Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBVs
2.3. Analysis of Physiochemical Characteristics of CBVs
2.4. Analysis of Anti-Oxidant Activities of CBVs
2.4.1. Total Phenol Content Analysis
2.4.2. Analysis of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.5. Analysis of Anti-Obesity Activities of CBVs
2.5.1. Cell Culture
2.5.2. Cell Viability Analysis
2.5.3. Intracellular Triglyceride Analysis
2.5.4. Oil Red O (ORO) Staining and Quantification
2.5.5. Anti-Obesity Related Biomarker Analysis (Real-Time PCR)
2.6. Analysis of Anti-Aging Activities of CBVs
2.6.1. Cell Culture
2.6.2. Cell Viability Analysis
2.6.3. Cell Lifespan Analysis
2.6.4. Anti-Aging-Related Biomarker Analysis (Real-Time PCR)
2.7. Statistical Analysis
3. Results and Discussion
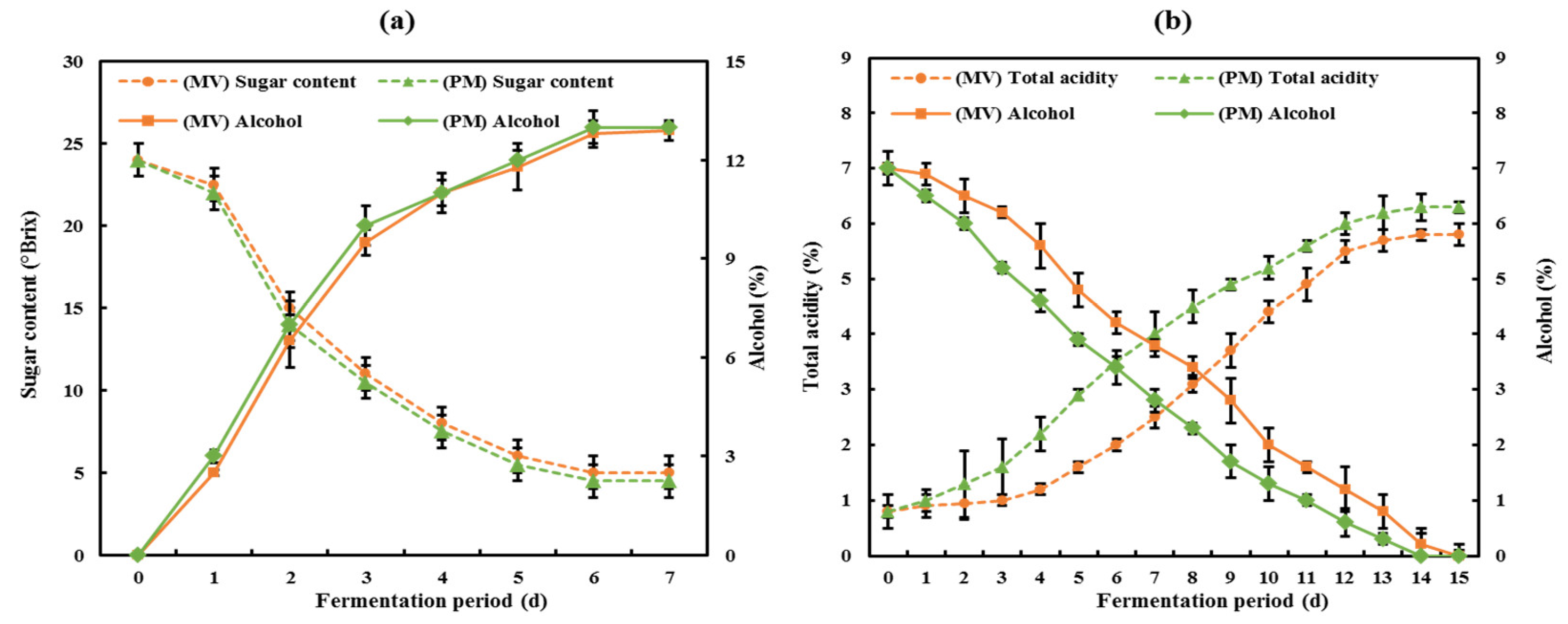
3.1. Physiochemical Characteristics of CBVs
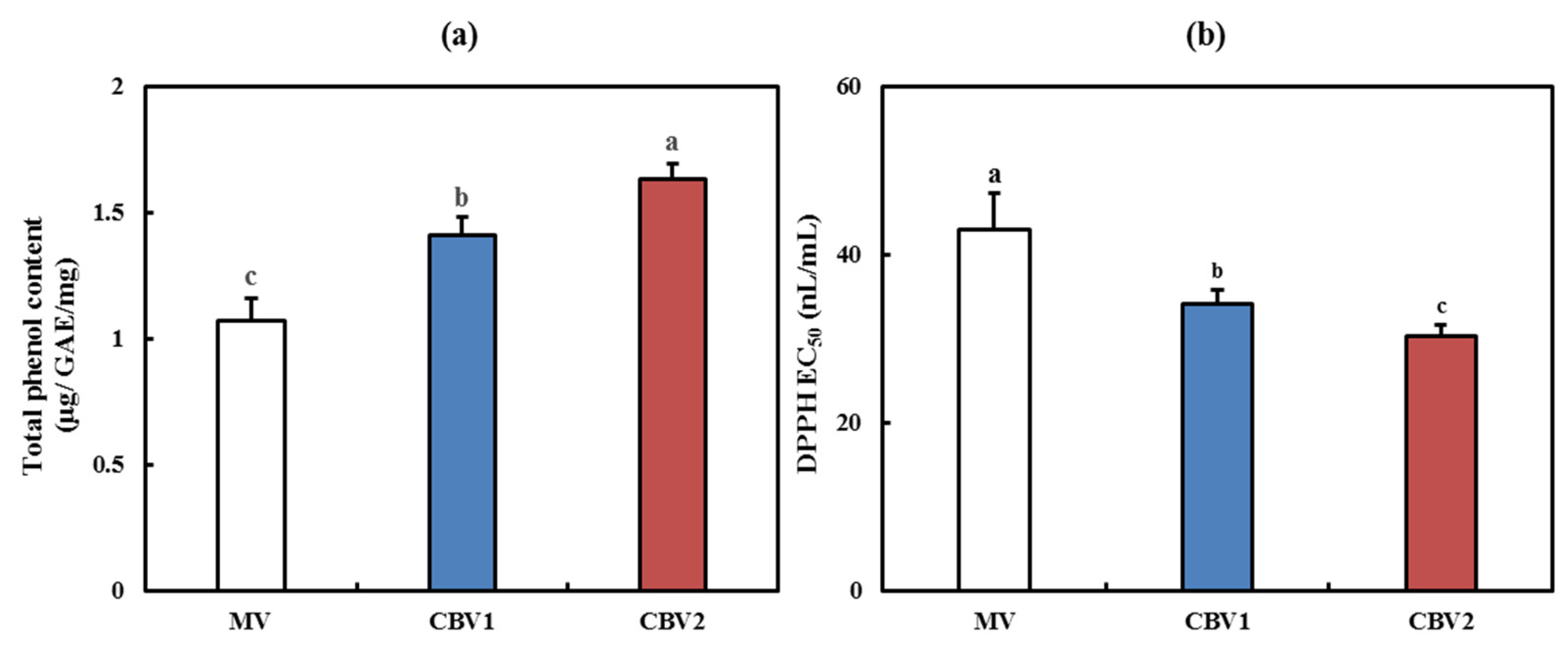
3.2. Antioxidant Activities of CBVs
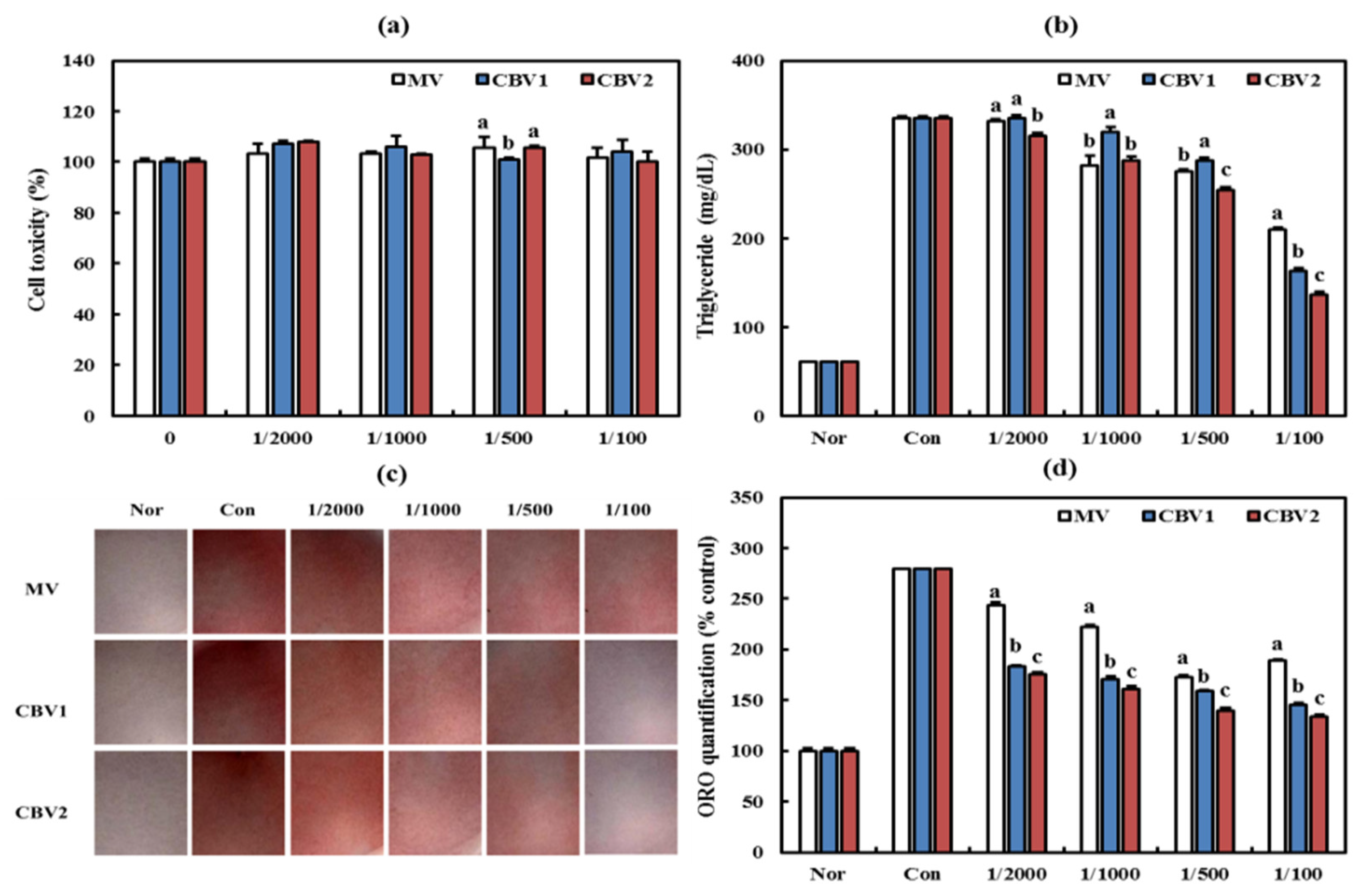
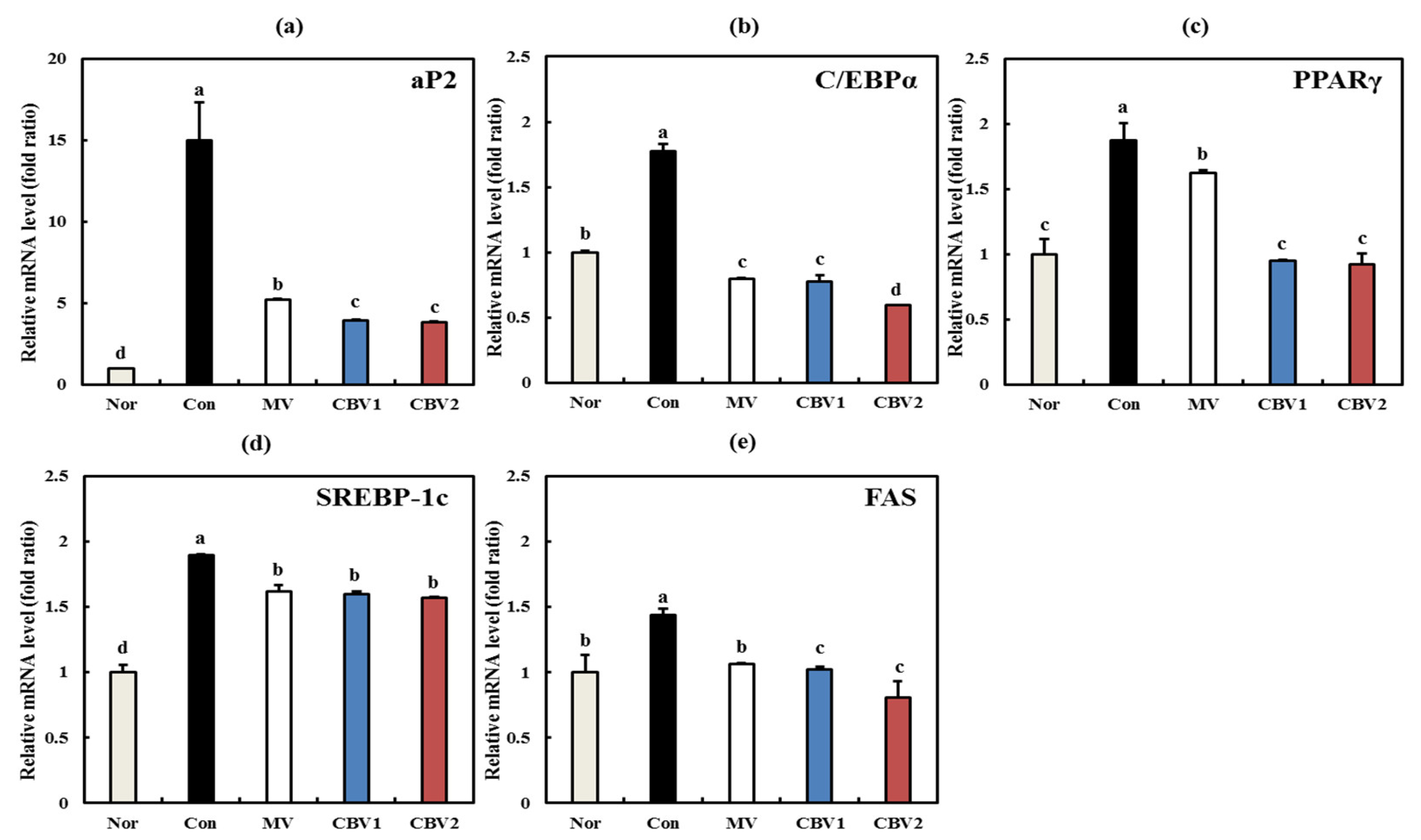
3.3. Anti-Obesity Activities of CBVs
3.4. Anti-Aging Activities of CBVs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horiuchi, J.; Kanno, T.; Kobayashi, M. Effective onion vinegar production by a two-step fermentation system. J. Biosci. Bioeng. 2000, 90, 289–293. [Google Scholar] [CrossRef]
- Gullo, M.; Giudici, P. Acetic acid bacteria in traditional Balsamic vinegar: Phenotypic traits relevant for starter cultures selection. Int. J. Food Microbiol. 2008, 125, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, S.; Kocher, G.S. Production of sugarcane blended apple vinegar under batch and Semi-Continuous fermentation conditions. Int. J. Food Ferment. Technol. 2017, 7, 271–278. [Google Scholar]
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT-Food Sci. Technol. 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, P.R.; Ling, M.Q.; Qi, M.Y.; García-Estévez, I.; Escribano-Bailón, M.T.; Chen, X.J.; Shi, Y.; Duan, C.Q. Blending strategies for wine color modificationⅠ: Color improvement by blending wines of different phenolic profiles testified under extreme oxygen exposures. Food Res. Int. 2020, 130, 108885. [Google Scholar] [CrossRef]
- Budak, H.B.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Shishehbor, F.; Mansoori, A.; Shirani, F. Vinegar consumption can attenuate postprandial glucose and insulin responses; a systematic review and meta-analysis of clinical trials. Diabetes Res. Clin. Pract. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Budak, H.N.; Kumbul, D.; Savas, C.M.; Seydim, A.C.; Kok, T.; Ciris, M.I.; Guzel-Seydim, Z.B. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J. Agric. Food Chem. 2011, 59, 6638–6644. [Google Scholar] [CrossRef]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef]
- Setorki, M.; Asgary, S.; Eidi, A.; Rohani, A.H.; Khazaei, M. Acute effects of vinegar intake on some biochemical risk factors of atherosclerosis in hypercholesterolemic rabbits. Lipids Health Dis. 2010, 9, 10. [Google Scholar] [CrossRef]
- Oikeh, E.I.; Omoregie, E.S.; Oviasogie, F.E.; Oriakhi, K. Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci. Nutr. 2015, 4, 103–109. [Google Scholar] [CrossRef]
- Lim, H.; Yeo, E.; Song, E.; Chang, Y.H.; Han, B.K.; Choi, H.J.; Hwang, J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr. Res. Pract. 2015, 9, 599–605. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, H.C.; Ko, S.Y.; Hwang, J.H.; Park, J.G.; Kang, S.H.; Han, S.H.; Yun, S.H.; Kim, S.J. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol. Pharm. Bull. 2007, 30, 772–778. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.; Zhou, M.; Gao, B.; Wang, C.; Li, D.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT-Food Sci. Technol. 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Y.; Yang, Y.; Jin, Z.; Luo, X. Effect of fermentation modes on nutritional and volatile compounds of Huyou vinegar. J. Food Sci. Technol. 2018, 55, 2631–2640. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Azzini, E.; Maiani, G.; Carcea, M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013, 140, 666–671. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Yi, M.R.; Hwang, J.H.; Oh, Y.S.; Oh, H.J.; Lim, S.B. Quality characteristics and antioxidant activity of immature Citrus unshiu vinegar. Korean J. Food Nutr. 2014, 43, 250–257. [Google Scholar] [CrossRef]
- Song, E.Y.; Choi, Y.H.; Kang, K.H.; Koh, J.S. Free sugar, organic acid, hesperidin, naringin and inorganic elements changes of Cheju citrus fruits according to harvest date. Korean J. Food Sci. Technol. 1998, 30, 306–312. [Google Scholar]
- Azuma, K.; Nakayama, M.; Koshioka, M.; Ippoushi, K.; Yamaguchi, Y.; Kohata, K.; Yamauchi, Y.; Ito, H.; Higashio, H. Phenolic antioxidants from the leaves of Corchorus olitorius L. J. Agric. Food Chem. 1999, 47, 3963–3966. [Google Scholar] [CrossRef]
- Na, H.S.; Choi, G.C.; Yang, S.I.; Lee, J.H.; Cho, J.Y.; Ma, S.J.; Kim, J.Y. Comparison of characteristics in commercial fermented vinegars made with different ingredients. Korean J. Food Preserv. 2013, 20, 482–487. [Google Scholar] [CrossRef]
- Yim, S.H.; Cho, K.S.; Choi, J.H.; Lee, J.H.; Lee, B.; Kim, M.S.; Jiang, G.H.; Eun, J.B. Physicochemical characteristics and antioxidant activity of pear vinegars using ‘Wonhwang’, ‘Niitaka’ and ‘Chuhwangbae’ fruits. Korean J. Food Preserv. 2016, 23, 174–179. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; ElGhouizi, A.; Aboulghazi, A.; Lyoussi, B.; ElArabi, I. Beneficial effects of apple vinegar on hyperglycemia and hyperlipidemia in hypercaloric-fed rats. J Diabetes Res. 2020, 2020, 9284987. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, H.J.; Byun, M.W.; Yook, H.S. Antioxidant and antimicrobial activities of ethanol extract from six vegetables containing different sulfur compounds. Korean J. Food Nutr. 2012, 41, 577–583. [Google Scholar] [CrossRef]
- Park, Y.H.; Choi, J.H.; Whang, K.; Lee, S.O.; Yang, S.A.; Yu, M.H. Inhibitory effects of lyophilized dropwort vinegar powder on adipocyte differentiation and inflammation. J. Life Sci. 2014, 24, 476–484. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Cho, H.D.; Jeong, J.H.; Lee, M.K.; Jeong, Y.K.; Shim, K.H.; Seo, K.I. New vinegar produced by tomato suppresses adipocyte differentiation and fat accumulation in 3T3-L1 cells and obese rat model. Food Chem. 2013, 141, 3241–3249. [Google Scholar] [CrossRef]
- Hosoda, S.; Kawazoe, Y.; Shiba, T.; Numazawa, S.; Manabe, A. Anti-obesity effect of ginkgo vinegar, a fermented product of ginkgo seed coat, in mice fed a high-fat diet and 3T3-L1 preadipocyte cells. Nutrients 2020, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Son, H.K.; Kim, Y.K.; Shin, H.W.; Lim, H.J.; Moon, B.S.; Lee, J.J. Comparison of anti-obesity effects of sprit vinegar and natural fermented vinegar products on the differentiation of 3T3-L1 cells and obese rats fed a high-fat diet. J. Food Nutr. Res. 2017, 5, 594–605. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBP alpha induces adipogenesis through PPAR gamma: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Son, E.; Im, G.; Kim, D.S. Herbal combination of Phyllostachys pubescens and Scutellaria baicalensis inhibits adipogenesis and promotes browning via AMPK activation in 3T3-L1 adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Kang, K.A.; Lee, K.H.; Zhang, R.; Piao, M.; Chae, S.; Kim, K.N.; Jeon, Y.J.; Park, D.B.; You, H.J.; Kim, J.S.; et al. Caffeic acid protects hydrogen peroxide induced cell damage in WI-38 human lung fibroblast cells. Biol. Pharm. Bull. 2006, 29, 1820–1824. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Su, H.; Pan, Y.; Han, J.; Zhang, T.; Mao, G. Effect of sulfated galactan from Porphyra haitanesis on H2O2-induced premature senescence in WI-38 cell. Int. J. Biol. Macromol. 2018, 106, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.R.; Choi, M.J.; Choi, J.M.; Ko, J.C.; Ko, J.Y.; Cho, E.J. Malvidin protects WI-38 human fibroblast cells against stress-induced premature senescence. J. Cancer Prev. 2016, 21, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Mawal-Dewan, M.; Frisoni, L.; Cristofalo, V.J.; Sell, C. Extension of replicative lifespan in WI-38 human fibroblasts by dexamethasone treatment is accompanied by suppression of p21 Waf1/Cip1/Sdi1 levels. Exp. Cell Res. 2003, 285, 91–98. [Google Scholar] [CrossRef]
- Campisi, J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef]
- Seshadri, T.; Campisi, J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science 1990, 247, 205–209. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Borlon, C.; Pascal, T.; Royer, V.; Eliaers, F.; Ninane, N.; Carrard, G.; Friguet, B.; de Longueville, F.; Boffe, S.; et al. Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. J. Cell Sci. 2005, 118, 743–758. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | MV | CBV1 | CBV2 |
|---|---|---|---|
| pH | 3.45 ± 0.11 a | 3.37 ± 0.40 a | 3.46 ± 0.19 a |
| Total acidity (%) | 4.69 ± 0.11 b | 5.04 ± 0.20 ab | 5.40 ± 0.22 a |
| Sugar content (%) | 68.40 ± 0.00 a | 54.93 ± 0.23 b | 53.60 ± 0.00 c |
| H2O2 | H2O2 Plus MV | H2O2 Plus CBV1 | H2O2 Plus CBV2 |
|---|---|---|---|
| 24 19 | 24 22 | 24 26 | 24 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.-R.; Park, B.-Y.; Kim, S.-H.; Jung, J.-H. Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar. Foods 2021, 10, 1441. https://doi.org/10.3390/foods10071441
Yun Y-R, Park B-Y, Kim S-H, Jung J-H. Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar. Foods. 2021; 10(7):1441. https://doi.org/10.3390/foods10071441
Chicago/Turabian StyleYun, Ye-Rang, Bo-Yeon Park, Sung-Hyun Kim, and Ji-Hye Jung. 2021. "Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar" Foods 10, no. 7: 1441. https://doi.org/10.3390/foods10071441
APA StyleYun, Y.-R., Park, B.-Y., Kim, S.-H., & Jung, J.-H. (2021). Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar. Foods, 10(7), 1441. https://doi.org/10.3390/foods10071441