Effect of Microwave Maceration and SO2 Free Vinification on Volatile Composition of Red Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microwave Treatment and Microvinification
2.2. Solid Phase Extraction
2.2.1. Free Volatile Compounds
2.2.2. Glycosidically Bound Volatile Compounds
2.3. Gas Chromatography-Mass Spectrometry
2.4. Descriptive Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Microwave Treatment on Volatile Compounds of Musts
3.2. Effect of Microwave Treatment on Volatile Compounds of Wines Fermented with and without SO2.
3.3. Effect of Microwave Treatment on Volatile Compounds Formed during Alcoholic Fermentation with and without SO2.
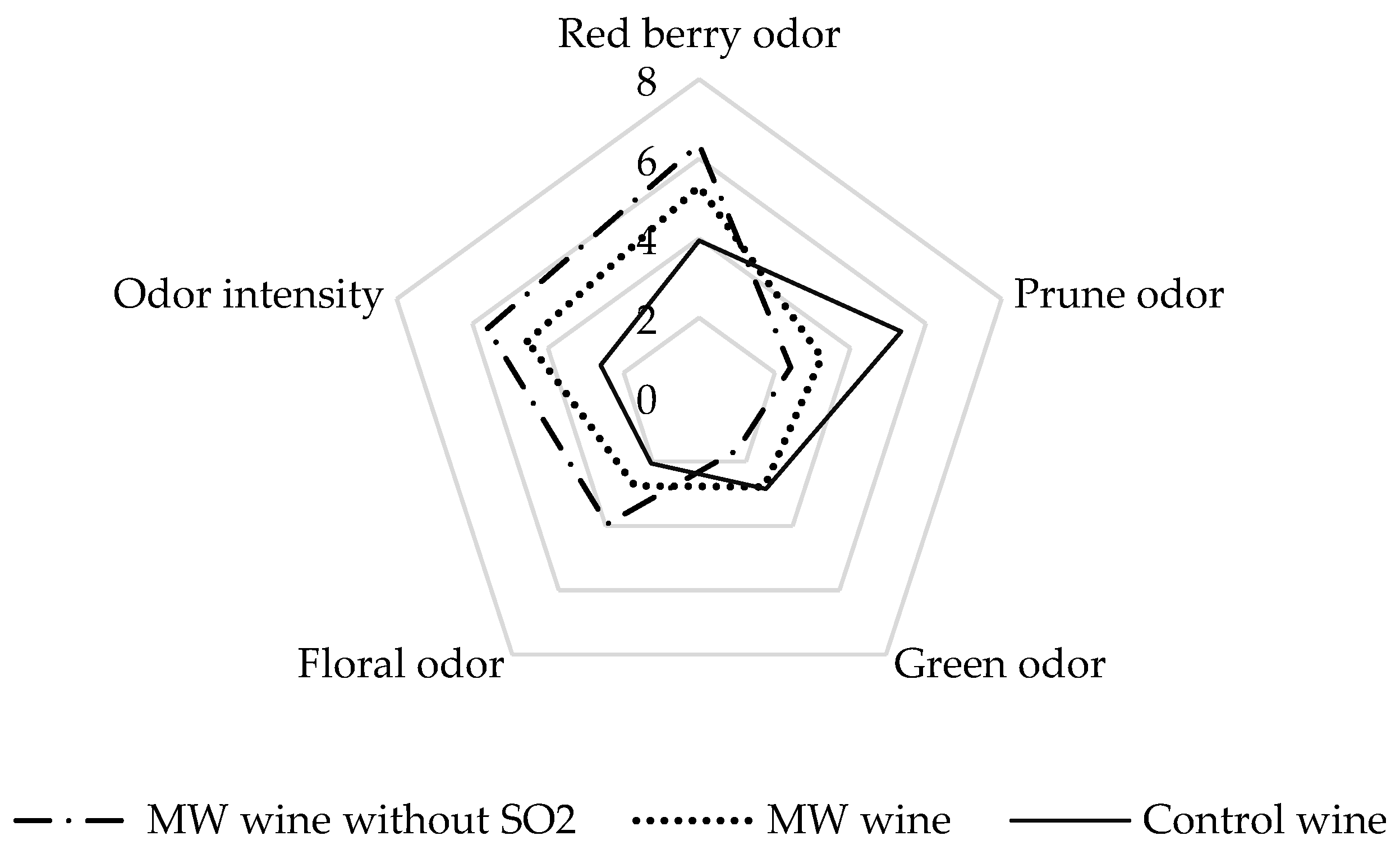
3.4. Sensory Analysis of Control and Microwave-Treated Wine Elaborated with and without SO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging technology to develop novel red winemaking practices: An overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Cui, Y.; Lv, W.; Liu, J.F.; Wang, B.J. Effect of different ending fermentation technologies on microbial-stability of Italian Riesling low alcohol sweet white wine. Adv. Mater. Res. 2012, 393, 1165–1168. [Google Scholar] [CrossRef]
- Rougier, C.; Prorot, A.; Chazal, P.; Leveque, P.; Lepratb, P. Thermal and Nonthermal Effects of Discontinuous Microwave Exposure (2.45 Gigahertz) on the Cell Membrane of Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 4832–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumes, R. Wine aroma precursors. In Wine Chemistry and Biochemistry; Moreno Arribas, M.V., Polo, M.C., Eds.; Springer Science & Business Media LLD: New York, NY, USA, 2009; pp. 251–274. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Atanackovic, M.; Petrovic, A.; Jovic, S.; Gojkovic-Bukarica, L.; Bursac, M.; Cvejic, J. Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chem. 2012, 131, 513–518. [Google Scholar] [CrossRef]
- Comuzzo, P.; Marconi, M.; Zanella, G.; Querzè, M. Pulsed electric field processing of white grapes (cv. Garganega): Effects on wine composition and volatile compounds. Food Chem. 2018, 264, 16–23. [Google Scholar] [CrossRef]
- Jiranek, V.; Grbin, P.; Yap, A.; Barnes, M.; Bates, D. High power ultrasonics as a novel tool offering new opportunities for managing wine microbiology. Biotechnol. Lett. 2008, 30, 1–6. [Google Scholar] [CrossRef]
- García Martín, J.F.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, B.; Jurado, R.; Gómez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT-Food Sci. Technol. 2021, 138, 110645–110653. [Google Scholar] [CrossRef]
- Parenti, A.; Spugnoli, P.; Calamai, L.; Ferrari, S.; Gori, C. Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur. Food Res. Technol. 2004, 218, 360–366. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in analytical and volatile compositions of red wines induced by pre fermentation heat treatment of grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geffroy, O.; Lopez, R.; Feilhes, C.; Violleau, F.; Kleiber, D.; Favarel, J.L.; Ferreira, V. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 2018, 257, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Rodríguez, A.; Carrera, C.; Palma-Lovillo, M.; García Barroso, C. Ultrasonic treatments during the alcoholic fermentation of red wines: Effects on ‘Syrah’ wines. Vitis 2019, 58, 83–88. [Google Scholar] [CrossRef]
- Oliver Simancas, R.; Díaz-Maroto, M.C.; Alañón Pardo, M.E.; Pérez Porras, P.; Bautista Ortín, A.B.; Gómez-Plaza, E.; Pérez-Coello, M.S. Effect of power ultrasounds treatment on free and glycosidically-bound volatile compounds and sensorial profile of red wines. Molecules 2021, 26, 1193. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of high-powder ultrasounds during red wine vinification. Int. J. Food Sci. Technol. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Letellier, M.; Budzinski, H. Microwave assisted extraction of organic compounds. Analysis 1999, 27, 259–271. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Bittar, S.A.; Perino-Issartier, S.; Dangles, O.; Chemat, F. An innovative grape juice enriched in polyphenols by microwave-assisted extraction. Food Chem. 2013, 141, 3268–3272. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. Part A 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Carew, A.L.; Close, D.C.; Dambergs, R.G. Yeast strain affects phenolic concentration in Pinot noir wines made by microwave maceration with early pressing. J. Appl. Microbiol. 2015, 118, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Carew, A.L.; Gill, W.; Close, D.C.; Dambergs, R.G. Microwave maceration with early pressing improves phenolics and fermentation kinetics in Pinot noir. Am. J. Enol. Vitic. 2014, 65, 401–406. [Google Scholar] [CrossRef]
- Carew, A.L.; Sparrow, A.M.; Curtin, C.D.; Close, D.C.; Dambergs, R.G. Microwave maceration of Pinot Noir grapemust: Sanitation and extraction effects and wine phenolics outcomes. Food Bioproc. Technol. 2014, 7, 954–963. [Google Scholar] [CrossRef]
- Casassa, L.F.; Sari, S.E.S.; Bolcato, E.A.; Fanzone, M.L. Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color. Fermentation 2019, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Tartian, A.C.; Cotea, V.V.; Niculaua, M.; Zamfir, C.I.; Colibaba, C.L.; Moros, A.M. The influence of the different techniques of maceration on the aromatic and phenolic profile of the Busuioaca de Bohotin wine. BIO Web of Conferences. In Proceedings of the 40th World Congress of Vine and Wine, Sofia, Bulgaria, 29 May–2 June 2017. [Google Scholar]
- Jankovic, S.M.; Milosev, M.Z.; Novakovic, M.L.J. The effects of microwave radiation on microbial culture. Hosp. Pharmacol. Int. Multidiscip. J. 2014, 1, 102–108. [Google Scholar] [CrossRef] [Green Version]
- González-Arenzana, L.; Santamaría, P.; López, R.; Garijo, P.; Gutiérrez, A.R.; Garde-Cerdán, T.; López-Alfaro, I. Microwave technology as a new tool to improve microbial control of oak barrels: A preliminary study. Food Control 2013, 30, 536–539. [Google Scholar] [CrossRef]
- Sánchez Palomo, E.; González-Viñas, M.A.; Díaz-Maroto, M.C.; Soriano Pérez, A.; Pérez-Coello, M.S. Aroma potential of Albillo wines and effect of skin-contact treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Roman, T.; Tonidandel, L.; Nicolini, G.; Bellantuono, E.; Barp, L.; Larcher, R.; Celotti, E. Evidence of the possible interaction between ultrasound and thiol precursors. Foods 2020, 9, 104. [Google Scholar] [CrossRef] [Green Version]
- Bayonove, C.; Baumes, R.; Crouzet, J.; Günata, Z. Aromas. In Enología: Fundamentos Científicos y Tecnológicos; Flanzy, C., Ed.; AMV Ediciones, Mundi Prensa: Madrid, Spain, 2000; pp. 137–176. [Google Scholar]
- Guth, H. Quantification and sensory studies of character impact odorants of different white varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ugliano, M.; Bartowsky, E.J.; Mccarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V. Managing Wine Quality; Volume 2: Oenology and Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 3–28. Volatile aroma compounds and wine sensory attributes. [Google Scholar] [CrossRef]
- Garde-Cerdan, T.; Ancin-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. Multidimensional chromatographic approach applied to the identification of novel aroma compounds in wine. Identification of ethyl cyclohexanoate, ethyl 2-hydroxy-3-methylbutyrate and ethyl 2-hydroxy-4-methylpentanoate. J. Chromatogr. A 2006, 1137, 223–230. [Google Scholar] [CrossRef]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdan, T.; Puertas, B.; Moreno-Rojas, J.M.; Gonzalo-Diago, A.; Cantos-Villar, E. Effect of hydroxytyrosol on quality of sulfur dioxide-free red wine. Food Chem. 2016, 192, 25–33. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.P.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Jurado, R.; Gómez-Plaza, E. A new approach to the reduction of alcohol content in red wines: The use of high-power ultrasounds. Foods 2020, 9, 726. [Google Scholar] [CrossRef]
- De la Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Valentin, V.; Ferreira, V. Fourteen ethyl esters of wine can be replaced by simpler ester vectors without compromising quality but at the expense of increasing aroma concentration. Food Chem. 2020, 307, 125553–125564. [Google Scholar] [CrossRef] [Green Version]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdan, T.; Puertas, B.; Moreno-Rojas, J.M.; Zafrilla, P.; Cantos-Villar, E. Replacement of sulfur dioxide by hydroxytyrosol in white wine: Influence on both quality parameters and sensory. LWT Food Sci. Technol. 2016, 65, 214–221. [Google Scholar] [CrossRef]
- Sonni, F.; Cejudo Bastante, M.J.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of sulfur dioxide by lysozyme and oenological tannins during fermentation: Influence on volatile composition of white wines. J. Sci. Food Agric. 2008, 89, 688–696. [Google Scholar] [CrossRef]
- Pons, A.; Lavigne, V.; Eric, F.; Darriet, F.; Dubourdieu, D. Identification of volatile compounds responsible for prune aroma in prematurely aged red wines. J. Agric. Food Chem. 2008, 56, 5285–5290. [Google Scholar] [CrossRef] [PubMed]
- Pozzatti, M.; Guerra, C.C.; Martins, G.; dos Santos, I.D.; Wagner, R.; Ferrão, M.F.; Manfroi, V. Effects of winemaking on ‘Marselan’ red wines: Volatile compounds and sensory aspects. Ciência Téc. Vitiv. 2020, 35, 63–75. [Google Scholar] [CrossRef]
- Gracin, L.; Jambrak, A.R.; Juretic, H.; Dobrovic, S.; Barukcic, I.; Grozdanovic, M.; Smoljanic, G. Influence of high power ultrasound on Brettanomyces and lactic acid bacteria in wine in continuous flow treatment. Appl. Acoust. 2016, 103, 143–147. [Google Scholar] [CrossRef]
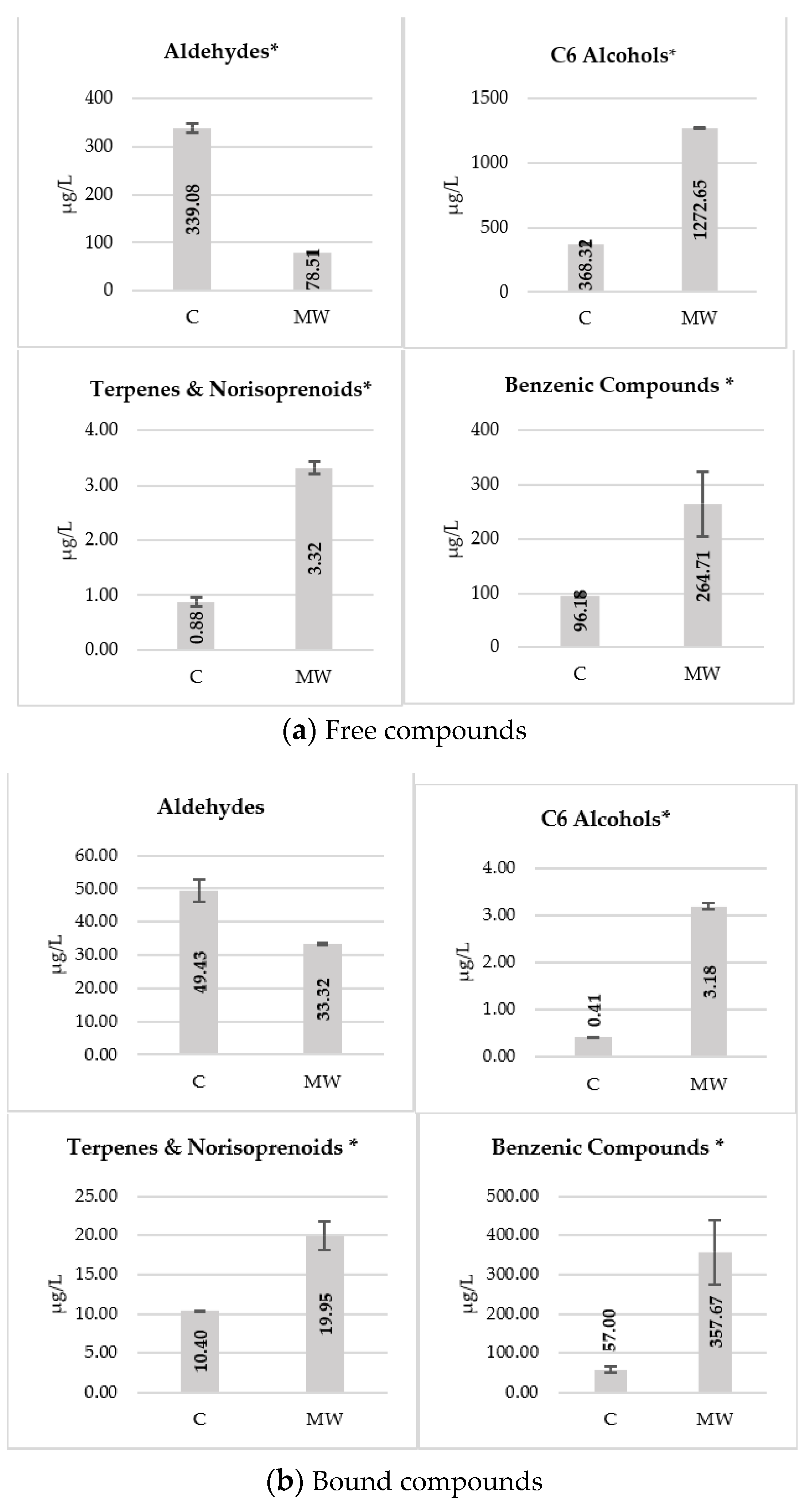

| Volatile Compounds | Control Wine | MW Wine | MW Wine without SO2 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 1-hexanol | 227.32 ± 48.70 a | 812.81 ± 33.61 c | 553.42 ± 76.76 b |
| cis-3-hexen-1-ol | 19.92 ± 2.00 a | 41.78 ± 2.35 b | 17.09 ± 1.67 a |
| trans-3-hexen-1-ol | 4.25 ± 0.58 a | 10.54 ± 0.72 b | 4.92 ± 0.49 a |
| cis-2-hexen-1-ol | 8.94 ± 1.06 c | 4.18 ± 1.06 b | 1.48 ± 0.28 a |
| trans-2-hexen-1-ol | 2.89 ± 0.69 a | 4.97 ± 0.38 b | 4.02 ± 0.88 a,b |
| Σ C6 alcohols | 263.32 ± 50.24 a | 874.28 ± 37.19 c | 580.93 ± 77.86 b |
| linalool | 5.65 ± 0.99 a | 5.61 ± 0.15 a | 12.00 ± 0.58 b |
| α-terpineol | 3.10 ± 0.37 a | 2.94 ± 0.84 a | 3.74 ± 0.26 a |
| β-damascenone | 72.97 ± 2.20 b | 25.79 ± 2.80 a | 23.12 ± 1.74 a |
| trans-geraniol | 10.51 ± 2.69 a | 16.07 ± 2.02 b | 9.77 ± 2.59 a |
| geranial | 40.27 ± 6.03 b | 132.88 ± 8.47 c | 15.88 ± 1.14 a |
| nerolidol | 20.91 ± 3.43 a | 29.97 ± 0.26 a | 42.17 ± 7.38 b |
| 6,7-dehydro-7,8-dihydro-3-oxo-ionol | 33.44 ± 4.05 b | 23.53 ± 0.22 a | 28.24 ± 2.09 a,b |
| 3-oxo-α-ionol | 102.25 ± 2.09 a | 100.39 ± 26.65 a | 60.97 ± 12.63 a |
| Σ Terpenes and norisoprenoids | 289.11 ± 2.58 b | 337.18 ± 19.73 c | 195.89 ± 8.72 a |
| benzaldehyde | 6.53 ± 1.20 a | 5.40 ± 0.21 a | 6.31 ± 1.52 a |
| guaiacol | 45.59 ± 13.28 b | 25.75 ± 3.10 a | 54.59 ± 5.01 b |
| benzyl alcohol | 184.07 ± 46.40 b | 105.67 ± 3.38 a | 214.91 ± 8.77 b |
| vinylguaicol | 72.25 ± 13.59 b | 27.08 ± 3.73 a | 64.14 ± 5.35 b |
| syringol | 170.76 ± 32.34 a | 222.23 ± 23.22 a | 187.71 ± 12.31 a |
| vanillin | 7.71 ± 1.66 a | 6.31 ± 1.15 ª | 6.27 ± 0.31 ª |
| methyl vanillate | 77.23 ± 17.87 b | 37.60 ± 2.36 ª | 44.89 ± 5.73 a |
| ethyl vanillate | 3.15 ± 0.11 a | 24.54 ± 0.81 b | 95.49 ± 4.11 c |
| methyl vanillyl ether | 76.73 ± 5.23 a | 79.13 ± 3.44 a | 84.09 ± 15.05 a |
| Σ Benzenic compounds | 644.00 ± 99.16 a,b | 533.72 ± 27.22 a | 758.40 ± 29.56 b |
| Volatile Compounds | Control Wine | MW Wine | MW Wine without SO2 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 1-hexanol | 3.73 ± 0.72 a | 5.47 ± 1.33 a | 4.43 ± 1.04 a |
| trans-3-hexen-1-ol | 0.73 ± 0.10 a | 6.04 ± 0.08 b | 0.79 ± 0.18 a |
| cis-2-hexen-1-ol | 2.10 ± 0.17 c | 0.83 ± 0.05 a | 1.60 ± 0.07 b |
| trans-2-hexen-1-ol | 0.66 ± 0.13 b | 0.39 ± 0.01 a | 0.56 ± 0.04 b |
| Σ C6 alcohols | 7.22 ± 0.82a | 12.73 ± 1.25 b | 7.38 ± 1.02 a |
| α-terpineol | 0.07 ± 0.01 b | Nd | 0.04 ± 0.01 a |
| β-damascenone | 0.58 ± 0.13 a | 0.60 ± 0.07 a | 1.65 ± 0.26 b |
| trans-geraniol | 0.83 ± 0.03 a | 1.10 ± 0.09 b | 1.41 ± 0.43 c |
| geranic acid | 4.45 ± 0.62 a | 5.96 ± 0.21 b | 3.82 ± 0.10 a |
| 3-oxo-α-ionol | 1.77 ± 0.09 a | 4.00 ± 1.13 b | 2.27 ± 0.51 a |
| 3-hydroxy-7,8-dihydro-β-ionol | 0.80 ± 0.09 a | 2.00 ± 0.35 b | 1.13 ± 0.02 a |
| Σ Terpenes and norisoprenoids | 8.50 ± 0.81 a | 13.65 ± 1.69 b | 10.33 ± 1.19 c |
| benzaldehyde | 0.70 ± 0.02 b | 0.19 ± 0.03 a | 0.63 ± 0.17 b |
| guaiacol | 9.09 ± 1.47 b | 0.80 ± 0.09 a | 16.20 ± 5.01 c |
| benzyl alcohol | 43.46 ± 2.44 c | 15.29 ± 0.73 b | 6.70 ± 0.57 a |
| 4-vinylguaicol | 15.48 ± 0.26 a | 20.46 ± 0.24 a | 28.90 ± 4.47 b |
| syringol | 116.54 ± 18.60 a | 112.95 ± 17.39 a | 165.14 ± 34.44 a |
| benzoic acid | 6.72 ± 0.16 a | 7.09 ± 1.02 a | 10.80 ± 0.89 b |
| vanillin | 1.77 ± 0.07 a | 2.12 ± 0.29 b | 1.67 ± 0.02 a |
| methyl vanillate | 6.82 ± 1.12 a | 5.53 ± 1.05 a | 10.51 ± 0.97 b |
| ethyl vanillate | nd | 14.16 ± 1.41 | nd |
| Σ Benzenic compounds | 200.57 ± 19.66 a | 178.58 ± 20.84 a | 240.56 ± 41.44 a |
| Volatile Compounds | Control Wine | MW Wine | MW Wine without SO2 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| 2-methyl-1-propanol | 38.68 ± 4.74 a | 154.91 ± 14.26 b | 245.16 ± 9.03 c |
| butanol | 13.16 ± 5.41 a,b | 16.17 ± 0.32 b | 7.47 ± 0.01 a |
| 3-methyl-1-pentanol | 4.13 ± 0.67 a | 33.20 ± 6.28 c | 15.09 ± 3.61 b |
| 4-methyl-1-pentanol | 17.58 ± 0.79 a | 102.74 ± 21.09 b | 26.80 ± 4.26 a |
| 3-octanol | 17.75 ± 3.57 b | 1.85 ± 0.48 a | 3.69 ± 0.40 a |
| 1-octen-3-ol | 2.55 ± 0.15 b | 1.66 ± 0.31 a | 4.01 ± 0.52 c |
| 1-heptanol | 2.12 ± 0.39 a | 25.25 ± 0.45 c | 11.90 ± 3.24 b |
| 1-octanol | 1.41 ± 0.18 a | 4.30 ± 0.19 b | 5.85 ± 1.33 b |
| Σ Alcohols | 97.38 ± 3.12 a | 340.07 ± 28.58 b | 319.96 ± 2.22 b |
| isobutyric acid | 53.62 ± 7.38 a | 64.50 ± 3.78 ª | 57.53 ± 5.72 a |
| butanoic acid | 5.87 ± 0.18 a | 36.47 ± 1.30 c | 13.10 ± 0.31 b |
| isovaleric acid | 435.90 ± 48.07 a | 474.04 ± 13.00 a | 438.50 ± 25.45 a |
| pentanoic acid | 2.84 ± 0.44 a | 19.65 ± 1.81 b | 3.26 ± 0.14 a |
| octanoic acid | 1020.51 ± 19.23 b | 1296.23 ± 48.52 c | 621.99 ± 12.47 a |
| dodecanoic acid | 57.93 ± 6.19 ª | 66.54 ± 11.11 ª | 78.01 ± 5.93 a |
| Σ Acids | 1576.66 ± 74.02 b | 1957.43 ± 56.69 c | 1212.39 ± 20.09 a |
| ethyl butyrate | 12.62 ± 1.09 a | 61.87 ± 1.02 c | 40.84 ± 0.77 b |
| ethyl isovalerate | 4.14 ± 0.43 a | 10.32 ± 2.44 b | 15.00 ± 0.50 c |
| isoamyl acetate | 1437.24 ± 53.61 a | 2066.95 ± 41.66 b | 946.51 ± 12.57 a |
| ethyl hexanoate | 217.70 ± 38.39 a | 327.35 ± 37.71 b | 236.58 ± 15.79 a |
| ethyl piruvate | 3.42 ± 0.20 a | 7.76 ± 0.01 b | 8.11 ± 1.12 b |
| hexyl acetate | 1.88 ± 0.52 a | 18.15 ± 3.29 b | 5.25 ± 1.06 ª |
| ethyl octanoate | 262.41 ± 26.43 b | 285.79 ± 15.27 b | 84.76 ± 20.38 a |
| ethyl decanoate | 22.68 ± 4.74 a | 114.82 ± 9.64 b | 33.76 ± 5.83 a |
| ethyl succinate | 473.01 ± 5.05 b | 443.08 ± 15.26 b | 273.41 ± 73.23 a |
| ethyl 2-(OH)-4-methylpentanoate | 41.38 ± 1.29 a | 32.22 ± 0.98 a | 36.59 ± 9.42 a |
| ethyl 3-(OH)-hexanoate | 7.74 ± 0.31 b | 7.45 ± 1.21 b | 1.40 ± 0.38 a |
| ethyl 4-(OH)-butanoate | 6594.31 ± 155.03 c | 3116.845 ± 234.87 b | 1991.30 ± 412.43 a |
| Σ Esters | 9078.54 ± 217.17 c | 6492.62 ± 222.50 b | 3673.50 ± 482.15 a |
| 2-phenylethyl acetate | 76.36 ± 7.55 a | 425.68 ± 15.83 c | 175.51 ± 47.70 b |
| 2-phenylethanol | 24303.25 ± 555.69 a | 35620.5 ± 2701.47 b | 43608.55 ± 1702.30 e |
| 4-vinyl-phenol | 311.78 ± 24.28 a | 417.51 ± 15.50 b | 431.89 ± 39.91 b |
| tyrosol | 3485.19 ± 839.37 c | 2411.07 ± 7.49 b | 1284.24 ± 374.96 a |
| benzeneacetic acid | 10.19 ± 2.34 a | 24.20 ± 0.81 b | 39.38 ± 5.46 c |
| (2-phenylethyl) acetamide | 56.51 ± 7.36 a | 49.32 ± 5.69 a | 99.23 ± 15.67 b |
| Σ Benzenic compounds | 28243.26 ± 1370.95 a | 38948.27 ± 2684.29 b | 45638.81 ± 1402.11 c |
| 3-(methylthio)-1-propanol | 105.56 ± 13.62 c | 38.72 ± 4.69 a | 78.50 ± 10.71 b |
| 3-(2H)-thiophenone, dihydro-2-methyl | 204.19 ± 15.73 c | 136.46 ± 2.75 b | 50.87 ± 7.30 a |
| γ-butyrolactone | 646.20 ± 50.68 b | 49.33 ± 14.33 a | 27.55 ± 4.40 a |
| γ-nonalactone | 10.28 ± 1.35 a | 15.29 ± 2.23 ª | 15.83 ± 3.99 ª |
| pantolactone | 13.26 ± 1.63 b | 6.87 ± 2.36 a | 14.09 ± 2.40 b |
| γ-decalactone | 0.43 ± 0.03 a | 2.05 ± 0.07 c | 0.84 ± 0.13 b |
| Σ Furans & sulfur compounds | 979.92 ± 60.10 b | 248.72 ± 13.59 a | 187.69 ± 9.18 a |
| Volatile Compound | Odor Threshold μg/L * | Odor Descriptor | OAV ** | ||
|---|---|---|---|---|---|
| Control Wine | MW Wine | MW Wine without SO2 | |||
| linalool | 15 | Citrus, floral, sweet | 0.4 | 0.4 | 0.8 |
| β-damascenone | 0.05 | Honey, sweet, | 1459.4 | 515.8 | 462.4 |
| guaiacol | 10 | Smoke, sweet, medicine | 4.6 | 2.6 | 5.5 |
| vinylguaicol | 40 | Spices, curry | 1.8 | 0.7 | 1.6 |
| ethyl butyrate | 20 | Fruity, strawberry, sweet, | 0.6 | 3.1 | 2.0 |
| ethyl isovalerate | 3 | Apple, sweet | 1.4 | 3.4 | 5.0 |
| isoamyl acetate | 30 | Banana, fruity, sweet | 47.9 | 68.9 | 31.5 |
| ethyl hexanoate | 5 | Fruity, green, apple. | 43.5 | 65.5 | 47.3 |
| ethyl octanoate | 5 | Sweet, fruity, pear | 52.5 | 57.2 | 16.9 |
| 2-phenylethyl acetate | 250 | Flowery | 0.3 | 1.7 | 0.7 |
| 2-phenylethanol | 10,000 | Rose, honey | 2.4 | 3.5 | 4.3 |
| γ-nonalactone | 25 | soft coconut, sweet | 0.4 | 0.6 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz García, R.; Oliver Simancas, R.; Díaz-Maroto, M.C.; Alañón Pardo, M.E.; Pérez-Coello, M.S. Effect of Microwave Maceration and SO2 Free Vinification on Volatile Composition of Red Wines. Foods 2021, 10, 1164. https://doi.org/10.3390/foods10061164
Muñoz García R, Oliver Simancas R, Díaz-Maroto MC, Alañón Pardo ME, Pérez-Coello MS. Effect of Microwave Maceration and SO2 Free Vinification on Volatile Composition of Red Wines. Foods. 2021; 10(6):1164. https://doi.org/10.3390/foods10061164
Chicago/Turabian StyleMuñoz García, Raquel, Rodrigo Oliver Simancas, María Consuelo Díaz-Maroto, María Elena Alañón Pardo, and María Soledad Pérez-Coello. 2021. "Effect of Microwave Maceration and SO2 Free Vinification on Volatile Composition of Red Wines" Foods 10, no. 6: 1164. https://doi.org/10.3390/foods10061164
APA StyleMuñoz García, R., Oliver Simancas, R., Díaz-Maroto, M. C., Alañón Pardo, M. E., & Pérez-Coello, M. S. (2021). Effect of Microwave Maceration and SO2 Free Vinification on Volatile Composition of Red Wines. Foods, 10(6), 1164. https://doi.org/10.3390/foods10061164







