Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Fruit Purees
2.3. Reactivation of LAB Cultures and Fermentation of Fruit Purees
2.4. Physicochemical Properties of Fermented and Non-Fermented Papaya Puree
2.5. Determination of Microbial Count and Survival of LABs
2.6. Organoleptic Properties of Non-Fermented and Fermented Stored Papaya Purees
2.7. Determination of Total Phenolic Content
2.8. Simulated In Vitro Gastrointestinal Digestion
2.9. Determination of FRAP Activity
2.10. Effect of Digestion on the Phenolic Profile of Fermented and Non-Fermented Papaya Purees
2.11. Determination of α-Glucosidase Inhibition
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of LAB-Fermented Papaya Purees
3.2. Effect of Fermentation and Storage on Colour Characteristics of Papaya Puree
3.3. Survival of LABs in Papaya Purees after Fermentation and Storage
3.4. Organoleptic Properties of Fermented and Non-Fermented Papaya Puree
3.5. Changes in Phenolic Compounds and Antioxidant Power in Papaya Puree after Fermentation and Storage
3.6. Effect of LAB-Fermented Papaya Puree on In Vitro α-Glucosidase Inhibition Activity
3.7. In Vitro-Simulated Gastrointestinal (GI) Digestion and Antioxidant Power of Fermented and Non-Fermented Papaya Puree
3.8. Effect of Fermentation and In Vitro Digestion on the Antioxidant Capacity of Papaya Puree
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slavin, L.J.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Report of a Joint FAO/WHO Workshop, 1–3 September 2004, Kobe, Japan. Available online: https://apps.who.int/iris/bitstream/handle/10665/43143/9241592818_eng.pdf;jsessionid=6D4B987B3DCB6B4F0306374D1EA9A570?sequence=1 (accessed on 21 February 2021).
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic fermentation as an efficient tool to enhance the antioxidant activity of tropical fruit juices and teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef]
- Di Cagno, R.; Minervini, G.; Rizzello, C.G.; De Angelis, M.; Gobbetti, M. Effect of lactic acid fermentation on antioxidant, texture, color and sensory properties of red and green smoothies. Food Microbiol. 2011, 28, 1062–1071. [Google Scholar] [CrossRef]
- Filannino, P.; Azzi, L.; Cavoski, I.; Vincentini, O.; Rizzello, C.G.; Gobbetti, M.; Di Cagno, R. Exploitation of the health-promoting and sensory properties of organic pomegranate (Punica granatum L.) juice through lactic acid fermentation. Int. J. Food Microbiol. 2013, 163, 184–192. [Google Scholar] [CrossRef]
- Evans, E.A.; Ballen, F.H.; Crane, J.H. An Overview of US Papaya Production, Trade, and Consumption. FE914, one of a series of the Food and Resource Economics Department, UF/IFAS Extension. Available online: https://edis.ifas.ufl.edu/pdffiles/FE/FE91400.pdf (accessed on 23 February 2021).
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Science and Human Wellness, a fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Nuncio-Jáuregui, N.; José Frutos, M. Influence of fermentation with different lactic acid bacteria and in vitro digestion on the biotransformation of phenolic compounds in fermented pomegranate juices. J. Agric. Food Chem. 2017, 65, 6488–6496. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef] [Green Version]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Patthamakanokporn, O.; Puwastien, P.; Nitithamyong, A.; Sirichakwal, P.P. Changes of antioxidant activity and total phenolic compounds during storage of selected fruits. J. Food Compos. Anal. 2008, 21, 241–248. [Google Scholar] [CrossRef]
- Gayosso-Garcia Sancho, L.E.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of phenols, carotenoids and vitamin C from papaya (Carica papaya L. cv. Maradol) fruit determined by HPLC-DAD-MS/MS ESI. Food Res. Int. 2011, 44, 1284–1291. [Google Scholar] [CrossRef]
- Casirola, D.M.; Ferraris, R.P. α-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism 2006, 55, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Danese, C.; Esposito, D.; D’Alfonso, V.; Cirene, M.; Ambrosino, M.; Colotto, M. Plasma glucose level decreases as collateral effect of fermented papaya preparation use. Clin. Ter. 2006, 157, 195–198. [Google Scholar] [PubMed]
- Managa, G.M.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis). Foods 2019, 8, 399. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. Influence of storage temperature and packaging on bacteria and yeast viability in a plant-based fermented food. Foods 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.D.N.; Ramos, A.M.; Minim, V.P.R.; Chaves, J.B.P. Sensory stability of whole mango juice: Influence of temperature and storage time. Food Sci. Technol. 2012, 32, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzymes and their antioxidant properties and phenolic composition. Food Chem. 2010, 119, 592–599. [Google Scholar] [CrossRef]
- Ayed, L.; Abid, S.B.; Hamdi, M. Development of a beverage from red grape juice fermented with the Kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kokkinomagoulos, E.; Hatzikamari, M.; Bekatorou, A. Emmer-Based Beverage Fortified with Fruit Juices. Appl. Sci. 2021, 1, 3116. [Google Scholar] [CrossRef]
- Soibam, H.; Ayam, V.S.; Chakraborty, I. Preparation, and evaluation of wine from sugarcane and beet juice. Adv. Biores. 2017, 8. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mukherjee, S. Effects of fruit juice blending ratios on kinnow juice preservation at ambient storage condition. Afr. J. Food Sci. 2011, 5, 281–286. [Google Scholar]
- Jan, A.; Masih, E.D. Development and quality evaluation of pineapple juice blend with carrot and orange juice. Int. J. Sci. Res. 2012, 2, 1–8. [Google Scholar]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Mellican, R.I.; Li, J.; Mehansho, H.; Nielsen, S.S. The role of iron and the factors affecting off-color development of polyphenols. J. Agric. Food Chem. 2003, 51, 2304–2316. [Google Scholar] [CrossRef]
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; de Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Cebeci, A.; Gürakan, C. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 2003, 20, 511–518. [Google Scholar] [CrossRef]
- Srisukchayakul, P.; Charalampopoulos, D.; Karatzas, K.A. Study on the effect of citric acid adaptation toward the subsequent survival of Lactobacillus plantarum NCIMB 8826 in low pH fruit juices during refrigerated storage. Food Res. Int. 2018, 111, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Codex Standard, Codex General Standard for Fruit Juices and Nectars. 2005. Available online: www.codexalimentarius.net/ (accessed on 23 February 2021).
- Chen, P.T.; Hong, Z.S.; Cheng, C.L.; Ng, I.S.; Lo, Y.C.; Nagarajan, D.; Chang, J.S. Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum 23 using microalgae as feedstock. Bioresour. Technol. 2020, 308, 123266. [Google Scholar] [CrossRef] [PubMed]
- Managa, G.M.; Akinola, S.A.; Remize, F.; Garcia, C.; Sivakumar, D. Lactobacillus fermentation and bioaccessibility changes physicochemical parameters and bioaccessibility of lactic acid bacteria fermented chayote leave (Sechium edule) and pineapple (Ananas comosus) smoothie. Front. Nutr. 2021, 8, 120. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, Y.; Kim, Y.; Choi, I.; Kim, G. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, R.; de Las Rivas, B.; de Felipe Toledano, F.L.; Reverón, I. Biotransformation of phenolics by Lactobacillus plantarum in fermented foods. In Fermented Foods in Health and Disease Prevention; Frías, J., Martínez-Villaluenga, C., Peñas, E., Eds.; 2017; pp. 63–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Landete, J.M.; Reveron, I.; Santamaria, L.; de Las Rivas, B.; Muñoz, R. A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Appl. Environ. Microbiol. 2015, 81, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavia, Z.E.; Mousavia, S.M.; Razavia, S.H.; Hadinejada, M.; Emam-Djomeha, Z.; Mirzapoura, M. Effect of Fermentation of Pomegranate Juice by Lactobacillus plantarum and Lactobacillus acidophilus on the Antioxidant Activity and Metabolism of Sugars, Organic Acids and Phenolic Compounds. Food Biotechnol. 2013, 27, 1–13. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.Y. Stability of Green tea catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kiso, M.; Goto, T. Effect of pH and tea concentration on extraction of catechins from Japanese green tea. ACS Symp. Ser. Am. Chem. Soc. 2000, 754, 347–354. [Google Scholar] [CrossRef]
- Adebo, A.A.; Medina-Menza, I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains. A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [Green Version]
- Truchado, P.; Larrosa, M.; García-Conesa, M.T.; Cerdá, B.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012, 60, 5749–5754. [Google Scholar] [CrossRef]
- Rodríguez, H.; Landete, J.M.; Curiel, J.A.; de las Rivas, B.; Mancheño, J.M.; Muñoz, R. Characterization of the p-coumaric acid decarboxylase from Lactobacillus plantarum CECT 748T. J. Agric. Food Chem. 2008, 56, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Pinto, D.; Marzani, B.; Filannino, P.; Farris, G.A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb. Cell Fact. 2015, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of phenolic compounds, carotenoids, amino acid composition, in vitro antioxidant and anti-diabetic activities in the leaves of seven cowpea (Vigna unguiculata) cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Srinivasan, K.; Gupta, S.; Gupta, P.K.; Lal, J. Effect of mangiferin on hyperglycemia and atherogenicity in streptozotocin diabetic rats. J. Ethnopharmacol. 2005, 97, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Mulet-Cabero, A.I.; Torcello-Gómez, A. Simulating human digestion: Developing our knowledge to create healthier and more sustainable foods. Food Funct. 2020, 11, 9397–9431. [Google Scholar] [CrossRef]
- Rodriguez-Roque, M.J.; Rojas-Grauü, M.A.; Elez-Martinez, P.; Martin-Belloso, O. Changes in vitamin C, phenolic, and carotenoid profiles throughout in vitro gastrointestinal digestion of a blend fruit juice. J. Agric. Food Chem. 2013, 61, 1859–1867. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 28, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Hernanz, D.; Heredia, F.J. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking by products extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, W.; Hu, L.; Zhao, L. Mild alkaline hydrolysis is an efficient and low cost method for improving the free phenolic content and health benefit of pomegranate peel extract. J. Food Process Pres. 2013, 37, 694–700. [Google Scholar] [CrossRef]
- Neilson, A.P.; Hopf, A.S.; Cooper, B.R.; Pereira, M.A.; Bomser, J.A.; Ferruzzi, M.G. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Garcia-Conesa, M.T.; Espin, J.C.; Tomas-Barberan, F.A. Ellagitannins, 524 ellagic acid and vascular health. Mol. Aspects Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Zhaoping, L.; Summanen, P.H.; Komoriya, T.; Henning, S.M.; Lee, R.P.; Carlson, E.; Heber, D.; Finegold, S.M. Pomegranate ellagitannins stimulate growth of gut bacteria in vitro: Implications for prebiotic and metabolic effects. Anaerobe 2015, 34, 164–168. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.; Kim, D.; Chun, S.; Lee, S. Onion extract structural changes during in vitro digestion and its potential antioxidant e_ect on brain lipids obtained from low-and high-fat-fed mice. Free Radic. Res. 2013, 47, 1009–1015. [Google Scholar] [CrossRef]
- Degrain, A.; Manhivi, V.; Remize, V.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of lactic acid fermentation on colour, phenolic compounds and antioxidant activity in African nightshade. Microorganisms 2020, 8, 1324. [Google Scholar] [CrossRef] [PubMed]
- Mateo Anson, N.; Nordlund, E.; Havenaar, R.; Aura, A.-M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
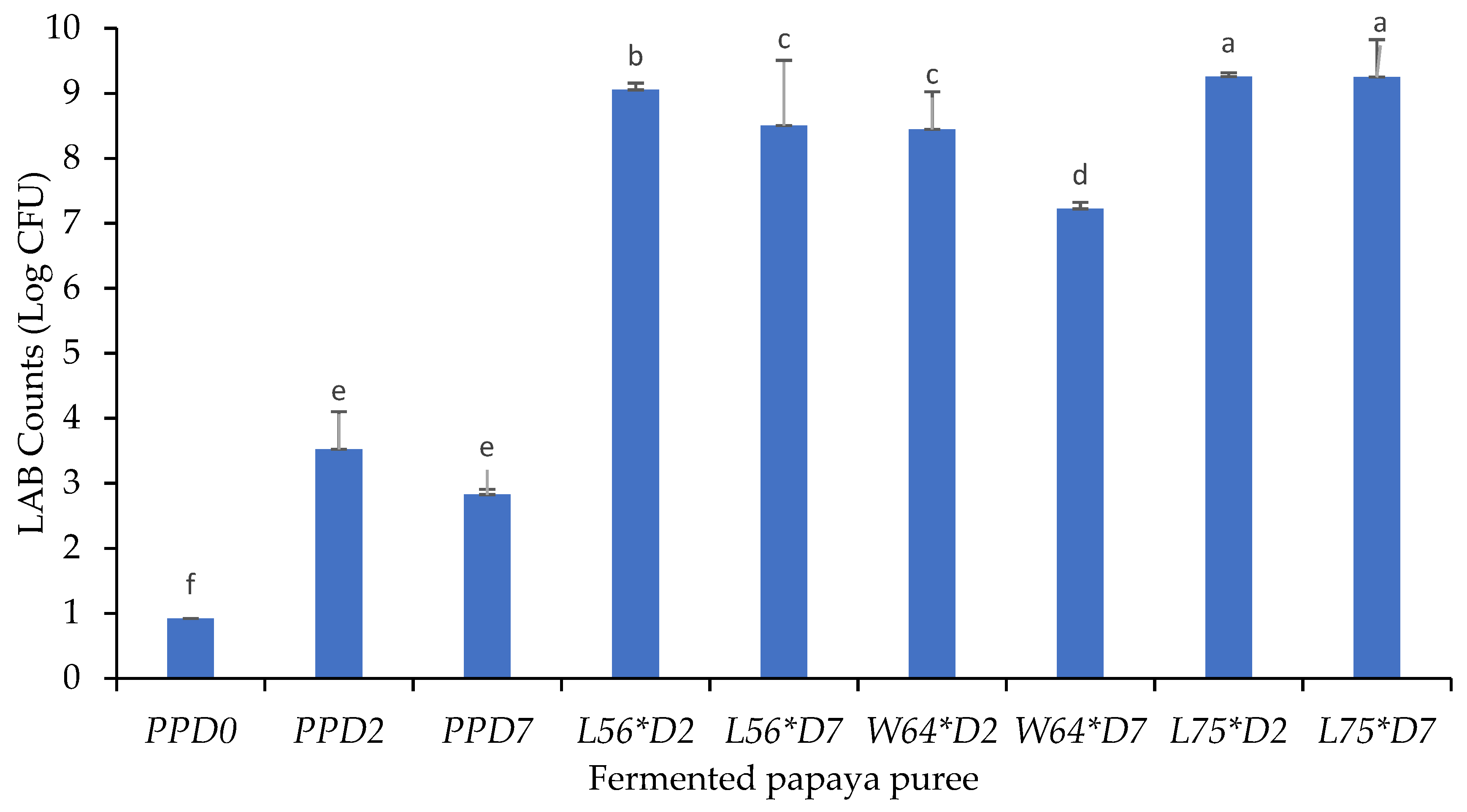

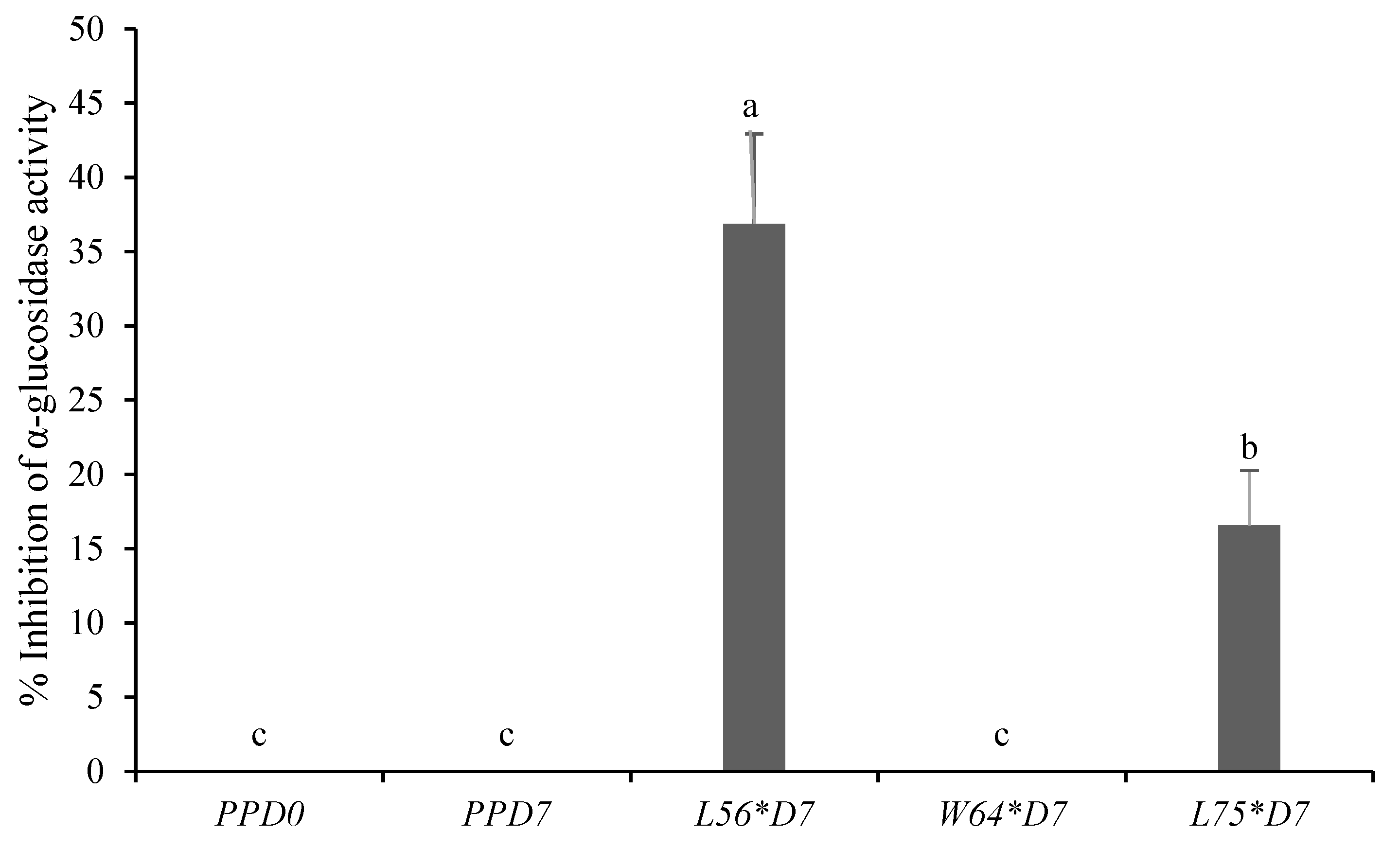
| Fruit Puree | pH | Titratable Acidity (mg/mL) | Total Soluble Solids (Brix°) |
|---|---|---|---|
| PPD0 | 5.08 ± 0.01 a | 0.59 ± 0.04 d | 8.03 ± 0.06 a,b |
| PPD2 | 4.99 ± 0.01 a,b | 0.63 ± 0.02 d | 8.07 ± 0.06 a,b |
| PPD7 | 4.36 ± 0.01 b | 0.68 ± 0.02 c | 8.50 ± 0.02 a |
| L56*D2 | 3.03 ± 0.01 d | 0.76 ± 0.02 b | 6.87 ± 0.06 d |
| L56*D7 | 3.88 ± 0.01 c | 0.73 ± 0.02 b | 8.60 ± 0.02 a |
| W64*D2 | 3.76 ± 0.01 c | 0.80 ± 0.02 a,b | 7.13 ± 0.06 c |
| W64*D7 | 4.09 ± 0.01 b | 0.71 ± 0.02 b | 7.80 ± 0.17 b |
| L75*D2 | 3.16 ± 0.01 d | 0.94 ± 0.04 a | 6.83 ± 0.06 d |
| L75*D7 | 3.36 ± 0.01 c | 0.84 ± 0.04 a,b | 7.13 ± 0.07 c |
| Fruit Puree | L* | a* | b* | ΔE |
|---|---|---|---|---|
| PPD0 | 43.21 ± 0.01 a | 23.15 ± 0.01 c | 52.76 ± 0.01 b,c | |
| PPD2 | 35.07 ± 0.06 b | 24.48 ± 0.01 b | 58.02 ± 0.01 a | 9.8 ± 0.5 g |
| PPD7 | 32.43 ± 0.01 d | 25.62 ± 0.01 a,b | 54.42 ± 0.01 b | 11.2 ± 1.0 d |
| L56*D2 | 33.91 ± 0.08 c | 23.87 ± 0.03 c | 52.33 ± 0.01 c | 9.3 ± 0.1 h |
| L56*D7 | 32.13 ± 0.01 d | 25.33 ± 0.01 a,b | 53.86 ± 0.01 b | 11.3 ± 1.0 c |
| W64*D2 | 32.99 ± 0.02 c | 24.97 ± 0.02 b | 53.13 ± 0.15 b,c | 10.4 ± 0.1 e |
| W64*D7 | 33.65 ± 0.01 c | 24.35 ± 0.02 b | 55.72 ± 0.01 a,b | 10.1 ± 0.7 f |
| L75*D2 | 31.29 ± 0.01 d,e | 24.97 ± 0.02 b | 50.62 ± 0.01 d | 12.3 ± 2.3 b |
| L75*D7 | 29.96 ± 0.05 e | 26.38 ± 0.02 a | 50.61 ± 0.01 d | 13.8 ± 0.4 a |
| Parameters | PPD0 | PPD7 | L56*D7 | W64*D7 | L75*D7 |
|---|---|---|---|---|---|
| Total phenol (mg GAE/100 g FW) | 303.9 ± 0.7 e | 408.7 ± 0.8 d | 451.0 ± 0.6 c | 467.1 ± 0.5 b | 475.1 ± 1.9 a |
| FRAP (µmol TEAC/100 g FW) | 1.4 ± 0.2 c | 2.0 ± 0.3 b | 2.0 ± 0.3 b | 2.7 ± 0.5 a | 2.8 ± 0.2 a |
| Phenolic compounds (mg/kg) | |||||
| Gallic acid | 4.4 ± 1.0 | 6.7± 1.5 | 5.6 ± 0.1 | 2.9 ± 0.2 | 6.4 ± 0.5 |
| Gallocatechin gallate | 581.4 ± 2.7 a | 564.2 ± 1.8 a | 218.9 ± 2.4 d | 462.5 ± 9.8 b | 330.4 ± 11.0 b |
| Protocatechuic acid | 19.4 ± 0.4 b | 19.3± 0.6 b | 44.4 ± 2.0 a | 19.3 ± 0.8 b | 17.4 ± 0.1 b |
| Catechin | 14.21 ± 2.3 c | 14.7 ± 1.5 c | 66.1 ± 2.7 a | 58.2 ± 0.7 b | 51.7 ± 1.3 b |
| Epicatechin | 7.7 ± 0.9 b | 6.2 ± 1.2 b,c | 16.9 ± 0.7 a | 7.7 ± 1.1 b | 5.4 ± 0.4 c |
| Chlorogenic acid | 19.9 ± 0.1 a,b | 17.2± 0.4 b | 17.7 ± 0.2 b | 1.3 ± 0.8 c | 1.7 ± 0.2 c |
| Vanillic acid | 5.6 ± 0.6 a | 4.5± 0.8 a | 4.5 ± 0.4 a | 2.5 ± 0.5 b | 2.5 ± 1.1 b |
| Syringic acid | 4.3 ± 0.1 | 4.9± 0.1 | 7.2 ± 0.4 | 2.5 ± 0.1 e,f | 4.8 ± 1.2 ** |
| Ellagic acid | 3.9 ± 2.0 c | 5.4± 1.1 b | 6.2 ± 1.5 b | 3.1 ± 0.2 c | 12.4 ± 0.2 a |
| Quercetin | 104.9 ± 0.1 a | 103.8± 16.3 a | 38.1 ± 1.5 c | 52.8 ± 5.0 b | 57.7 ± 2.3 b |
| p-Coumaric acid | 26.9 ± 0.2 b | 23.7± 2.5 b | 37.4 ± 3.2 a | 17.3 ± 0.8 c | 25.4 ± 0.8 b |
| Ferulic acid | 15.5 ± 0.2 b | 12.6± 0.1 d | 20.5 ± 0.1 a | 13.5 ± 0.2 c,d | 14.3 ± 0.3 b,c |
| Phenolic compounds in papaya puree (mg/kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPD7 | L56*D7 | W64*D7 | L75*D7 | |||||||||||||
| Compounds | BD | GP | IP | DP | BD | GP | IP | DP | BD | GP | IP | DP | BD | GP | IP | DP |
| Total phenol | 303.9 ± 1.3 g | 407.4 ± 1.7 de | 396.3 ± 2.3 e | 135.4 ± 0.3 i | 294.7 ± 0.1 g | 481.3 ± 0.4 b | 411.3 ± 0.1 d | 400.1 ± 1.1 e | 380.0 ± 0.3 f | 468.2 ± 0.1 c | 367.4 ± 1.3 f | 273.4 ± 0.5 h | 395.4 ± 0.4 e | 502.4 ± 0.4 a | 372.0 ± 2.4 f | 371.3 ± 0.6 f |
| FRAP (µmol TEAC/100 g FW) | 1.2 ± 0.1 ij | 3.7 ± 0.1 cd | 3.5 ± 1.1 d | 1.1 ± 1.0 j | 2.0 ± 0.3 h | 2.9 ± 0.2 e | 2.5 ± 0.7 f | 1.4 ± 0.0 i | 2.7 ± 0.5 ef | 3.9 ± 1.6 bc | 4.0 ± 0.1 b | 2.0 ± 0.3 h | 2.8 ± 0.2 e | 4.7 ± 1.0 a | 3.7 ± 1.2 c | 2.3 ± 0.1 g |
| Gallic acid | 6.7± 1.5 f | 6.1 ± 0.2 f | 185.0 ± 1.0 a | 73.7 ± 2.6 d | 5.6 ± 0.1 f | 140.2 ± 2.1 b | 150.9 ± 13.5 b | 47.6 ± 0.1 e | 2.9 ± 0.2 f | 89.2 ± 1.2 d | 126.8 ± 0.6 c | 42.9 ± 6.0 e | 6.4 ± 0.5 f | 57.5 ± 0.4 e | 139.6 ± 0.2 bc | 43.2 ± 1.3 e |
| Gallocatechin gallate | 564.2 ± 1.8 a | 534.6 ± 7.2 b | 264.3 ± 4.4 f | 32.9 ± 7.6 ij | 218.9 ± 2.4 f | 216.3 ± 1.1 f | 100.4 ± 0.7 h | 16.6 ± 1.3 j | 462.5 ± 9.8 c | 447.3 ± 7.4 c | 158.6 ± 2.3 g | 33.2 ± 1.1 ij | 330.4 ± 1.9 d | 311.7 ± 22.2 e | 111.4 ± 9.5 h | 47.8 ± 4.4 i |
| Protocatechuic acid | 19.3± 0.6 f | 18.5 ± 0.4 fg | 15.4 ± 0.5 fg | 8.9 ± 0.2 g | 44.4 ± 2.0 c | 32.1 ± 0.4 de | 39.8 ± 1.8 cd | 15.1 ± 0.1 fg | 19.3 ± 0.8 g | 39.4 ± 0.7 cd | 66.1 ± 6.0 a | 24.7 ± 0.6 e | 17.4 ± 0.1 fg | 29.3 ± 2.7 e | 56.6 ± 0.3 b | 18.4 ± 0.2 fg |
| Catechin | 14.7 ± 1.5 h | 13.4 ± 1.1 h | 26.6 ± 3.5 gh | 5.5 ± 0.2 h | 66.1 ± 2.7 de | 139.2 ± 5.5 c | 211.4 ± 9.8 b | 39.5 ± 1.2 fg | 58.2 ± 0.7 ef | 84.2 ± 6.4 d | 137.4 ± 7.9 c | 41.1 ± 2.3 fg | 51.7 ± 1.3 ef | 121.9 ± 5.4 c | 277.5 ± 6.6 a | 68.3 ± 2.7 de |
| Chlorogenic acid | 17.2± 0.4 b | 22.1 ±0.2 a | 8.0 ± 0.1 c | 2.2 ± 0.1 e | 17.7 ± 0.2 b | 3.5 ± 1.4 e | 7.6 ± 0.1 c | 0.7 ± 0.2 e | 1.3 ± 0.8 ef | 5.9 ± 2.2 d | 10.0 ± 3.3 c | 4.8 ± 0.4 de | 1.7 ± 0.2 e | 5.6 ± 1.0 d | 10.0 ± 0.1 c | 4.2 ± 0.2 de |
| Vanillic acid | 4.5± 0.8 c | 4.9 ± 0.5 c | 2.5 ± 0.1 cd | 1.1 ± 0.3 d | 4.5 ± 0.4 c | 4.1 ± 0.8 c | 4.8 ± 0.2 c | 2.1 ± 0.1 cd | 2.5 ± 0.5 cd | 5.4 ± 2.0 bc | 7.5 ± 0.3 bc | 2.5 ± 0.1 cd | 2.5 ± 1.1 cd | 9.7 ± 0.9 ab | 12.7 ± 0.2 a | 2.5 ± 0.3 cd |
| Syringic acid | 4.9± 0.1 e | 5.1 ± 1.2 e | 17.3 ± 0.4 cd | 8.2 ± 1.0 e | 7.2 ± 0.4 e | 7.9 ± 1.6 e | 20.3 ± 1.5 c | 13.5 ± 0.8 d | 2.5 ± 0.1 ef | 3.7 ± 0.6 ef | 19.3 ± 0.9 cd | 3.4 ± 0.1 ef | 4.8 ± 1.2 ef | 35.2 ± 1.4 a | 29.3 ± 0.9 b | 1.2 ± 1.2 f |
| Ellagic acid | 5.4± 1.1 d | 5.6 ± 0.3 cd | 11.2 ± 0.7 c | 8.1 ± 0.7 cd | 6.2 ± 1.5 cd | 4.3 ± 2.0 d | 13.3 ± 0.9 bc | 7.1 ± 0.1 cd | 3.1 ± 0.2 d | 4.8 ± 1.3 d | 12.2 ± 0.4 bc | 6.2 ± 0.4 cd | 12.4 ± 0.2 bc | 19.3 ± 2.4 b | 29.1 ± 0.7 a | 2.8 ± 0.6 d |
| Quercetin | 103.8± 16.3 a | 87.5 ± 2.3 b | 62.8 ± 0.9 c | 38.1 ± 0.8 f | 38.1 ± 1.5 f | 31.7 ± 1.7 fg | 27.4 ± 2.9 gh | 15.9 ± 0.4 i | 52.8 ± 5.0 e | 58.1 ± 2.4 de | 63.2 ± 3.1 cd | 25.9 ± 0.8 g | 57.7 ± 2.3 de | 68.9 ± 2.1 bc | 73.5 ± 3.1 b | 18.8 ± 2.3 hi |
| p-Coumaric acid | 23.7± 2.5 ef | 21.6 ± 0.7 ef | 113.8 ± 10.0 a | 54.4 ± 0.9 c | 37.4 ± 3.2 d | 32.5 ± 0.6 de | 29.0 ± 1.6 de | 16.7 ± 0.5 f | 17.3 ± 0.8 f | 74.2 ± 1.6 c | 87.2 ± 0.7 b | 24.0 ± 5.8 ef | 25.4 ± 0.8 ef | 58.1± 3.7 c | 84.4 ± 2.7 b | 17.5 ± 0.9 f |
| Ferulic acid | 12.6± 0.1 f | 13.4 ± 0.9 ef | 15.8 ± 0.2 e | 6.0 ± 0.1 g | 20.5 ± 0.1 bc | 18.9 ± 1.1 cd | 17.2 ± 0.5 de | 11.5 ± 0.3 f | 13.5 ± 0.ef | 15.0 ± 0.8 e | 18.9 ± 0.1 cd | 7.0 ± 0.7 g | 14.3 ± 0.3 e | 19.2 ± 1.1 c | 27.4 ± 0.4 a | 12.1 ± 0.2 f |
| PPD7 | L56*D7 | W64*D7 | L75*D7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery% | Bioaccessibility% | Recovery% | Bioaccessibility% | Recovery% | Bioaccessibility% | Recovery% | Bioaccessibility% | |||||
| Phenolic Compounds | GP | IP | DP | GP | IP | DP | GP | IP | DP | GP | IP | DP |
| Gallic acid | 91.0 ± 3.0 i | 2761.2 ± 5.7 c | 1100.0 ± 6.2 f | 2503.6 ± 6.0 c | 2694.6 ± 3.9 c | 850.0 ± 3.0 g | 3075.9 ± 1.8 b | 4372.4 ± 5.9 a | 1479.3 ± 2.8 e | 898.4 ± 2.9 g | 2181.3 ± 3.3 d | 675.0 ± 1.5 h |
| Gallocatechin gallate | 94.8 ± 1.0 ab | 46.8 ± 0.8 b | 5.8 ± 0.4 f | 98.8 ± 1.9 a | 45.9 ± 2.0 b | 7.6 ± 0.4 e | 96.7 ± 2.5 a | 34.3 ± 1.9 c | 7.2 ± 0.5 e | 94.3 ± 1.7 ab | 33.7 ± 2.4 c | 14.5 ± 1.7 d |
| Protocatechuic acid | 95.9 ± 2.1 e | 79.8 ± 3.0 f | 46.1 ± 2.2 h | 72.3 ± 2.4 g | 89.6 ± 3.1 e | 34.0 ± 0.8 i | 204.1 ± 3.1 b | 342.5 ± 2.8 a | 128.0 ± 1.9 cd | 168.4 ± 2.9 c | 325.3 ± 2.0 ab | 105.7 ± 3.3 d |
| Catechin | 91.2 ± 1.7 g | 181.0 ± 2.0 d | 37.4 ± 0.5 j | 210.6 ± 1.8 cd | 319.8 ± 3.4 b | 59.8 ± 3.7 i | 144.7 ± 2.9 e | 236.1 ± 2.2 c | 70.6 ± 2.9 h | 235.8 ± 3.6 c | 536.8 ± 1.8 a | 132.1 ± 2.0 f |
| Epicatechin | 87.1 ± 2.1 e | 164.5 ± 2.7 b | 59.7 ± 0.6 f | 86.4 ± 1.0 e | 114.8 ± 2.1 c | 32.5 ± 0.8 h | 109.1 ± 1.5 d | 137.7 ± 1.6 bc | 40.3 ± 0.8 g | 140.7 ± 1.9 bc | 207.4 ± 2.2 a | 79.6 ± 1.6 e |
| Caffeic acid | 89.1 ± 0.8 b | 100.0 ± 2.9 a | 28.3 ± 1.2 e | 37.1 ± 1.1 d | 43.8 ± 0.9 c | 12.4 ± 0.5 f | 97.8 ± 2.0 ab | 100.0 ± 2.3 a | 37.0 ± 4.0 d | 93.5 ± 1.5 b | 100.0 ± 2.0 a | 106.5 ± 3.0 a |
| Chlorogenic acid | 128.5 ± 1.1 e | 46.5 ± 0.8 f | 12.8 ± 0.1 h | 19.8 ± 0.9 g | 42.9 ± 0.8 f | 4.0 ± 0.1 i | 453.8 ± 2.1 bc | 769.2 ± 1.8 a | 369.2 ± 3.1 c | 329.4 ± 2.4 c | 588.2 ± 1.5 b | 247.1 ± 0.7 d |
| Vanillic acid | 108.9 ± 3.3 d | 55.6 ± 2.0 f | 24.4 ± 0.7 h | 91.1 ± 2.9 e | 106.7 ± 1.0 d | 46.7 ± 2.2 g | 216.0 ± 0.9 c | 300.0 ± 3.4 bc | 100.0 ± 0.9 d | 388.0 ± 2.0 b | 508.0 ± 1.0 a | 100.0 ± 0.4 d |
| Syringic acid | 104.1 ± 2.9 g | 353.1 ± 2.7 c | 167.3 ± 2.0 e | 109.7 ± 3.1 g | 281.9 ± 1.2 d | 187.5 ± 4.5 de | 148.0 ± 3.4 f | 772.0 ± 6.0 a | 136.0 ± 1.8 f | 733.3 ± 3.0 ab | 610.4 ± 1.9 b | 25.0 ± 0.5 h |
| Ellagic acid | 103.7 ± 2.9 f | 207.4 ± 0.8 c | 150.0 ± 1.9 e | 69.4 ± 1.7 g | 214.5 ± 1.1 c | 114.5 ± 0.7 f | 154.8 ± 1.2 e | 393.5 ± 2.0 a | 200.0 ± 1.9 d | 155.6 ± 2.1 e | 234.7 ± 2.8 b | 22.6 ± 0.9 h |
| Quercetin | 84.3 ± 2.0 c | 60.5 ± 1.1 d | 36.7 ± 0.6 f | 83.2 ± 1.4 c | 71.9 ± 2.2 cd | 41.7 ± 0.7 e | 110.0 ± 2.8 b | 119.7 ± 1.6 ab | 49.1 ± 0.1 e | 119.4 ± 2.0 ab | 127.4 ± 1.9 a | 32.6 ± 2.2 f |
| p-Coumaric acid | 91.1 ± 2.0 f | 480.2 ± 2.7 ab | 229.5 ± 3.5 d | 86.9 ± 2.5 f | 77.5 ± 1.8 g | 44.7 ± 2.6 h | 428.9 ± 4.1 b | 504.0 ± 2.1 a | 138.7 ± 1.9 e | 228.7 ± 2.8 d | 332.3 ± 3.3 c | 68.9 ± 1.7 g |
| Ferulic acid | 106.3 ± 1.3 d | 125.4 ± 0.9 c | 47.6 ± 1.2 h | 92.2 ± 2.5 e | 83.9 ± 0.9 e | 56.1 ± 1.3 f | 111.1 ± 0.9 d | 140.0 ± 2.8 b | 51.9 ± 3.6 g | 134.3 ± 1.9 b | 191.6 ± 2.1 a | 84.6 ± 3.5 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion. Foods 2021, 10, 962. https://doi.org/10.3390/foods10050962
Mashitoa FM, Akinola SA, Manhevi VE, Garcia C, Remize F, Slabbert RM, Sivakumar D. Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion. Foods. 2021; 10(5):962. https://doi.org/10.3390/foods10050962
Chicago/Turabian StyleMashitoa, Florence M., Stephen A. Akinola, Vimbainashe E. Manhevi, Cyrielle Garcia, Fabienne Remize, Retha. M. Slabbert, and Dharini Sivakumar. 2021. "Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion" Foods 10, no. 5: 962. https://doi.org/10.3390/foods10050962
APA StyleMashitoa, F. M., Akinola, S. A., Manhevi, V. E., Garcia, C., Remize, F., Slabbert, R. M., & Sivakumar, D. (2021). Influence of Fermentation of Pasteurised Papaya Puree with Different Lactic Acid Bacterial Strains on Quality and Bioaccessibility of Phenolic Compounds during In Vitro Digestion. Foods, 10(5), 962. https://doi.org/10.3390/foods10050962









