.001 * | 1.875 | |
| h | storage time | 3.142 | ˂0.001 * | 2.486 |
| cheese variant | 1.532 | ˂0.001 | 2.719 | |
| interaction | 0.135 | 0.208 | 1.875 | |
| C | storage time | 14.751 | ˂0.001 * | 2.486 |
| cheese variant | 2.934 | ˂0.001 * | 2.719 | |
| interaction | 1.421 | ˂0.001 | 1.875 | |
| Overall sensory quality | storage time | 8.476 | ˂0.001 * | 2.557 |
| cheese variant | 1.627 | ˂0.001 | 2.557 | |
| interaction | 0.823 | ˂0.001 | 1.850 |
| Sample | Week | L* | a* | b* | h | C |
|---|---|---|---|---|---|---|
| 0% ALP | 0 | 94.84 ± 0.09 | 1.64 ± 0.49 | 9.71 ± 0.11 | 1.40 ± 0.01 | 9.84 ± 0.36 |
| 1 | 93.48 ± 0.71 | 1.70 ± 0.17 | 10.11 ± 0.07 | 1.40 ± 0.01 | 10.25 ± 0.21 | |
| 2 | 92.87 ± 0.03 | 1.99 ± 0.03 | 10.55 ± 0.46 | 1.38 ± 0.02 | 10.74 ± 0.23 | |
| 3 | 90.85 ± 0.63 | 1.86 ± 0.09 | 9.76 ± 0.21 | 1.38 ± 0.02 | 9.94 ± 0.18 | |
| 4 | 93.14 ± 0.40 | 2.30 ± 0.26 | 10.16 ± 0.27 | 1.38 ± 0.01 | 10.42 ± 0.15 | |
| 0.25% ALP | 0 | 92.21 ± 0.67 | 2.58 ± 0.02 | 11.72 ± 0.40 | 1.35 ± 0.01 | 12.00 ± 0.32 |
| 1 | 91.85 ± 0.17 | 2.56 ± 0.10 | 11.47 ± 0.16 | 1.35 ± 0.03 | 11.75 ± 0.24 | |
| 2 | 91.36 ± 0.45 | 2.65 ± 0.08 | 11.40 ± 0.18 | 1.34 ± 0.01 | 11.74 ± 0.41 | |
| 3 | 89.26 ± 0.62 | 2.46 ± 0.03 | 11.85 ± 0.57 | 1.37 ± 0.01 | 12.10 ± 0.21 | |
| 4 | 91.68 ± 0.85 | 2.53 ± 0.09 | 12.22 ± 0.45 | 1.37 ± 0.02 | 12.52 ± 0.31 | |
| 0.50% ALP | 0 | 90.94 ± 0.75 | 3.99 ± 0.16 | 13.02 ± 0.30 | 1.27 ± 0.02 | 13.62 ± 0.25 |
| 1 | 90.46 ± 0.21 | 4.09 ± 0.21 | 13.02 ± 0.28 | 1.27 ± 0.01 | 13.65 ± 0.13 | |
| 2 | 88.80 ± 0.23 | 4.55 ± 0.16 | 13.21 ± 0.19 | 1.24 ± 0.03 | 13.97 ± 0.41 | |
| 3 | 88.99 ± 0.49 | 3.99 ± 0.17 | 13.23 ± 0.40 | 1.28 ± 0.01 | 13.82 ± 0.20 | |
| 4 | 90.13 ± 0.38 | 4.52 ± 0.22 | 13.49 ± 0.17 | 1.25 ± 0.03 | 14.23 ± 0.25 | |
| 1.0% ALP | 0 | 88.48 ± 0.59 | 6.69 ± 0.35 | 15.35 ± 0.81 | 1.16 ± 0.01 | 16.77 ± 0.12 |
| 1 | 87.48 ± 0.23 | 7.25 ± 0.23 | 15.85 ± 0.24 | 1.14 ± 0.02 | 17.43 ± 0.31 | |
| 2 | 86.70 ± 0.88 | 7.40 ± 0.53 | 15.38 ± 0.45 | 1.12 ± 0.05 | 17.07 ± 0.23 | |
| 3 | 88.11 ± 0.51 | 7.22 ± 0.60 | 16.64 ± 0.95 | 1.16 ± 0.01 | 18.14 ± 0.15 | |
| 4 | 88.06 ± 0.85 | 7.40 ± 0.73 | 15.89 ± 0.72 | 1.14 ± 0.01 | 17.53 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmytrów, I.; Szymczak, M.; Szkolnicka, K.; Kamiński, P. Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment. Foods 2021, 10, 895. https://doi.org/10.3390/foods10040895
Dmytrów I, Szymczak M, Szkolnicka K, Kamiński P. Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment. Foods. 2021; 10(4):895. https://doi.org/10.3390/foods10040895
Chicago/Turabian StyleDmytrów, Izabela, Mariusz Szymczak, Katarzyna Szkolnicka, and Patryk Kamiński. 2021. "Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment" Foods 10, no. 4: 895. https://doi.org/10.3390/foods10040895