Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards
2.2. Biological Material and Mushroom Cultivation Substrates
2.3. Extraction of Aroma Volatile Compounds
2.4. Gas Chromatography-Mass Spectrometry Analysis
2.5. Identification and Semi-Quantification of Volatile Compounds
2.6. Cooking of Mushroom Samples
2.7. Total Lipids Content
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Volatiles Contents
3.2. Aldehydes
3.3. Alcohols
3.4. Ketones
3.5. Fatty Acid Methyl Esters (FAME)
3.6. Alkanes
3.7. Terpenes
3.8. Other Compounds—Toluene
3.9. Eight-Carbon Compounds
3.10. Effect of Cooking on Volatiles’ Profiles
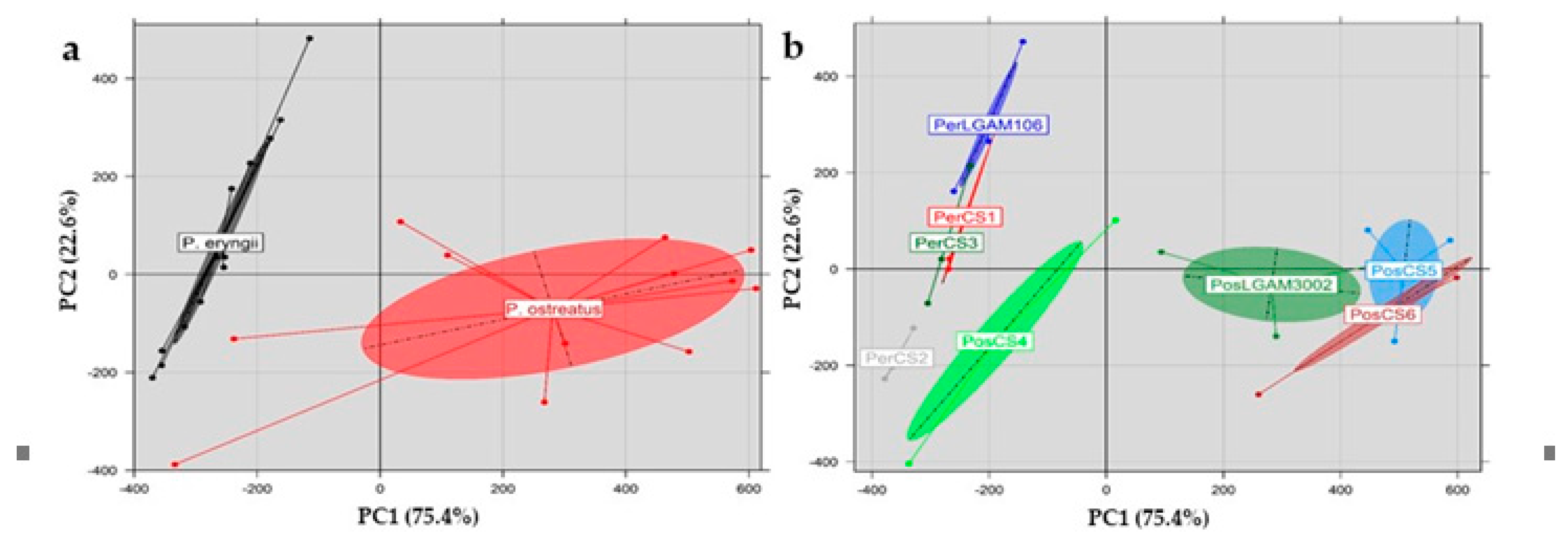
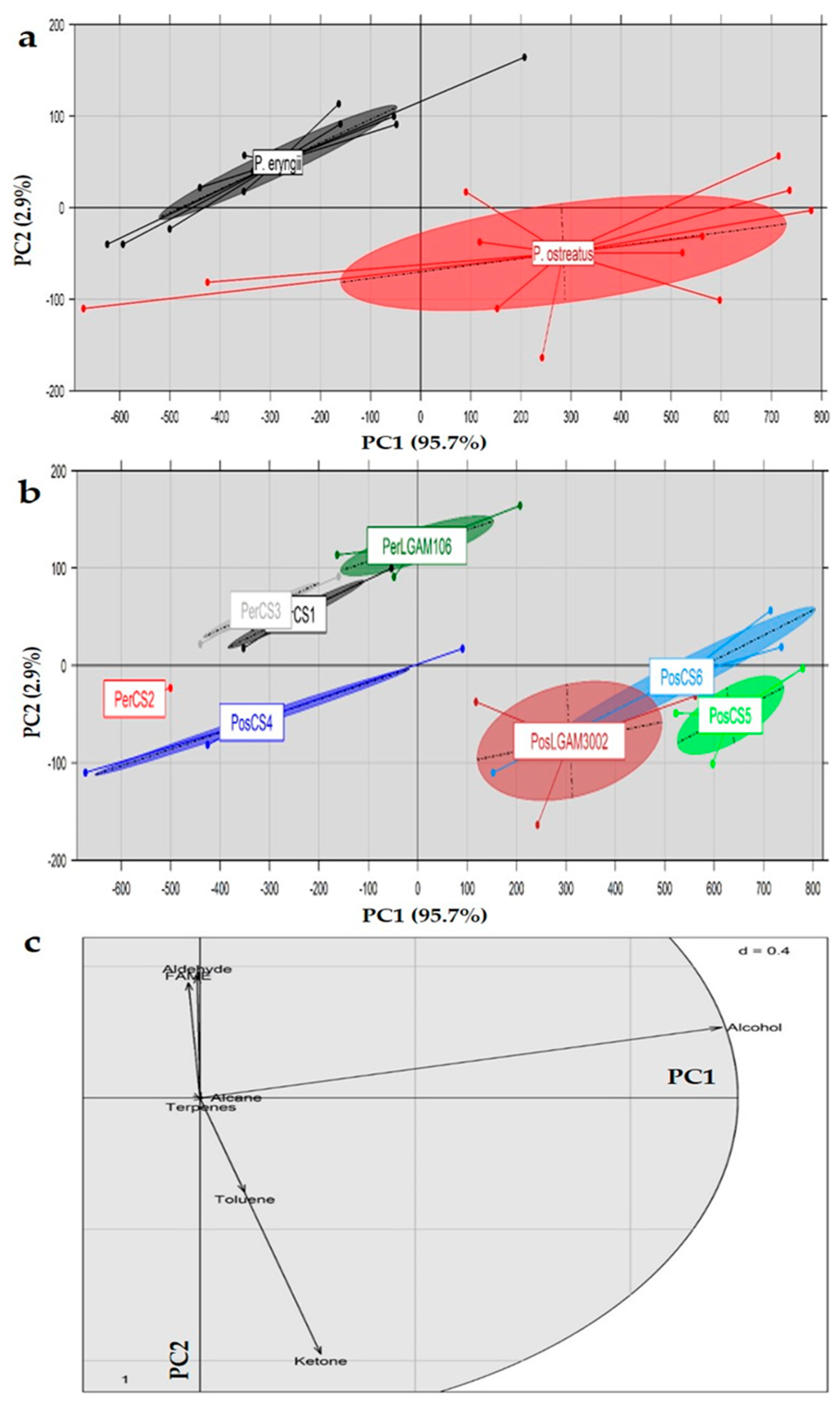
3.11. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant. Biosyst An Int. J. Deal. With All Asp. Plant. Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, D.E.; Cheng, H.Y.; Chung, I.-M.; Kim, S.-H.; Han, J.-G.; Kong, W.-S. Characterization of volatile profiles of six popular edible mushrooms using headspace-solid-phase microextraction coupled with gas chromatography combined with chemometric analysis. J. Food Sci. 2019, 84, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. In Edible and Medicinal Mushrooms; John Wiley & Sons, Ltd: Chichester, UK, 2017; Volume 2010, pp. 5–13. [Google Scholar]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an increased functionality in oyster (Pleurotus) mushrooms produced on grape marc or olive mill wastes serving as sources of bioactive compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Tsiantas, K.; Tsiaka, T.; Koutrotsios, G.; Siapi, E.; Zervakis, G.I.; Kalogeropoulos, N.; Zoumpoulakis, P. On the identification and quantification of ergothioneine and lovastatin in various mushroom species: Assets and challenges of different analytical approaches. Molecules 2021, 26, 1832. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G. Solid-state fermentation of plant residues and agro-industrial wastes for the production of medicinal mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer: Singapore, 2017; pp. 365–396. [Google Scholar]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus raw olive mill waste as substrates for the production of medicinal mushrooms: An assessment of selected cultivation and quality parameters. Biomed. Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ditengou, F.A.; Müller, A.; Rosenkranz, M.; Felten, J.; Lasok, H.; van Doorn, M.M.; Legué, V.; Palme, K.; Schnitzler, J.-P.; Polle, A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 2015, 6, 6279. [Google Scholar] [CrossRef]
- Hynes, J.; Müller, C.T.; Jones, T.H.; Boddy, L. Changes in volatile production during the course of fungal mycelial interactions between Hypholoma fasciculare and Resinicium bicolor. J. Chem. Ecol. 2006, 33, 43–57. [Google Scholar] [CrossRef]
- Aisala, H.; Laaksonen, O.; Manninen, H.; Raittola, A.; Hopia, A.; Sandell, M. Sensory properties of Nordic edible mushrooms. Food Res. Int. 2018, 109, 526–536. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xin, G.; Sun, B.; Bao, X.; Wei, Y.; Zhao, X.; Xu, H. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Changes in the key aroma compounds of raw shiitake mushrooms (Lentinula edodes) induced by pan-frying as well as by rehydration of dry mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef]
- Provost, J.J.; Colabroy, K.L.; Kelly, B.S.; Wallert, M.A. The Science of Cooking: Understanding the Biology and Chemistry behind Food and Cooking; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef]
- Maga, J.A. Mushroom flavor. J. Agric. Food Chem. 1981, 29, 1–4. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. Aroma-volatile profile of black morel (Morchella importuna) grown in Israel. J. Sci. Food Agric. 2018, 98, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Namgung, H.-J.; Choi, H.-K.; Kim, Y.-S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Feng, T.; Yang, M.; Ma, B.; Zhao, Y.; Zhuang, H.; Zhang, J.; Chen, D. Volatile profiles of two genotype Agaricus bisporus species at different growth stages. Food Res. Int. 2021, 140, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Guedes de Pinho, P.; Soares, S.; César da Silva Ferreira, A.; Baptista, P. Volatile biomarkers for wild mushrooms species discrimination. Food Res. Int. 2013, 54, 186–194. [Google Scholar] [CrossRef]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Costa, R.; Tedone, L.; De Grazia, S.; Dugo, P.; Mondello, L. Multiple headspace-solid-phase microextraction: An application to quantification of mushroom volatiles. Anal. Chim. Acta 2013, 770, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chemie Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Headspace solid-phase microextraction. Anal. Chem. 1993, 65, 1843–1852. [Google Scholar] [CrossRef]
- Chen, G.; Wu, F.; Pei, F.; Cheng, S.; Muinde, B.; Hu, Q.; Zhao, L. Volatile components of white Hypsizygus marmoreus detected by electronic nose and HS-SPME-GC-MS: Influence of four drying methods. Int. J. Food Prop. 2017, 20, 2901–2910. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, D.E.; Baek, S.H.; Lim, S.M.; Chung, I.-M.; Han, J.-G.; Kim, S.-H. An unattended HS-SPME-GC–MS/MS combined with a novel sample preparation strategy for the reliable quantitation of C8 volatiles in mushrooms: A sample preparation strategy to fully control the volatile emission. Food Chem. 2021, 347, 128998. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC–MS and electronic nose. LWT Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Bar, E.; Lewinsohn, D.; Shavit, E.; Roth-Bejerano, N.; Kagan-Zur, V.; Barak, Z.; Guy, O.; Zaady, E.; Lewinsohn, E.; et al. Characterization of morphology, volatile profiles, and molecular markers in edible desert truffles from the Negev desert. J. Agric. Food Chem. 2017, 65, 2977–2983. [Google Scholar] [CrossRef]
- Aisala, H.; Linderborg, K.M.; Sandell, M. Fiber depth, column coating and extraction time are major contributors in the headspace solid-phase microextraction–gas chromatography analysis of Nordic wild mushrooms. Eur. Food Res. Technol. 2018, 244, 841–850. [Google Scholar] [CrossRef]
- Guedes de Pinho, P.; Ribeiro, B.; Gonçalves, R.F.; Baptista, P.; Valentão, P.; Seabra, R.M.; Andrade, P.B. Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms. J. Agric. Food Chem. 2008, 56, 1704–1712. [Google Scholar] [CrossRef]
- San Román, I.; Alonso, M.L.; Bartolomé, L.; Alonso, R.M.; Fañanás, R. Analytical strategies based on multiple headspace extraction for the quantitative analysis of aroma components in mushrooms. Talanta 2014, 123, 207–217. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscop, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 9–51. [Google Scholar]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 15 February 2021).
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Siberchicot, A.; Julien-Laferrière, A.; Dufour, A.-B.; Thioulouse, J.; Dray, S. Adegraphics: An S4 lattice-based package for the representation of multivariate data. R. J. 2017, 9, 198. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z.; Shi, D.; Yao, F.; Gao, H. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushrooms. J. Sci. Food Agric. 2019, 99, 1691–1699. [Google Scholar] [CrossRef]
- Mau, J.L.; Lin, Y.P.; Chen, P.T.; Wu, Y.H.; Peng, J.T. Flavor compounds in king oyster mushrooms Pleurotus eryngii. J. Agric. Food Chem. 1998, 46, 4587–4591. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant. J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Formation of phenylacetic acid and benzaldehyde by degradation of phenylalanine in the presence of lipid hydroperoxides: New routes in the amino acid degradation pathways initiated by lipid oxidation products. Food Chem. X 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Tagkouli, D.; Kaliora, A.; Bekiaris, G.; Koutrotsios, G.; Christea, M.; Zervakis, G.I.; Kalogeropoulos, N. Free amino acids in three Pleurotus species cultivated on agricultural and agro-industrial by-products. Molecules 2020, 25, 4015. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, X.; Li, M.; Chen, J.; Wang, X. Monitoring oxidative stability and changes in key volatile compounds in edible oils during ambient storage through HS-SPME/GC–MS. Int. J. Food Prop. 2017, 20, S2926–S2938. [Google Scholar] [CrossRef]
- Kalač, P. Chemical composition and nutritional value of European species of wild growing mushrooms: A review. Food Chem. 2009, 113, 9–16. [Google Scholar] [CrossRef]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic Acid 10-Hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Biosci. Biotechnol. Biochem. 2003, 67, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Cadwallader, K.R. Aromas. In Encyclopedia of Food Chemistry, 1st ed.; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Cambridge, MA, USA, 2019; Volume 1, pp. 22–29. [Google Scholar]
- Rajarathnam, S.; Shashirekha, M.N. Mushrooms and truffles|Use of wild mushrooms. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Finglas, P.M., Caballero, B., Trugo, L.C., Eds.; Elsevier: Cambridge, MA, USA, 2003; Volume 26, pp. 4048–4054. [Google Scholar]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC–MS. J. Chromatogr. B 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Usami, A.; Nakaya, S.; Nakahashi, H.; Miyazawa, M. Chemical composition and aroma evaluation of volatile oils from edible mushrooms (Pleurotus salmoneostramineus and Pleurotus sajor-caju). J. Oleo Sci. 2014, 63, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Usami, A.; Motooka, R.; Nakahashi, H.; Marumoto, S.; Miyazawa, M. Chemical composition and character impact odorants in volatile oils from edible mushrooms. Chem. Biodivers. 2015, 12, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, M.; Racioppi, R.; Rana, G.L.; Laurita, A. Studies on volatile organic compounds of some truffles and false truffles. Nat. Prod. Res. 2014, 28, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Politowicz, J.; Lech, K.; Lipan, L.; Figiel, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agric. 2018, 98, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.H.; Kim, S.Y.; Choi, H.-K.; Kim, Y.-S. Characterization of aroma-active compounds in raw and cooked pine-mushrooms (Tricholoma matsutake Sing). J. Agric. Food Chem. 2006, 54, 6332–6335. [Google Scholar] [CrossRef]
- Johnathan, M.; Gan, S.H.; Ezumi, M.F.W.; Faezahtul, A.H.; Nurul, A.A. Phytochemical profiles and inhibitory effects of tiger milk mushroom (Lignosus rhinocerus) extract on ovalbumin-induced airway inflammation in a rodent model of asthma. BMC Complement. Altern. Med. 2016, 16, 167. [Google Scholar] [CrossRef]
- Šiškovič, N.; Strojnik, L.; Grebenc, T.; Vidrih, R.; Ogrinc, N. Differentiation between species and regional origin of fresh and freeze-dried truffles according to their volatile profiles. Food Control. 2021, 123, 1–10. [Google Scholar] [CrossRef]
- Min, D.B.S.; Ina, K.; Peterson, R.J.; Chang, S.S. The Alkylbenzenes in roast beef. J. Food Sci. 1977, 42, 503–505. [Google Scholar] [CrossRef]
- Van Ba, H.; Mi, P.K.; Cho, I.; Amna, T.; Hwang, I. Diversity in volatile components in beef tissue cooked at different temperature. In Proceedings of the 56th International Congress of Meat Science and Technology (56th ICoMST), Jeju, Korea, 15–20 August 2010. [Google Scholar]
- Giogios, I.; Kalogeropoulos, N.; Grigorakis, K. Volatile compounds of some popular Mediterranean seafood species. Mediterr. Mar. Sci. 2013, 14, 343. [Google Scholar] [CrossRef]
- Zhou, A.; McFeeters, R.F. Volatile Compounds in cucumbers fermented in low-salt conditions. J. Agric. Food Chem. 1998, 46, 2117–2122. [Google Scholar] [CrossRef]
- Wang, D.; Javed, H.U.; Shi, Y.; Naz, S.; Ali, S.; Duan, C.-Q. Impact of drying method on the evaluation of fatty acids and their derived volatile compounds in ‘thompson seedless’ raisins. Molecules 2020, 25, 608. [Google Scholar] [CrossRef]
- Eric, K.; Raymond, L.V.; Abbas, S.; Song, S.; Zhang, Y.; Masamba, K.; Zhang, X. Temperature and cysteine addition effect on formation of sunflower hydrolysate Maillard reaction products and corresponding influence on sensory characteristics assessed by partial least square regression. Food Res. Int. 2014, 57, 242–258. [Google Scholar] [CrossRef]
- Schönberg, A.; Moubacher, R. The Strecker degradation of α-amino acids. Chem. Rev. 1952, 50, 261–277. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Huber, K. Carbohydrates. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2008; Volume 39, pp. 83–154. [Google Scholar]
- Van Lancker, F.; Adams, A.N.; De Kimpe, N. Formation of pyrazines in maillard model systems of lysine-containing dipeptides. J. Agric. Food Chem. 2010, 58, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.C. Flavors. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K., Fenneman, O., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2008; Volume 39, pp. 732–785. [Google Scholar]
- Chen, X.; Yu, J.; Cui, H.; Xia, S.; Zhang, X.; Yang, B. Effect of temperature on flavor compounds and sensory characteristics of Maillard reaction products derived from mushroom hydrolysate. Molecules 2018, 23, 247. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, X.; Karangwa, E. Comparation sensory characteristic, non-volatile compounds, volatile compounds and antioxidant activity of MRPs by novel gradient temperature-elevating and traditional isothermal methods. J. Food Sci. Technol. 2015, 52, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.-W.; Yu, A.-N. Volatiles from the Maillard reaction of L-ascorbic acid with L-glutamic acid/L-aspartic acid at different reaction times and temperatures. Asia-Pac. J. Chem. Eng. 2012, 7, 563–571. [Google Scholar] [CrossRef]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Adamiec, J.; Rössner, J.; Velíšek, J.; Cejpek, K.; Šavel, J. Minor Strecker degradation products of phenylalanine and phenylglycine. Eur. Food Res. Technol. 2001, 212, 135–140. [Google Scholar] [CrossRef]
- Hwang, H.-I.; Hartman, T.G.; Rosen, R.T.; Lech, J.; Ho, C.-T. Formation of pyrazines from the Maillard reaction of glucose and lysine-alpha-amine-15N. J. Agric. Food Chem. 1994, 42, 1000–1004. [Google Scholar] [CrossRef]
- Shu, C.-K. Pyrazine formation from serine and threonine. J. Agric. Food Chem. 1999, 47, 4332–4335. [Google Scholar] [CrossRef]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Czerny, M.; Bielohradsky, J.; Grosch, W. Structure-odor-activity relationships of alkylpyrazines. Z. Lebensm. Unters. Forsch. 1999, 208, 308–316. [Google Scholar] [CrossRef]
- Misharina, T.A.; Mukhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I.; Medvedeva, I.B. The composition of volatile components of dry cepe and oyster mushroom. Appl. Biochem. Microbiol. 2009, 45, 544–549. [Google Scholar] [CrossRef]
- Misharina, T.A.; Muhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I.; Medvedeva, I.B. Formation of flavor of dry champignons (Agaricus bisporus L.). Appl. Biochem. Microbiol. 2010, 46, 108–113. [Google Scholar] [CrossRef]
- Miyazawa, M.; Matsuda, N.; Tamura, N.; Ishikawa, R. Characteristic fFlavor of volatile oil from dried fruiting bodies of Hericium erinaceus (Bull.: Fr.) Pers. J. Essent. Oil Res. 2008, 20, 420–423. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Misharina, T.A.; Muhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I. The composition of volatile components of cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus). Appl. Biochem. Microbiol. 2009, 45, 187–193. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.-H.; Claver, I.P.; Zhu, K.-X.; Peng, W.; Zhou, H.-M. Effect of different cooking methods on the flavor constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]

| No | Class of Compound | Compound Name | Rt (min) | RI | Odor Description |
|---|---|---|---|---|---|
| 1 | Aldehyde | 2-methylbutanal * | 3.24 | 908 | Musty, chocolate, nutty, furfural and isovaleraldehyde-like with malty and fermented nuances |
| 2 | Aldehyde | 3-methylbutanal * | 3.29 | 913 | Ethereal, aldehydic, chocolate peach, fatty |
| 3 | Alkane | 1,3-octadiene | 3.75 | 966 | |
| 4 | Aldehyde | pentanal | 3.89 | 977 | Diffusive, fermented, bready, fruity with berry nuances |
| 5 | Aromatic compound | toluene | 4.58 | 1048 | Sweet |
| 6 | sulfur compound | dimethyl disulfide * | 4.82 | 1071 | Sulfurous, vegetable, cabbage, onion |
| 7 | Aldehyde | hexanal | 4.98 | 1087 | Green, fatty, leafy, vegetative, fruity, with a woody nuance |
| 8 | Alkane | undecane | 5.11 | 1100 | Herbal, woody |
| 9 | Aromatic compound | ethylbenzene | 5.40 | 1129 | |
| 10 | Ketone | 2-heptanone | 5.89 | 1178 | Cheese, fruity, ketonic, green banana, with a creamy nuance |
| 11 | Aldehyde | n heptanal | 6.00 | 1189 | Green |
| 12 | Heterocyclic compound | 2,4,5-trimethyloxazole * | 6.07 | 1196 | Nutty, roasted, wasabi, shellfish, mustard, vegetable |
| 13 | Alkane | dodecane | 6.11 | 1200 | |
| 14 | Terpene | limonene | 6.16 | 1205 | Terpene, pine, herbal, peppery |
| 15 | Aldehyde | trans-2-hexenal | 6.37 | 1224 | Sweet, almond, fruity, green, leafy, apple, plum, vegetable |
| 16 | Heterocyclic compound | 2-pentyl-furan | 6.47 | 1233 | Fruity, green, earthy beany with vegetable like nuances |
| 17 | Ketone | 3-octanone | 6.75 | 1259 | Musty, mushroom, ketonic, moldy and cheesy fermented with a green, vegetative nuance |
| 18 | Pyrazine | methylpyrazine * | 6.83 | 1267 | Nutty, cocoa, roasted, green |
| 19 | Ketone | 2-octanone | 7.09 | 1291 | Musty, ketonic, bleu and parmesan cheese-like with earthy and dairy nuances |
| 20 | Aldehyde | octanal | 7.14 | 1295 | Aldehydic, waxy, citrus orange with a green peely nuance |
| 21 | Alkane | tridecane | 7.19 | 1300 | |
| 22 | Ketone | 1-octen-3-one | 7.29 | 1308 | Earthy, metallic, mushroom-like with vegetative nuances of cabbage and broccoli |
| 23 | Internal standard | 4-methyl-1-pentanol | 7.40 | 1317 | |
| 24 | Ketone | 2,3-octanedione | 7.50 | 1324 | Dill, asparagus, cilantro, herbal, aldehydic, earthy, fatty, cortex |
| 25 | Pyrazine | 2,5-dimethylpyrazine * | 7.50 | 1324 | Nutty, peanut, musty, earthy, powdery and slightly roasted with a cocoa powder nuance |
| 26 | Aldehyde | 2-heptenal | 7.60 | 1332 | Green, fatty |
| 27 | Pyrazine | 2,6-dimethylpyrazine * | 7.69 | 1339 | Cocoa, roasted nuts, roast beef, coffee |
| 28 | Ketone | 6-methyl-5-hepten-2-one | 7.72 | 1341 | Fruity, apple, musty, ketonic and creamy with slight cheesy and banana nuances |
| 29 | Pyrazine | ethylpyrazine * | 7.75 | 1344 | Nutty, musty, fermented, coffee, roasted, cocoa and meaty nuances |
| 30 | Pyrazine | 2,3-dimethylpyrazine * | 7.85 | 1352 | Musty, nut skins, cocoa powdery and roasted with potato and coffee nuances |
| 31 | Alcohol | 1-hexanol | 7.89 | 1355 | Pungent, ethereal, fusel oil, fruity and alcoholic, sweet with a green top note |
| 32 | Alcohol | 3-octanol | 8.42 | 1396 | Earthy, mushroom, dairy, musty, creamy, waxy with a slight fermented green minty nuance |
| 33 | Alkane | tetradecane | 8.47 | 1400 | Mild waxy |
| 34 | Aldehyde | nonanal | 8.48 | 1401 | Waxy, aldehydic, citrus, with a fresh slightly green lemon peel like nuance, and a cucumber fattiness |
| 35 | Pyrazine | 2-ethyl-methylpyrazine * | 8.59 | 1408 | Coffee bean, nutty, grassy, roasted |
| 36 | Ketone | 3-octen-2-one | 8.71 | 1416 | Earthy, oily, ketonic, sweet, with hay and mushroom nuances |
| 37 | Alkane | 3-ethyl-2-methyl-1,3-hexadiene | 8.91 | 1429 | |
| 38 | Aldehyde | 2-octenal | 9.04 | 1437 | Fatty, green, herbal |
| 39 | Pyrazine | 2,6-diethylpyrazine * | 9.12 | 1443 | Nutty, hazelnut |
| 40 | Alcohol | 1-octen-3-ol | 9.27 | 1453 | Earthy, green, oily, vegetative and fungal |
| 41 | Pyrazine | 3-ethyl-2,5-dimethylpyrazine * | 9.30 | 1455 | Potato, cocoa, roasted, nutty |
| 42 | Sulfur compound | methional * | 9.35 | 1458 | Creamy tomato, potato skin and French fry, yeasty, bready, limburger cheese with a savory meaty brothy nuance |
| 43 | Pyrazine | 2-ethyl-3,5-dimethylpyrazine * | 9.52 | 1469 | Peanut, nutty, caramel, coffee, musty, cocoa, pyrazine and roasted |
| 44 | Pyrazine | tetramethylpyrazine * | 9.53 | 1470 | Nutty, musty and vanilla with dry, brown cocoa nuances |
| 45 | Pyrazine | 2-methyl-5-propylpyrazine * | 9.71 | 1482 | |
| 46 | Alcohol | 2-ethyl-1-hexanol | 9.89 | 1494 | Citrus, fresh, floral, oily, sweet |
| 47 | Alkane | pentadecane | 9.99 | 1500 | Waxy |
| 48 | Pyrazine | 3,5-diethyl-2-methylpyrazine * | 10.07 | 1505 | Nutty, meaty, vegetable |
| 49 | Pyrazine | 2,3,5-Trimethyl-6-ethylpyrazine * | 10.26 | 1516 | |
| 50 | Aldehyde | benzaldehyde | 10.56 | 1533 | Almond, fruity, powdery, nutty and benzaldehyde-like |
| 51 | Pyrazine | 2,5-dimethyl-3-isobutylpyrazine * | 10.60 | 1535 | |
| 52 | Pyrazine | 2-acetyl-5-methylfuran * | 10.92 | 1554 | Sweet, musty, nutty with a caramellic nuance |
| 53 | Alcohol | n-octanol | 11.06 | 1562 | Waxy, green, citrus, aldehydic and floral with a sweet, fatty, coconut nuance |
| 54 | Alkane | hexadecane | 11.72 | 1600 | |
| 55 | Ketone | 2-undecanone | 11.81 | 1605 | Waxy, fruity, ketonic with fatty pineapple nuances |
| 56 | Alcohol | 2-octen-1-ol | 12.11 | 1621 | Green, vegetable |
| 57 | Pyrazine | 2-isoamyl-6-methylpyrazine * | 12.30 | 1631 | |
| 58 | Aldehyde | phenylacetaldehyde | 12.59 | 1646 | Honey, floral rose, sweet, fermented, chocolate with a slight earthy nuance |
| 59 | Pyrazine | pyrazine,2-butyl-3,5-dimethyl * | 12.78 | 1657 | Sweet, earthy |
| 60 | Ketone | acetophenone | 12.82 | 1658 | Sweet, cherry pit, marzipan and coumarinic, vanilla nuance |
| 61 | Terpene | pristane | 12.98 | 1667 | |
| 62 | Pyrazine | 2,5-dimethyl-3-cis-propenylpyrazine * | 13.16 | 1677 | |
| 63 | Alkane | heptadecane | 13.60 | 1700 | |
| 64 | Aldehyde | 2,4-nonadienal | 13.74 | 1707 | Fatty, melon, waxy, green, leaf, cucumber, tropical fruit |
| 65 | Aldehyde | undecenal | 14.68 | 1756 | Fresh, fruity, orange peel |
| 66 | Aldehyde | 3-dodecen-1-al | 14.68 | 1756 | Bitter orange, mandarin, coriander |
| 67 | Aldehyde | 2,4-decadienal | 14.92 | 1768 | Acrid, greasy |
| 68 | Terpene | phytane | 15.03 | 1774 | |
| 69 | Alkane | octadecane | 15.53 | 1800 | |
| 70 | FAME | methyl laurate | 15.65 | 1806 | Waxy, soapy, creamy, coconut, with mushroom nuances |
| 71 | Aldehyde | 2,4 decadienal | 15.81 | 1814 | Fatty, citrus, nutty |
| 72 | Alkane | n-nonadecane | 17.49 | 1900 | Bland |
| 73 | Alcohol | phenylethyl alcohol | 17.79 | 1916 | Sweet, floral, fresh and bready with a rosey honey nuance |
| 74 | Aldehyde | 2-phenyl-2-butenal | 18.23 | 1939 | Musty, floral, honey, powdery and cocoa |
| 75 | Heterocyclic compound | 2-acetylpyrrole * | 18.71 | 1964 | Musty, nutty-like with a coumarin nuance |
| 76 | Alcohol | 1-dodecanol | 18.89 | 1973 | Earthy, soapy, waxy, fatty, honey, coconut |
| 77 | Alkane | eicosane | 19.41 | 2000 | Waxy |
| 78 | FAME | methyl myristate | 19.64 | 2012 | Fatty, waxy, petal |
| 79 | Aldehyde | 5-methyl-2-phenyl-2-hexenal | 20.64 | 2065 | |
| 80 | Alkane | heneicosane | 21.30 | 2100 | |
| 81 | FAME | methyl pentadecanoate | 21.58 | 2116 | |
| 82 | Alkane | docosane | 23.10 | 2200 | |
| 83 | FAME | methyl palmitate | 23.46 | 2221 | Oily, waxy, fatty, orris |
| 84 | Alkane | tetracosane | 26.86 | 2400 | |
| 85 | FAME | methyl stearate | 27.02 | 2413 | Oily, waxy |
| 86 | FAME | methyl oleate | 27.36 | 2440 | Mild, fatty |
| 87 | FAME | methyl linoleate | 28.15 | 2503 | Oily, fatty, woody |
| (a) | ||||||||||||
| P. eryngii | ||||||||||||
| CS1 | CS2 | CS3 | LGAM106 | |||||||||
| WS | GM | OL | WS | GM | OL | WS | GM | OL | WS | GM | OL | |
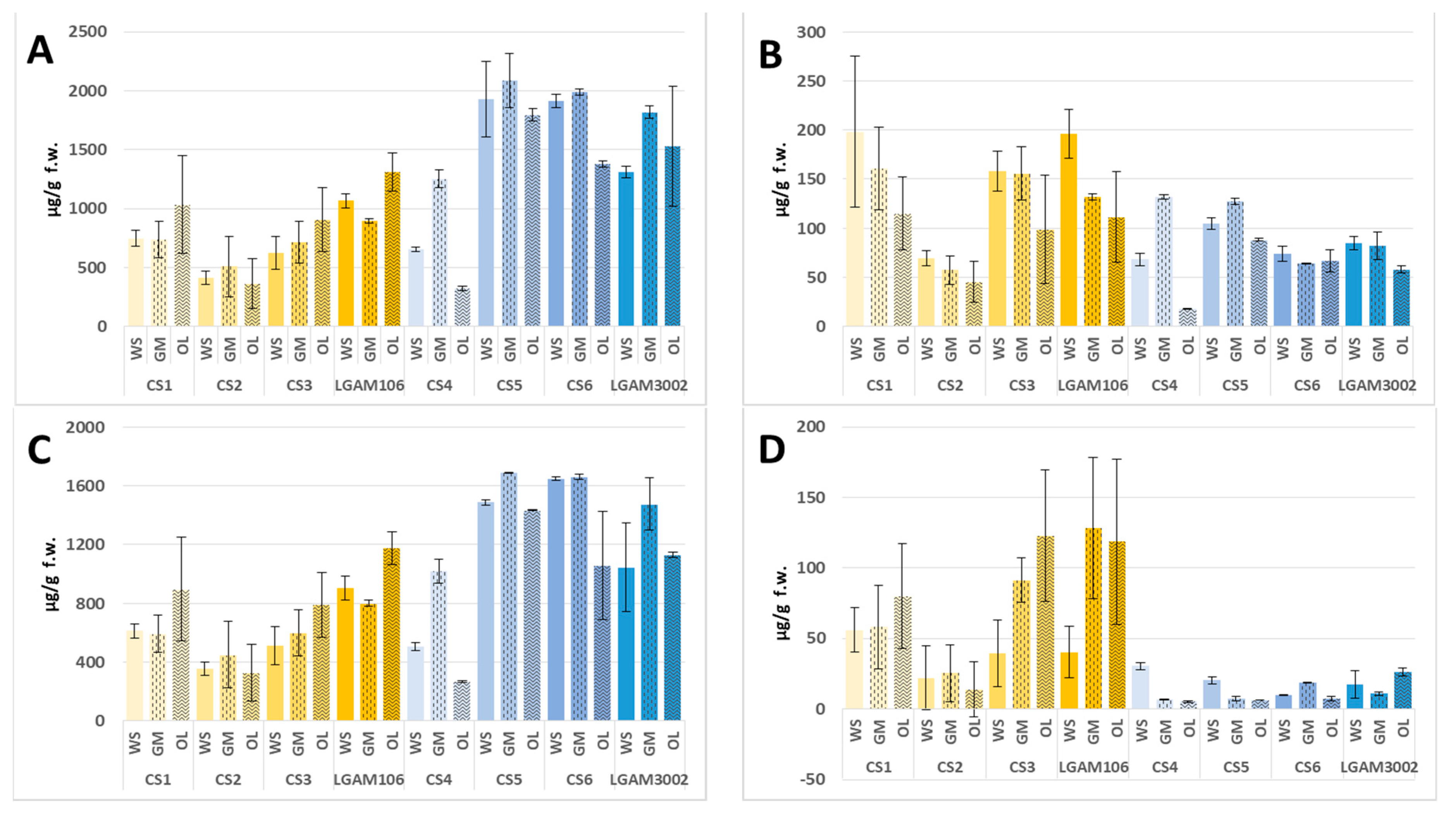
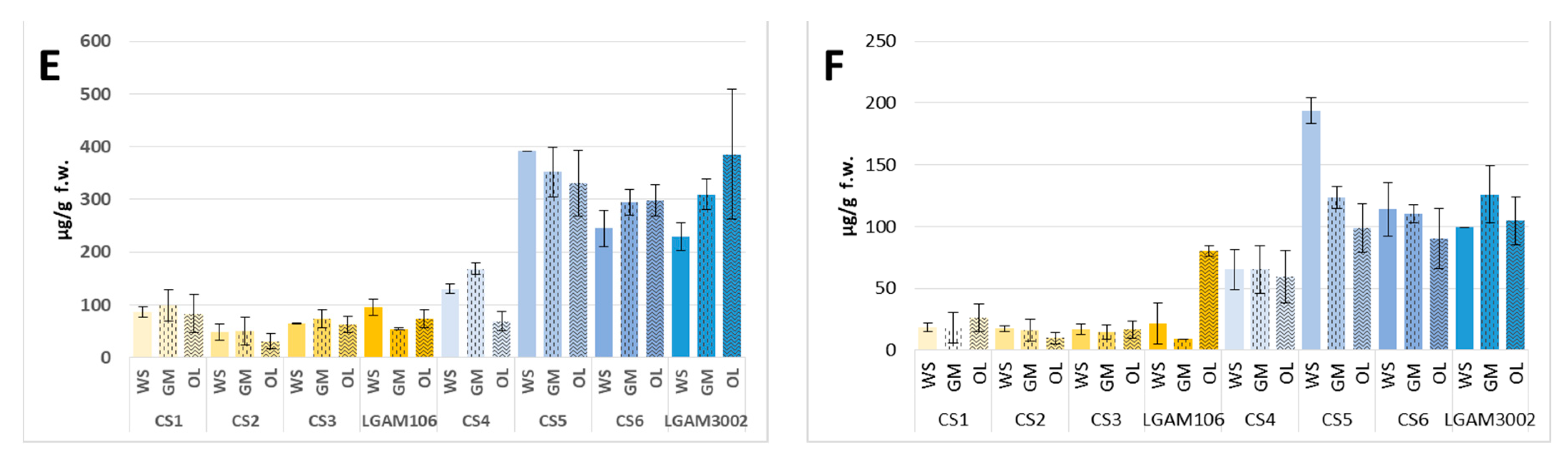
| Total * | 980.6 ± 134.3 a | 946.12 ± 171.9 a | 1220 ± 463 a | 527.8 ± 72.6 a | 611.6 ± 278.3 a | 446.3 ± 237.1 a | 805.0 ± 95.8 a | 967.9 ± 169.2 a | 1123 ± 274 a | 1268 ± 104 a | 1177 ± 83 a | 1512 ± 173 a |
| Alkanes | 8.98 ± 0.41 a | 17.79 ± 7.75 a | 17.97 ± 3.12 a | 16.69 ± 8.44 a | 13.25 ± 5.19 a | 20.99 ± 5.65 a | 16.82 ± 6.04 a | 29.83 ± 18.63 a | 26.09 ± 22.91 a | 9.84 ± 7.36 a | 51.34 ± 3.98b | 19.61 ± 6.78 a |
| Aldehydes | 198.1 ± 77.0 a | 160.6 ± 42.0 a | 115.0 ± 36.9 a | 69.21 ± 7.87 a | 57.40 ± 14.45 a | 45.57 ± 20.95 a | 158.3 ± 20.3 a | 155.6 ± 27.0 a | 98.86 ± 55.55 a | 196.2 ± 24.9 a | 131.8 ± 3.1 a | 111.3 ± 46.2 a |
| Alcohols | 611.9 ± 47.1 a | 590.9 ± 127.3 a | 896.4 ± 354.1 a | 353.1 ± 43.1 a | 449.2 ± 225.3 a | 325.7 ± 192.5 a | 508.8 ± 129.8 a | 598.9 ± 156.1 a | 790.0 ± 220.5 a | 904.6 ± 82.4 a | 798.4 ± 21.2 a | 1175 ± 114b |
| FAME | 56.07 ± 15.55 a | 58.02 ± 29.42 a | 79.88 ± 37.08 a | 22.09 ± 22.89 a | 25.48 ± 20.26 a | 13.80 ± 19.52 a | 39.49 ± 23.78 a | 91.32 ± 15.86 ab | 122.7 ± 46.5c | 40.25 ± 18.34 a | 128.3 ± 49.9 a | 118.5 ± 58.7 a |
| Ketones | 86.12 ± 9.38 a | 99.08 ± 30.40 a | 83.23 ± 35.87 a | 48.60 ± 15.47 a | 49.93 ± 26.42 a | 30.47 ± 14.40 a | 64.83 ± 1.44 a | 74.15 ± 17.03 a | 63.00 ± 14.96 a | 95.20 ± 14.68 b | 54.47 ± 1.91 a | 74.06 ± 17.55 ab |
| Furans | 0.56 ± 0.00 | 0.53 ± 0.11 | n.d. | 0.30 ± 0.08 | n.d. | n.d. | 0.17 ± 0.02 | 0.33 ± 0.05 | n.d. | 0.49 ± 0.21 | n.d. | n.d. |
| Toluene | 18.25 ± 3.35 a | 18.04 ± 12.40 a | 25.88 ± 11.05 a | 17.29 ± 2.03 a | 15.84 ± 9.03 a | 9.78 ± 4.61 a | 15.45 ± 4.20 a | 13.86 ± 5.93 a | 15.71 ± 6.96 a | 21.37 ± 16.34 a | 8.87 ± 0.00 a | 20.02 ± 4.12 a |
| Terpenes | n.d. | 0.45 ± 0.21 | 0.34 ± 0.13 | n.d. | n.d. | n.d. | n.d. | 13.72 ± 0.27 | 26.64 ± 6.53 | n.d. | 3.99 ± 2.39 | n.d. |
| C8 compounds | 747.1 ± 68.3 a | 734.9 ± 157.0 a | 1033 ± 415 a | 414.7 ± 55.9 a | 509.2 ± 256.2 a | 363.5 ± 208.9 a | 623.3 ± 142.3 a | 715.1 ± 175.5 a | 906.6 ± 268.5 a | 1065 ± 59 ab | 894.9 ± 16.8 a | 1311 ± 164 b |
| (b) | ||||||||||||
| P. ostreatus | ||||||||||||
| CS4 | CS5 | CS6 | LGAM3002 | |||||||||
| WS | GM | OL | WS | GM | OL | WS | GM | OL | WS | GM | OL | |
| Total * | 814.0 ± 45.8 b | 1403 ± 52 c | 424.3 ± 45.0 a | 2232 ± 70 b | 2327 ± 23 b | 1986 ± 42 a | 2118 ± 75 a | 2159 ± 40 a | 1545 ± 547 a | 1436 ± 362 a | 2035 ± 217 a | 1728 ± 70 a |
| Alkanes | 15.21 ± 0.45 a | 11.80 ± 0.73 b | 9.91 ± 1.27 b | 31.01 ± 0.36 a | 26.29 ± 1.77 a | 27.83 ± 11.67 a | 27.66 ± 8.66 a | 11.91 ± 1.47 a | 23.74 ± 18.80 a | 10.75 ± 0.89 a | 27.59 ± 1.44 b | 24.00 ± 3.45 b |
| Aldehydes | 68.20 ± 6.27 b | 131.9 ± 2.1 a | 17.54 ± 0.33 c | 104.7 ± 5.8 b | 127.5 ± 3.2 c | 88.06 ± 1.26 a | 73.80 ± 7.47 a | 63.89 ± 0.73 a | 66.88 ± 11.21 a | 84.56 ± 6.57 a | 82.16 ± 14.05 a | 57.87 ± 3.45 a |
| Alcohols | 504.3 ± 29.8 b | 1018 ± 84 a | 263.9 ± 6.7 c | 1485 ± 20 b | 1689 ± 3 c | 1431 ± 3 a | 1647 ± 11 a | 1659 ± 19 a | 1058 ± 369 a | 1043 ± 303 a | 1475 ± 177 a | 1129 ± 21 a |
| FAME | 30.47 ± 2.70 a | 6.92 ± 0.41 b | 5.19 ± 0.68 b | 20.50 ± 2.50 a | 7.41 ± 1.54 b | 6.27 ± 0.08 b | 9.91 ± 0.35 a | 18.62 ± 0.44 b | 7.35 ± 1.74 a | 17.44 ± 9.62 a | 10.85 ± 1.29 a | 26.05 ± 2.79 a |
| Ketones | 130.4 ± 9.7 a | 168.5 ± 10.0 a | 68.19 ± 18.20 b | 391.6 ± 34.2 a | 351.8 ± 24.9 a | 331.1 ± 30.1 a | 244.7 ± 26.2 a | 294.0 ± 29.1 a | 298.1 ± 122.7 a | 228.9 ± 0.2 a | 309.1 ± 47.3 ab | 385.9 ± 62 c |
| Furans | n.d. | 0.94 ± 0.44 | n.d. | 0.84 ± 0.64 | n.d. | 0.62 ± 0.15 | n.d. | n.d. | 0.64 ± 0.45 | 0.47 ± 0.49 | 0.56 ± 0.14 | n.d. |
| Toluene | 65.30 ± 16.30 a | 65.30 ± 19.37 a | 59.51 ± 21.04 a | 194.0 ± 10.4 b | 123.5 ± 9.2 a | 98.86 ± 19.51 a | 114.1 ± 21.6 a | 110.4 ± 7.3 a | 90.37 ± 24.33 a | 99.15 ± 0.13 a | 126.1 ± 23.3 a | 104.6 ± 19.3 a |
| Terpenes | n.d. | n.d. | n.d. | 3.75 ± 1.77 a | 2.22 ± 0.70 a | 1.79 ± 0.39 a | 0.09 ± 0.04 | n.d. | 0.19 ± 0.00 | 2.33 ± 0.02 ab | 4.01 ± 1.41 b | 0.44 ± 0.12 a |
| C8 compounds | 654.6 ± 21.1 b | 1253 ± 74 c | 323.0 ± 20.9 a | 1926 ± 54 b | 2087 ± 3 c | 1796 ± 27 a | 1912 ± 48 a | 1988 ± 51 a | 1376 ± 507 a | 1309 ± 322 a | 1818 ± 229 a | 1529 ± 50 a |
| Classes of Compounds | Rt | P. eryngii | P. ostreatus | |||
|---|---|---|---|---|---|---|
| Raw | Roasted | Raw | Roasted | |||
| Water content (%) | 90.57 ± 1.91 | 46.12 ± 2.8 | 90.95 ± 2.1 | 44.9 ± 3.1 | ||
| Total lipids (mg/g f.w.) | 7.29 ± 0.4 | -- | 3.73 ± 0.2 | -- | ||
| Total volatiles (μg/g f.w.) | 924.2 ± 233.3 | 398.1 ± 82.2 | 1165 ± 129 | 364.7 ± 22.0 | ||
| Alkanes | undecane | 5.11 | 25.88 ± 3.79 | 17.07 ± 5.76 | 16.98 ± 7.10 | 12.39 ± 5.02 |
| dodecane | 6.11 | 1.63 ± 0.12 | n.d. | 3.66 ± 3.65 | n.d. | |
| pentadecane | 9.99 | 8.47 ± 0.46 | n.d. | 5.74 ± 4.90 | n.d. | |
| hexadecane | 11.72 | 8.03 ± 1.06 | n.d. | 4.72 ± 3.14 | n.d. | |
| heptadecane | 13.60 | 6.26 ± 0.78 | n.d. | 3.83 ± 2.37 | n.d. | |
| octadecane | 15.53 | 5.61 ± 0.27 | n.d. | 3.15 ± 1.82 | n.d. | |
| nonadecane | 17.49 | 5.42 ± 0.94 | n.d. | 1.92 ± 0.95 | n.d. | |
| eicosane | 19.41 | 4.27 ± 0.39 | n.d. | 1.28 ± 0.36 | n.d. | |
| Alcohols | 1-hexanol | 7.89 | 2.37 ± 0.24 | n.d. | 6.09 ± 5.51 | n.d. |
| 3-octanol | 8.42 | 9.39 ± 7.27 | 6.32 ± 3.04 | 330.6 ± 75.9 | 65.42 ± 26.73 | |
| 1-octen-3-ol | 9.27 | 491.6 ± 208.5 | 178.3 ± 29.1 * | 341.4 ± 131.5 | 90.19 ± 16.59 | |
| 2-ethyl-1-hexanol | 9.89 | 1.68 ± 0.34 | 0.99 ± 0.44 | 3.06 ± 1.44 | n.d. | |
| 1-octanol | 11.06 | 23.26 ± 14.56 | 2.98 ± 1.22 | 9.47 ± 6.45 | 0.59 ± 0.03 | |
| phenylethyl alcohol | 17.79 | 1.83 ± 0.38 | n.d. | n.d. | n.d. | |
| Aldehydes | 2-methylbutanal | 3.17 | n.d. | 7.70 ± 9.16 | n.d. | n.d. |
| 3-methylbutanal | 3.29 | n.d. | 14.94 ± 2.35 | n.d. | 7.10 ± 1.55 | |
| pentanal | 3.89 | n.d. | n.d. | n.d. | 15.73 ± 6.19 | |
| hexanal | 4.98 | 78.91 ± 25.55 | 42.08 ± 27.17 | 17.26 ± 7.81 | 5.50 ± 2.03 | |
| trans-2-hexenal | 6.37 | 1.59 ± 0.39 | n.d. | 0.39 ± 0.19 | n.d. | |
| octanal | 7.14 | 8.04 ± 2.42 | n.d. | 4.89 ± 2.53 | 1.68 ± 1.10 | |
| 2-heptenal | 7.60 | 28.93 ± 15.73 | n.d. | 10.32 ± 1.61 | n.d. | |
| 2-octenal | 9.04 | 42.12 ± 16.00 | 15.47 ± 17.66 | 14.89 ± 4.34 | 1.17 ± 0.14 | |
| benzaldehyde | 10.56 | 15.40 ± 6.16 | 8.71 ± 4.57 | 3.60 ± 3.21 | 4.21 ± 1.62 | |
| phenylacetaldehyde | 12.59 | n.d. | 5.61 ± 2.88 | n.d. | 12.91 ± 6.05 | |
| 2,4-nonadienal | 13.74 | 11.13 ± 7.91 | n.d. | 1.53 ± 0.68 | n.d. | |
| 2,4-decadienal (E,E) | 15.81 | 2.92 ± 1.43 | n.d. | 1.73 ± 0.26 | n.d. | |
| 2-phenyl-2-butenal | 17.92 | n.d. | n.d. | n.d. | 1.87 ± 1.15 | |
| 5-methyl-2-phenyl-2-hexenal | 20.64 | n.d. | n.d. | n.d. | 1.39 ± 0.79 | |
| FAME | methyl laurate | 15.65 | n.d. | n.d. | 1.96 ± 0.11 | n.d. |
| methyl myristate | 19.64 | 3.21 ± 1.86 | n.d. | 2.50 ± 1.10 | n.d. | |
| methyl pentadecanoate | 21.58 | 4.67 ± 3.45 | n.d. | 7.83 ± 5.64 | n.d. | |
| methyl palmitate | 23.46 | 34.14 ± 33.47 | 2.06 ± 0.48 | 13.29 ± 5.59 | 0.77 ± 0.27 | |
| methyl stearate | 27.02 | 1.49 ± 0.16 | n.d. | n.d. | n.d. | |
| methyl oleate | 27.36 | 21.11 ± 20.21 | 1.32 ± 0.18 | 2.31 ± 1.09 | n.d. | |
| methyl linoleate | 28.15 | 18.20 ± 15.98 | 1.52 ± 0.22 | 10.67 ± 4.05 | 0.64 ± 0.25 | |
| Ketones | 2-heptanone | 5.95 | n.d. | n.d. | n.d. | 15.28 ± 13.38 |
| 3-octanone | 6.75 | 36.80 ± 5.36 | 9.53 ± 5.71 | 310.7 ± 125.3 | 25.08 ± 12.30 | |
| 2-octanone | 7.09 | 4.68 ± 2.52 | 6.60 ± 2.21 | n.d. | 7.31 ± 9.03 | |
| 2,3-octanedione | 7.50 | n.d. | n.d. | 1.21 ± 0.37 | n.d. | |
| 3-octen-2-one | 8.71 | 7.04 ± 6.87 | n.d. | n.d. | n.d. | |
| Aromatic compounds | toluene | 4.58 | 14.22 ± 5.26 | 60.73 ± 11.50 | 90.62 ± 12.05 | 27.20 ± 8.27 |
| Pyrazines | methylpyrazine | 6.83 | n.d. | n.d. | n.d. | 0.63 ± 0.18 |
| 2,5-dimethylpyrazine | 7.60 | n.d. | 4.33 ± 0.86 | n.d. | 5.44 ± 3.39 | |
| 2,6-dimethylpyrazine | 7.69 | n.d. | 1.92 ± 1.14 | n.d. | 3.09 ± 1.94 | |
| ethylpyrazine | 7.75 | n.d. | n.d. | n.d. | 0.79 ± 0.51 | |
| 2,3-dimethylpyrazine | 7.85 | n.d. | 0.89 ± 0.41 | n.d. | 0.78 ± 0.56 | |
| 2-ethyl-methylpyrazine | 8.59 | n.d. | 3.69 ± 0.43 | n.d. | 4.45 ± 2.98 | |
| 2,6-diethylpyrazine | 9.12 | n.d. | n.d. | n.d. | 1.76 ± 0.88 | |
| 3-ethyl-2,5-dimethylpyrazine | 9.30 | n.d. | 8.76 ± 0.31 | n.d. | 21.22 ± 5.11 | |
| 2-ethyl-3,5-dimethylpyrazine | 9.52 | n.d. | 2.85 ± 0.10 | n.d. | 6.00 ± 2.36 | |
| tetramethylpyrazine | 9.53 | n.d. | n.d. | n.d. | 0.56 ± 0.06 | |
| 2-methyl-5-propylpyrazine | 9.71 | n.d. | n.d. | n.d. | 0.67 ± 0.25 | |
| 3,5-diethyl-2-methyl pyrazine | 10.07 | n.d. | n.d. | n.d. | 5.94 ± 1.85 | |
| 2,3,5-trimethyl-6-ethylpyrazine | 10.40 | n.d. | n.d. | n.d. | 2.40 ± 0.47 | |
| 2,5-dimethyl-3-isobutylpyrazine | 10.60 | n.d. | n.d. | n.d. | 1.81 ± 0.51 | |
| 2-isoamyl-6-methylpyrazine | 12.30 | n.d. | n.d. | n.d. | 2.00 ± 0.49 | |
| 2-butyl-3,5-dimethyl pyrazine | 12.94 | n.d. | n.d. | n.d. | 4.49 ± 1.23 | |
| 2,5-dimethyl-3-propenylpyrazine | 13.27 | n.d. | n.d. | n.d. | 1.15 ± 0.65 | |
| Sulfur compounds | dimethyl disulfide | 4.82 | n.d. | n.d. | n.d. | 2.67 ± 2.19 |
| methional | 9.35 | n.d. | 1.96 ± 0.36 | n.d. | 2.59 ± 1.62 | |
| Terpenes | limonene | 6.16 | 5.21 ± 0.56 | 1.07 ± 0.41 | 0.30 ± 0.01 | n.d. |
| pristane | 12.98 | 0.18 ± 0.02 | n.d. | 4.32 ± 3.47 | n.d. | |
| phytane | 15.03 | n.d. | n.d. | 3.94 ± 1.49 | n.d. | |
| Other heterocyclic compounds | 2,4,5-trimethyloxazole | 6.07 | n.d. | n.d. | n.d. | 1.72 ± 1.48 |
| 2-pentyl-furan | 6.47 | 4.43 ± 2.31 | 1.50± 0.32 | 1.05 ± 0.48 | 0.51 ± 0.36 | |
| 2-acetyl-5-methylfuran | 10.92 | n.d. | 3.00 ± 0.45 | n.d. | n.d. | |
| 2-acetylpyrrole | 18.71 | n.d. | n.d. | n.d. | 1.66 ± 0.56 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagkouli, D.; Bekiaris, G.; Pantazi, S.; Anastasopoulou, M.E.; Koutrotsios, G.; Mallouchos, A.; Zervakis, G.I.; Kalogeropoulos, N. Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products. Foods 2021, 10, 1287. https://doi.org/10.3390/foods10061287
Tagkouli D, Bekiaris G, Pantazi S, Anastasopoulou ME, Koutrotsios G, Mallouchos A, Zervakis GI, Kalogeropoulos N. Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products. Foods. 2021; 10(6):1287. https://doi.org/10.3390/foods10061287
Chicago/Turabian StyleTagkouli, Dimitra, Georgios Bekiaris, Stella Pantazi, Maria Eleni Anastasopoulou, Georgios Koutrotsios, Athanasios Mallouchos, Georgios I. Zervakis, and Nick Kalogeropoulos. 2021. "Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products" Foods 10, no. 6: 1287. https://doi.org/10.3390/foods10061287
APA StyleTagkouli, D., Bekiaris, G., Pantazi, S., Anastasopoulou, M. E., Koutrotsios, G., Mallouchos, A., Zervakis, G. I., & Kalogeropoulos, N. (2021). Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products. Foods, 10(6), 1287. https://doi.org/10.3390/foods10061287