Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch
Abstract
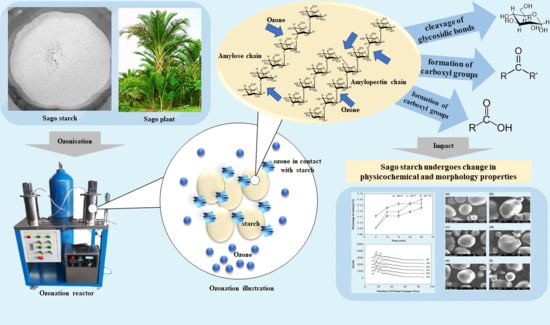
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sago Starch Modification
2.3. Evaluation of Modified Sago Starch
2.3.1. Swelling Power Test
2.3.2. Solubility of Sago Starch in Water
2.3.3. Carboxyl Test
2.3.4. Carbonyl Test
2.3.5. Morphology Analysis
2.3.6. Functional Group Analysis
2.3.7. Thermal Profile
2.4. Statistical Analysis
3. Results
3.1. Effect of pH, Time of Reaction, and Starch Concentration on Carbonyl Group Content
3.2. Effect of pH, Time of Reaction, and Starch Concentration on Carboxyl Group Content
3.3. Effect of pH, Time of Reaction, and Starch Concentration on Solubility of Starch
3.4. Effect of pH, Time of Reaction, and Starch Concentration on the Swelling Power of Starch
3.5. Effect of pH and Starch Concentration on Starch Granule
3.6. Effect of pH and Starch Concentration on the Functional Groups of Starch
3.7. Effect of pH and Concentration of Starch in Water on the Crystallinity of Starch
3.8. Effect of pH and Starch Concentration on the Thermal Properties of the Starch
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniel, J.R.; Vidovic, N. Carbohydrates, role in human nutrition. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Hobbs, L. Chapter 21 Sweeteners from starch: Production, properties and uses. In Starch, 3rd ed.; Bemiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 797–832. [Google Scholar] [CrossRef]
- Huang, F.; Liu, H.; Zhang, R.; Dong, L.; Liu, L.; Jia, X.; Zhang, M.; Ma, Y.; Wang, G. Physicochemical properties and prebiotic activities of polysaccharides from longan pulp based on different extraction techniques. Carbohydr. Polym. 2018, 206, 344–351. [Google Scholar] [CrossRef]
- Schwartz, D.; Whistler, R.L. Chapter 1 history and future of starch. In Starch, 3rd ed.; Bemiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 1–10. [Google Scholar] [CrossRef]
- Soto, D.; León, O.; Urdaneta, J.; Muñoz-Bonilla, A.; Fernández-García, M. Modified starch as a filter controller in water-based drilling fluids. Materials 2020, 13, 2794. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.-q.; Bao, Y.; Gu, W.; Lu, F.; Wu, Z. Preparation and application of high performance corn starch glue in straw decorative panel. Wood Fiber Sci. 2018, 50, 88–95. [Google Scholar] [CrossRef]
- Sumardiono, S.; Pudjihastuti, I.; Budiyono; Hartanto, H.; Sophiana, I.C. Combination process method of lactic acid hydrolysis and hydrogen peroxide oxidation for cassava starch modification. AIP Conf. Proc. 2017, 1840, 060006. [Google Scholar] [CrossRef]
- Karim, A.A.; Tie, A.P.L.; Manan, D.M.A.; Zaidul, I.S.M. Starch from the sago (Metroxylon sagu) palm tree properties, prospects, and challenges as a new industrial source for food and other uses. Compr. Rev. Food Sci. Food Saf. 2008, 7, 215–228. [Google Scholar] [CrossRef]
- Sumaryono; Muslihatin, W.; Ratnadewi, D. Effect of carbohydrate source on growth and performance of in vitro sago palm (Metroxylon sagu rottb.) plantlets. HAYATI J. Biosci. 2012, 19, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Sumardiono, S.; Rakhmawati, R.B. Physicochemical properties of sago starch under various modification process: An overview. Adv. Sci. Lett. 2017, 23, 5789–5791. [Google Scholar] [CrossRef]
- Zhu, F. Recent advances in modifications and applications of sago starch. Food Hydrocoll. 2019, 96, 412–423. [Google Scholar] [CrossRef]
- Sumardiono, S.; Rakhmawati, R.B.; Pudjihastuti, I. Physicochemical and rheological properties of sago (metroxylon sagu) starch modified with lactic acid hydrolysis and UV rotary drying. ASEAN J. Chem. Eng. 2018, 18, 41–53. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D. Synthesis and partial characterization of octenylsuccinic starch from Phaseolus lunatus. Food Hydrocoll. 2008, 22, 1467–1474. [Google Scholar] [CrossRef]
- Achudan, S.N.; Dos Mohamed, A.M.; Rashid, R.S.A.; Mittis, P. Yield and physicochemical properties of starch at different sago palm stages. Mater. Today Proc. 2020, 31, 122–126. [Google Scholar] [CrossRef]
- Hui, R.; Qi-he, C.; Ming-liang, F.; Qiong, X.; Guo-qing, H. Preparation and properties of octenyl succinic anhydride modified potato starch. Food Chem. 2009, 114, 81–86. [Google Scholar] [CrossRef]
- Abbas, K.A.; Khalil, S.K.; Meor Hussin, A.S. Modified starches and their usages in selected food products: A review study. J. Agric. Sci. 2010, 2, 90. [Google Scholar] [CrossRef] [Green Version]
- Fouladi, E.; Mohammadi Nafchi, A. Effects of acid-hydrolysis and hydroxypropylation on functional properties of sago starch. Int. J. Biol. Macromol. 2014, 68, 251–257. [Google Scholar] [CrossRef]
- Sumardiono, S.; Kusumawardani, N.I.; Abdurahman, A.U.; Arland, A.; Jos, B.; Pudjihastuti, I. The modification of sago (Metroxylon Sagu) starch by combination of lactic acid hydrolysis and H2O2 oxidation methods to increase baking expansion. AIP Conf. Proc. 2020, 2197, 070007. [Google Scholar] [CrossRef]
- Castanha, N.; da Matta Junior, M.D.; Augusto, P.E.D. Potato starch modification using the ozone technology. Food Hydrocoll. 2017, 66, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; da Rosa Zavarezeb, E. Oxidation of potato starch with different sodium hypochlorite concentrations and its effect on biodegradable films. LWT Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Ouellette, R.J.; Rawn, J.D. Organic Chemistry Study Guide: Key Concepts, Problems, and Solutions Features Hundreds of Problems from the Companion Book, Organic Chemistry, and Includes Solutions for Every Problem; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Castanha, N.; Santos, D.N.E.; Cunha, R.L.; Augusto, P.E.D. Properties and possible applications of ozone-modified potato starch. Food Res. Int. 2019, 116, 1192–1201. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Matta Junior, M.D.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Hydrogels based on ozonated Cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Sumardiono, S.; Pudjihastuti, I.; Jos, B.; Taufani, M.; Yahya, F. Modification of cassava starch using combination process lactic acid hydrolysis and microwave heating to increase coated peanut expansion quality. AIP Conf. Proc. 2017, 1840, 060005. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- a review. Chem. Eng. J. Adv. 2020, 3. [Google Scholar] [CrossRef]
- Wei, C.H.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33. [Google Scholar] [CrossRef]
- Obadi, M.; Zhu, K.X.; Peng, W.; Sulieman, A.A.; Mohammed, K.; Zhou, H.M. Effects of ozone treatment on the physicochemical and functional properties of whole grain flour. J. Cereal Sci. 2018, 81, 127–132. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Physicochemical and functional properties of ozone-oxidized starch. J. Agric. Food Chem. 2009, 57, 5965–5970. [Google Scholar] [CrossRef] [PubMed]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized Cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; Da Rosa Zavareze, E.; Dias, A.R.G. Ozone oxidation of Cassava starch in aqueous solution at different pH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Kesselmans, R.P.W.; Bleeker, I.P. Oxidizing Starch with Ozone in Absence of Catalyst by Passing Ozone through Aqueous Suspension or Solution of Starch at Constant pH, Giving High Yield in Short Reaction Time. WO Patent 9,735,890, 2 October 1997. [Google Scholar]
- Uthumporn, U.; Zaidul, I.S.M.; Karim, A.A. Hydrolysis of granular starch at sub-gelatinization temperature using a mixture of amylolytic enzymes. Food Bioprod. Process. 2010, 88, 47–54. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef]
- Pudjihastuti, I.; Handayani, N.; Sumardiono, S. Effect of pH on physicochemical properties of cassava starch modification using ozone. MATEC Conf. Proc. 2018, 156, 01027. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Williams, P.A.; Doublier, J.L.; Durand, S.; Buleon, A. Physico-chemical characterisation of sago starch. Carbohydr. Polym. 1999, 38, 361–370. [Google Scholar] [CrossRef]
- Widodo, R.T.; Hassan, A. Compression and mechanical properties of directly compressible pregelatinized sago starches. Powder Technol. 2015, 269, 15–21. [Google Scholar] [CrossRef]
- Pérez, S.; Baldwin, P.M.; Gallant, D.J. Structural features of starch granules I. Starch 2009, 149–192. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J. Discrimination of irradiated starch gels using FT-Raman spectroscopy and chemometrics. J. Agric. Food Chem. 2006, 54, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, M.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Almaraz-Abarca, N.; Castañeda-Pérez, E. Application of MIR-FTIR spectroscopy and chemometrics to the rapid prediction of fish fillet quality. CyTA J. Food 2014, 12, 369–377. [Google Scholar] [CrossRef]
- Dachriyanus, D. Analisis Struktur Senyawa Organik Secara Spektroskopi; Andalas University Press: Padang, Indonesia, 2004. [Google Scholar]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular structure, functionality and applications of oxidized starches: A review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Elena, J.; Lucia, M.D. X-ray diffraction study of hydration processes in the Portland cement. J. Appl. Eng. Sci. 2011, 1, 79–86. [Google Scholar]
- Khondker, A.; Lakhani, S. X-ray diffraction: A comprehensive explanation for multipurpose research. Int. J. Interdiscip. Res. Innov. 2015, 3, 60–64. [Google Scholar]
- Toraya, H. A new method for quantitative phase analysis using X-ray powder diffraction: Direct derivation of weight fractions from observed integrated intensities and chemical compositions of individual phases. J. Appl. Crystallogr. 2016, 49, 1508–1516. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Oladebeye, A.O.; Oshodi, A.A.; Amoo, I.A.; Karim, A.A. Functional, thermal and molecular behaviours of ozone-oxidised cocoyam and yam starches. Food Chem. 2013, 141, 1416–1423. [Google Scholar] [CrossRef]
- Wurzburg, O.B. Modified starches. In Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 87–118. [Google Scholar] [CrossRef]
- Fitria, E.; Hariyadi, P.; Andarwulan, N.; Triana, R.N. The physicochemical properties of modified sago starch by oxidation method using sodium hypochlorite. Indones. J. Food Qual. 2018, 5, 100–108. [Google Scholar]
- Vrieling, A.; Wielema, T.A.; Beugeling, J. Oxidation of Starch. Patent No. 3.205.763A, 3 September 2018. [Google Scholar]
- Zhou, F.; Liu, Q.; Zhang, H.; Chen, Q.; Kong, B. Potato starch oxidation induced by sodium hypochlorite and its effect on functional properties and digestibility. Int. J. Biol. Macromol. 2016, 84, 410–417. [Google Scholar] [CrossRef]
- Matsuguma, L.S.; Lacerda, L.G.; Schnitzler, E.; Carvalho Filho, M.A.S.; Franco, C.M.L.; Demiate, I.M. Characterization of native and oxidized starches of two varieties of Peruvian carrot (Arracacia Xanthorrhiza, B.) from two production areas of Paraná state, Brazil. Braz. Arch. Biol. Technol. 2009, 52, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Wardhani, D.H.; Cahyono, H.; Dwinanda, M.F.H.; Nabila, P.R.; Aryanti, N.; Pangestuti, D.R. Effect of KOH as deacetylation agent on physicochemical properties of glucomannan. J. Phys. Conf. Ser. 2019, 1295. [Google Scholar] [CrossRef]
- Daramola, B.; Osanyinlusi, S.A. Investigation on modification of Cassava starch using active components of ginger roots (Zingiber officinale Roscoe). Afr. J. Biotechnol. 2006, 5, 917–920. [Google Scholar] [CrossRef]
- Bamforth, C.W. Barley and malt starch in brewing: A general review. Master Brew. Assoc. Am. 2003, 40, 89–97. [Google Scholar]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Yuan, M.L.; Lu, Z.H.; Cheng, Y.Q.; Li, L.T. Effect of spontaneous fermentation on the physical properties of corn starch and rheological characteristics of corn starch noodle. J. Food Eng. 2008, 85, 12–17. [Google Scholar] [CrossRef]
- Wang, Y.J.; Den Truong, V.; Wang, L. Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohydr. Polym. 2003, 52, 327–333. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Lawal, O.S. Functional properties and retrogradation behaviour of native and chemically modified starch of Mucuna bean (Mucuna pruriens). J. Sci. Food Agric. 2003, 83, 1541–1546. [Google Scholar] [CrossRef]
- Çatal, H.; İbanoğlu, Ş. Ozonation of corn and potato starch in aqueous solution: Effects on the thermal, pasting and structural properties. Int. J. Food Sci. Technol. 2012, 47, 1958–1963. [Google Scholar] [CrossRef]
- Liu, Y.; Selomulyo, V.O.; Zhou, W. Effect of high pressure on some physicochemical properties of several native starches. J. Food Eng. 2008, 88, 126–136. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of plant proteins via maillard reaction: reaction chemistry, technofunctional properties, and potential food application. Foods 2021, 10, 376. [Google Scholar] [CrossRef]
- Atichokudomchai, N.; Varavinit, S.; Chinachoti, P. A study of annealing and freeze-thaw stability of acid-modified tapioca starches by differential scanning calorimetry (DSC). Starch Stärke 2002, 54, 343–349. [Google Scholar] [CrossRef]
- Boonna, S.; Tongta, S. Structural transformation of crystallized debranched cassava starch during dual hydrothermal treatment in relation to enzyme digestibility. Carbohydr. Polym. 2018, 191, 1–7. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Kaur, M.; Singh, N.; Lim, S.T. A comparison of native and oxidized normal and waxy corn starches: Physicochemical, thermal, morphological and pasting properties. LWT-Food Sci. Technol. 2008, 41, 1000–1010. [Google Scholar] [CrossRef]
- Garg, S.; Jana, A.K. Effect of propylation of starch with different degrees of substitution on the properties and characteristics of starch-low density polyethylene blend films. J. Appl. Polym. Sci. 2011, 122, 2197–2208. [Google Scholar] [CrossRef]















| Sago Starch | Variables | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| Ozone-oxidized sago starch | 10% pH 4 25 min | 67.53 ± 2.05 a | 103.18 ± 2.01 a | 151.94 ± 2.45 a | −280.24 ± 3.01 d |
| Ozone-oxidized sago starch | 20% pH 4 25 min | 83.87 ± 1.68 bc | 112.19 ± 3.35 c | 158.43 ± 3.25 b | −373.64 ± 2.76 a |
| Ozone-oxidized sago starch | 20% pH 7 25 min | 78.97 ± 1.58 b | 113.71 ± 2.31 c | 150.12 ± 1.46 a | −363.02 ± 5.02 b |
| Ozone-oxidized sago starch | 20% pH 10 25 min | 80.86 ± 1.60 b | 106.31 ± 3.54 b | 158.34 ± 3.05 b | −298.18 ± 2.50 d |
| Ozone-oxidized sago starch | 30% pH 4 25 min | 88.23 ± 1.76 c | 116.93 ± 3.01 d | 157.66 ± 3.21 | −361.39 ± 5.23 b |
| Native sago starch | No variable | 80.98 ± 1.32 b | 106.08 ± 2.95 b | 151.61 ± 2.92 a | −310.34 ± 4.07 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumardiono, S.; Jos, B.; Pudjihastuti, I.; Yafiz, A.M.; Rachmasari, M.; Cahyono, H. Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch. Foods 2021, 10, 1309. https://doi.org/10.3390/foods10061309
Sumardiono S, Jos B, Pudjihastuti I, Yafiz AM, Rachmasari M, Cahyono H. Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch. Foods. 2021; 10(6):1309. https://doi.org/10.3390/foods10061309
Chicago/Turabian StyleSumardiono, Siswo, Bakti Jos, Isti Pudjihastuti, Arvin M. Yafiz, Megaria Rachmasari, and Heri Cahyono. 2021. "Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch" Foods 10, no. 6: 1309. https://doi.org/10.3390/foods10061309
APA StyleSumardiono, S., Jos, B., Pudjihastuti, I., Yafiz, A. M., Rachmasari, M., & Cahyono, H. (2021). Physicochemical Properties of Sago Ozone Oxidation: The Effect of Reaction Time, Acidity, and Concentration of Starch. Foods, 10(6), 1309. https://doi.org/10.3390/foods10061309