Effects of Inclusion of Schizochytrium spp. and Forage-to-Concentrate Ratios on Goats’ Milk Quality and Oxidative Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets and Experimental Design
2.2. Sample Collection
2.3. Milk Chemical Composition
2.4. Milk and Blood Fatty Acid Analysis
2.5. Antioxidant Enzymes Activities and Oxidative Status Indicators
2.6. Statistics
3. Results
3.1. Feed Intake and Body Weight
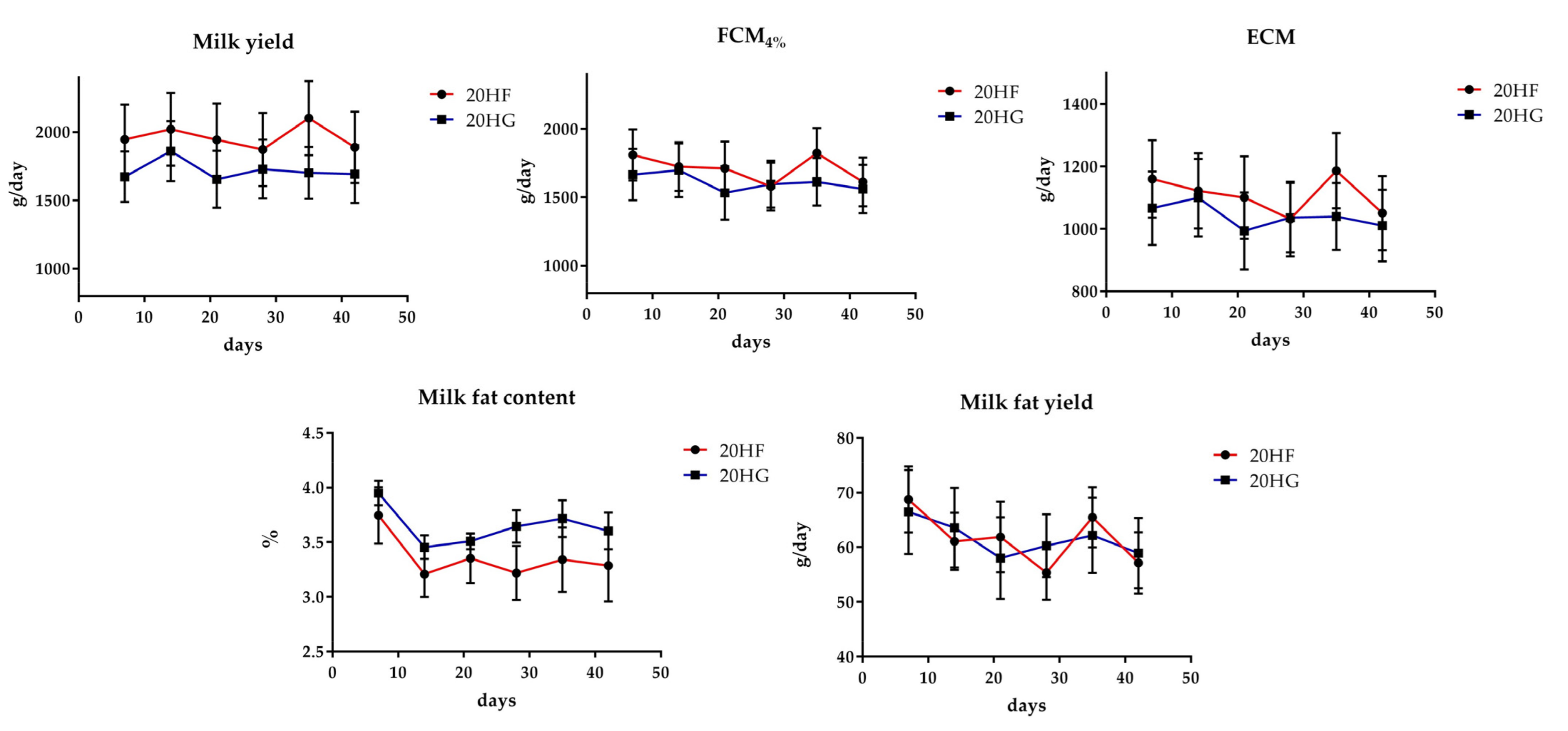
3.2. Milk Performance and Chemical Composition
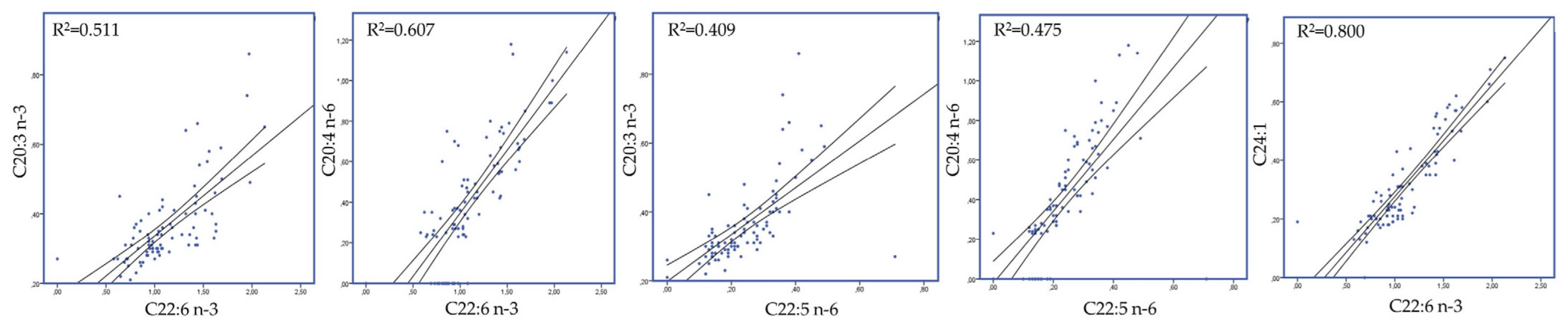
3.3. Blood Plasma and Milk Fatty Acid Profile
3.4. Blood Plasma and Milk Oxidative Status
4. Discussion
4.1. High Microalgae Level Decreased Feed Intake While F:C Ratio Remained Identical
4.2. The Interaction between Fat-Rich Microalgae and the F:C Ratio Did Not Affect Milk Performance
4.3. High Microalgae and Concentrate Levels Improved Milk Fatty Acid Profile
4.4. The Oxidative Status Subverted the State of Affairs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemahieu, C.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of feed supplementation with different omega-3 rich microalgae species on enrichment of eggs of laying hens. Food Chem. 2013, 141, 4051–4059. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing Omega-3 Long-Chain Polyunsaturated Fatty Acid Content of Dairy-Derived Foods for Human Consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Hervás, G.; Gómez-Cortés, P.; Frutos, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acid profile and dairy sheep performance in response to diet supplementation with sunflower oil plus incremental levels of marine algae. J. Dairy Sci. 2010, 93, 1655–1667. [Google Scholar] [CrossRef]
- Bichi, E.; Hervás, G.; Toral, P.G.; Loor, J.J.; Frutos, P. Milk fat depression induced by dietary marine algae in dairy ewes: Persistency of milk fatty acid composition and animal performance responses. J. Dairy Sci. 2013, 96, 524–532. [Google Scholar] [CrossRef]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. 2014, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp.; on milk chemical composition and fatty acid profile of both blood plasma and milk of goats. Small Rum. Res. 2020, 193, 106252. [Google Scholar] [CrossRef]
- Altomonte, I.; Salari, F.; Licitra, R.; Martini, M. Use of microalgae in ruminant nutrition and implications on milk quality—A review. Livest. Sci. 2018, 214, 25–35. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The Dark Side of Microalgae Biotechnology: A Heterotrophic Biorefinery Platform Directed to ω-3 Rich Lipid Production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Skliros, D.; Simoni, M.; Righi, F.; Flemetakis, E.; Tsiplakou, E. Alterations in the Rumen Particle-Associated Microbiota of Goats in Response to Dietary Supplementation Levels of Schizochytrium spp. Sustainability 2021, 13, 607. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Sotirakoglou, K.; Skliros, D.; Flemetakis, E.; Tsiplakou, E. Dose and time response of dietary supplementation with Schizochytrium sp. on the abundances of several microorganisms in the rumen liquid of dairy goats. Lives. Sci. 2021, 247. [Google Scholar] [CrossRef]
- Angeles-Hernandez, J.C.; Vieyra Alberto, R.; Kebreab, E.; Appuhamy, J.A.D.R.N.; Dougherty, H.C.; Castelan-Ortega, O.; Gonzalez-Ronquillo, M. Effect of forage to concentrate ratio and fat supplementation on milk composition in dairy sheep: A meta-analysis. Livest. Sci. 2020, 238, 104069. [Google Scholar] [CrossRef]
- Sterk, A.; Johansson, B.E.; Taweel, H.Z.; Murphy, M.; van Vuuren, A.M.; Hendriks, W.H.; Dijkstra, J. Effects of forage type, forage to concentrate ratio, and crushed linseed supplementation on milk fatty acid profile in lactating dairy cows. J. Dairy Sci. 2011, 94, 6078–6091. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A. Dietary lipids and forages interactions on cow and goat milk fatty acid composition and sensory properties. Reprod. Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Awada, M.; Soulage, C.O.; Meynier, A.; Debard, C.; Plaisancié, P.; Benoit, B.; Picard, G.; Loizon, E.; Chauvin, M.A.; Estienne, M.; et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: Role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012, 53, 2069–2080. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Chronopoulou, G.; Sotirakoglou, K.; Labrou, N.; Zervas, G.; Tsiplakou, E. The impact of the dietary supplementation level with Schizochytrium sp, on the oxidative capacity of both goats’ organism and milk. Livest. Sci. 2018, 218, 37–43. [Google Scholar] [CrossRef]
- Ma, N.; Abaker, J.A.; Bilal, M.S.; Dai, H.; Shen, X. Sodium butyrate improves antioxidant stability in sub-acute ruminal acidosis in dairy goats. BMC Vet. Res. 2018, 14. [Google Scholar] [CrossRef]
- Gabai, G.; Testoni, S.; Piccinini, R.; Marinelli, L.; Stradaioli, G. Oxidative stress in primiparous cows in relation to dietary starch and the progress of lactation. Anim. Sci. 2004, 79, 99–108. [Google Scholar] [CrossRef]
- Rode, L.M.; McAllister, T.A.; Cheng, K.J. Microbial degradation of vitamin A in rumen fluid from steers fed concentrate hay or straw diets. Can. J. Anim. Sci. 1990, 70, 227–233. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mavrommatis, A.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Zervas, G. The effects of dietary supplementation with rumen-protected amino acids on the expression of several genes involved in the immune system of dairy sheep. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1437–1449. [Google Scholar] [CrossRef]
- Cannas, A.; Tedeschi, L.O.; Fox, D.G.; Pell, A.N.; Van Soest, P.J. A mechanistic model for predicting the nutrient requirements and feed biological values for sheep. J. Anim. Sci. 2004, 82, 149–169. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J Anim Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Various Groups of Experts. DF: Bulletin of the IDF No. 285/1993—Reference Materials and Interlaboratory Collaborative Studies (Third Series); The International Dairy Federation: Brussels, Belgium, 1993; ISSN 0250-5118. [Google Scholar]
- The Royal Society of Chemistry. British Standards Institution. Available online: https://pubs.rsc.org/en/Content/ArticleLanding/1952/AN/an952770546a#!divAbstract (accessed on 15 April 2021).
- Bondia-Pons, I.; Castellote, A.I.; López-Sabater, M.C. Comparison of conventional and fast gas chromatography in human plasma fatty acid determination. J. Chromatogr. Analyt. Technol. Biomed. Life Sci. 2004, 809, 339–344. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Chadio, S.; Zervas, G. The effect of long term under- and over-feeding of sheep on milk and plasma fatty acid profiles and on insulin and leptin concentrations. J. Dairy Res. 2012, 79, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nourooz-Zadeh, J.; Appelqvist, L.A. Cholesterol oxides in Swedish foods and ingredients: Milk powder products. J. Food Sci. 1998, 53, 74–82. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mountzouris, K.C.; Zervas, G. Concentration of conjugated linoleic acid in grazing sheep and goat milk fat. Livest. Sci. 2006, 103, 74–84. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Mitsiopoulou, C.; Mavrommatis, A.; Karaiskou, C.; Chronopoulou, E.G.; Mavridis, G.; Sotirakoglou, K.; Labrou, N.E.; Zervas, G. Effect of under- and overfeeding on sheep and goat milk and plasma enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 2018, 102, 288–298. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grand-Jean, P. Plasma malondialdehyde as bio-marker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Karabinas, D.; Nenov, V.; Zervas, G.; Tsiplakou, E. Dietary Supplementation of a Live Yeast Product on Dairy Sheep Milk Performance, Oxidative and Immune Status in Peripartum Period. J. Fungi 2020, 6, 334. [Google Scholar] [CrossRef]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; Matsokis, N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazolinduced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Li, P.; Huo, L.; Su, W.; Lu, R.; Deng, C.; Li, L.; He, C. Free radical scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serbian Chem. Soc. 2011, 76, 709–717. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Alrawashdeh, M.; Radwan, T. Wilk’s lambda based on robust method. AIP Conf. Proc. 2017, 1842, 030032. [Google Scholar]
- Allen, M.S. Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Baumont, R. Palatability and feeding behaviour in ruminants. A review. Annales Zootech. 1996, 45, 385–400. [Google Scholar] [CrossRef]
- Liu, K.; Hao, X.; Li, Y.; Luo, G.; Zhang, Y.; Xin, H. The relationship between odd- and branched-chain fatty acids and microbial nucleic acid bases in rumen. Asian Australas. J. Anim. Sci. 2017, 30, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Dewanckele, L.; Toral, P.G.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression: An update. J. Dairy Sci. 2020, 103, 7655–7681. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; de la Fuente, M.A.; Toral, P.G.; Frutos, P.; Juárez, M.; Hervás, G. Effects of different forage:concentrate ratios in dairy ewe diets supplemented with sunflower oil on animal performance and milk fatty acid profile. J. Dairy Sci. 2011, 94, 4578–4588. [Google Scholar] [CrossRef] [PubMed]
- Schmidely, P.; Andrade, P.V.D. Dairy performance and milk fatty acid composition of dairy goats fed high or low concentrate diet in combination with soybeans or canola seed supplementation. S. Rum. Res. 2011, 99, 135–142. [Google Scholar] [CrossRef]
- Toral, P.G.; Frutos, P.; Carrenño, D.; Hervaás, G. Endogenous synthesis of milk oleic acid in dairy ewes: In vivo measurement using 13 C-labeled stearic acid. J. Dairy Sci. 2017, 100, 5880–5887. [Google Scholar] [CrossRef] [PubMed]
- Thanh, L.P.; Suksombat, W. Milk Yield, Composition, and Fatty Acid Profile in Dairy Cows Fed a High-concentrate Diet Blended with Oil Mixtures Rich in Polyunsaturated Fatty Acids. Asian Australas. J. Anim. Sci. 2015, 28, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Farina, G.; Cattaneo, D.; Lecchi, C.; Invernizzi, G.; Savoini, G.; Agazzi, A. A review on the role of EPA and DHA through goat nutrition to human health: Could they be effective both to animals and humans? J. Dairy Vet. Anim. Res. 2015, 2, 35–39. [Google Scholar] [CrossRef][Green Version]
- Lanier, S.; Suagee, J.K.; Becvar, O.; Corl, B.A. Mammary Uptake of Fatty Acids Supplied by Intravenous Triacylglycerol Infusion to Lactating Dairy Cows. Lipids 2013, 48, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, J.; Escobar, M.; Wallace, R.J.; Fievez, V.; Vlaeminck, B. Biohydrogenation of 22:6n-3 by butyrivibrio proteoclasticus p18. BMC Microbiol. 2016, 16, 104. [Google Scholar] [CrossRef]
- Barclay, W.; Abril, R.; Abril, P.; Weaver, C.; Ashford, A. Production of docosahexaenoic acid from microalgae and its benefits for use in animal feeds A.P. Simopoulos (Ed.), The Return of ω3 Fatty Acids into the Food Supply. I. Land-Based Animal Food Products and Their Health Effects. In World Review of Nutrition and Diet; Omega Tech Inc.: Karger, Basel, Switzerland, 1998; Volume 83, pp. 61–76. [Google Scholar]
- Franklin, S.T.; Martin, K.R.; Baer, R.J.; Schingoethe, D.J.; Hippen, A.R. Dietary marine algae (Schizochytrium sp.) increases concentrations of conjugated linoleic, docosahexaenoic and trans vaccenic acids in milk of dairy cows. J. Nutr. 1999, 129, 2048–2054. [Google Scholar] [CrossRef]
- Mele, M.; Buccioni, A.; Petacchi, F.; Serra, A.; Banni, S.; Antongiovanni, M.; Secchiari, P. Effect of forage/concentrate ratio and soybean oil supplementation on milk yield, and composition from Sarda ewes. Anim. Res. 2006, 55, 273–285. [Google Scholar] [CrossRef]
- Piperova, L.S.; Teter, B.B.; Bruckental, I.; Sampugna, J.; Mills, S.E.; Yurawecz, M.P.; Fritsche, J.; Ku, K.; Erdman, R.A. Mammary lipogenic enzyme activity, trans fatty acids and conjugated linoleic acids are altered in lactating dairy cows fed a milk fat-depressing diet. J. Nutr. 2000, 130, 2568–2574. [Google Scholar] [CrossRef]
- Byelashov, O.A.; Sinclair, A.J.; Kaur, G. Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol. 2015, 27, 79–82. [Google Scholar] [CrossRef]
- Qiao, Q.; Wang, X.; Ren, H.; An, K.; Feng, Z.; Cheng, T.; Sun, Z. Oil Content and Nervonic Acid Content of Acer truncatum Seeds from 14 Regions in China. Hortic. Plant J. 2019, 5, 24–30. [Google Scholar] [CrossRef]
- Calder, P.C.; Campoy, C.; Eilander, A.; Fleith, M.; Forsyth, S.; Larsson, P.O.; Mensink, R.P. A systematic review of the effects of increasing arachidonic acid intake on PUFA status, metabolism and health-related outcomes in humans. Br. J. Nutr. 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhang, Y.; Xu, Q.; Zheng, N.; Zhao, S.; Liu, K.; Qu, X.; Yu, J.; Wang, J. DHA content in milk and biohydrogenation pathway in rumen: A review. PeerJ 2020, 8, e10230. [Google Scholar] [CrossRef]
- Keady, T.W.J.; Mayne, C.S.; Fitzpatrick, D.A. Effects of supplementation of dairy cattle with fish oil on silage intake, milk yield and milk composition. J. Dairy Res. 2000, 67, 137–153. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Doreau, M. Effect of different types of forages, animal fat or marine oils in cow’s diet on milk fat secretion and composition, especially conjugated linoleic acid (CLA) and polyunsaturated fatty acids. Lives. Prod. Sci. 2001, 70, 31–48. [Google Scholar] [CrossRef]
- Huws, S.A.; Kim, E.J.; Lee, M.R.F.; Scott, M.B.; Tweed, J.K.S.; Pinloche, E.; Scollan, N.D. As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ. Microbiol. 2011, 13, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Oh, J.J.; Lim, J.N.; Hong, J.E.; Kim, J.H.; Kim, J.H.; Kang, H.S.; Choi, Y.J.; Lee, H.G. Effects of Lactation Stage and Individual Perfor-mance on Milk cis-9, trans-11 Conjugated Linoleic Acids Content in Dairy Cows. Asian Australas. J. Anim. Sci. 2013, 26, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Porter, N.A. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signal. 2005, 7, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M.; Hsu, Y.M.; Ying, M.C. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr. Metab. 2019, 16, 36. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of Mycotoxins on Animals’ Oxidative Status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef]
- Đuričić, I.; Kotur-Stevuljević, J.; Miljković, M.; Kerkez, M.; Đorđević, V.; Đurašić, L.; Šobajić, S. Effect of Nutritionally Relevant Doses of Long-Chain N-3 Pufa on Lipid Status, Oxidative Stress and Inflammatory Markers in an Average Middle-Aged Serbian Population. J. Med. Biochem. 2015, 34, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, H.; Liu, J.; Lau, C.W.; Liu, P.; Chen, Z.Y.; Lee, H.K.; Tipoe, G.L.; Ho, H.M.; Yao, X.; et al. Cyclooxygenase-2-dependent oxidative stress mediates palmitate-induced impairment of endothelium-dependent relaxations in mouse arteries. Biochem. Pharmacol. 2014, 91, 474–482. [Google Scholar] [CrossRef]
- Katsuyama, M. NOX/NADPH oxidase, the superoxide-generating enzyme: Its transcriptional regulation and physiological roles. J. Pharmacol. Sci. 2010, 114, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef]
- Song, M.Q.; Shi, C.M.; Lin, S.M.; Chen, Y.J.; Shen, H.M.; Luo, L. Effect of starch sources on growth, hepatic glucose metabolism and antioxidant capacity in juvenile largemouth bass, Micropterus salmoides. Aquaculture 2018, 490, 355–361. [Google Scholar] [CrossRef]
- Larsson, K.; Harrysson, H.; Havenaar, R.; Alminger, M.; Undeland, I. Formation of malondialdehyde (MDA), 4-hydroxy-2-hexenal (HHE) and 4-hydroxy-2-nonenal (HNE) in fish and fish oil during dynamic gastrointestinal in vitro digestion. Food Funct. 2016, 7, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Mitsiopoulou, C.; Christodoulou, C.; Kariampa, P.; Simoni, M.; Righi, F.; Tsiplakou, E. Effects of Supplementing Rumen-Protected Methionine and Lysine on Milk Performance and Oxidative Status of Dairy Ewes. Antioxidants 2021, 10, 654. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Qaid, H.A.Y.; Al-Mohy, Y.H.; Ghannam, M.M. The Protective Roles of Vitamin E and α-Lipoic Acid Against Nephrotoxicity, Lipid Peroxidation, and Inflammatory Damage Induced by Gold Nanoparticles. Int. J. Nanomed. 2020, 15, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Appel, L.J.; Croft, K.D.; Miller, E.R.; Mori, T.A.; Puddey, I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef]
- Yuan, S.B.; Chen, D.W.; Zhang, K.Y.; Yu, B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian Australas. J. Anim. Sci. 2007, 20, 1600–1605. [Google Scholar] [CrossRef]
- Pechova, A.; Misurova, L.; Pavlata, L.; Dvorak, R. The influence of supplementation of different forms of zinc in goats on the zinc concentration in blood plasma and milk. Biol. Trace Elem. Res. 2009, 132, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Castañeda, P.C.; Gaxiola-Robles, R.; Labrada-Martagón, V.; Acosta Vargas, B.; Méndez-Rodríguez, L.C.; Zenteno-Savín, T. Oxidative damage to proteins related to metals and antioxidant defenses in breastmilk. Nutr. Hosp. 2017, 34, 59–64. [Google Scholar] [CrossRef] [PubMed]



| Treatment | ||||
|---|---|---|---|---|
| 20 HF | 20 HG | 40 HF | 40 HG | |
| Diet components (Kg per goat) | ||||
| Alfalfa hay | 1.2 | 0.7 | 1.2 | 0.7 |
| Wheat straw | 0.3 | 0.18 | 0.3 | 0.18 |
| Concentrate mix | 1 | 1.3 | 1 | 1.3 |
| Schizochytrium spp. (g) | 20 | 20 | 40 | 40 |
| Forage to Concentrate (F:C) ratio | 1.5:1 (60:40) | 0.88:1.3 (40:60) | 1.5:1 (60:40) | 0.88:1.3 (40:60) |
| Dry Matter | 2282 | 1989 | 2298 | 2000 |
| Ash | 188 | 144 | 192 | 142 |
| Crude Protein | 312 | 311 | 312 | 311 |
| Ether Extract | 82.3 | 87.9 | 90.3 | 97.0 |
| Ash-free NDF treated with amylase | 932 | 712 | 931 | 710 |
| Acid Detergent Fiber | 608 | 399 | 605 | 409 |
| Non Fibrous Carbohydrate | 987 | 925 | 976 | 920 |
| Starch | 474 | 542 | 462 | 542 |
| NDF/Starch | 2.0 | 1.3 | 2.0 | 1.3 |
| Fatty Acid | Concentrates | Forages | ||||
|---|---|---|---|---|---|---|
| 20 HF | 20 HG | 40 HF | 40 HG | Alfalfa Hay | Wheat Straw | |
| Myristic acid (C14:0) | 2.48 | 2.12 | 3.1 | 3.18 | 6.2 | 0 |
| Palmitic acid (C16:0) | 21.94 | 22.99 | 20.22 | 23.77 | 36.77 | 29.88 |
| Stearic acid (C18:0) | 1.92 | 2.06 | 1.55 | 1.87 | 2.33 | 4.86 |
| Oleic acid (C18:1 cis-9) | 28.95 | 31.83 | 22.13 | 27.08 | 2.49 | 34.77 |
| Linoleic acid (C18:2 n-6 cis) | 31.96 | 31.04 | 31.09 | 27.76 | 18.27 | 21.95 |
| Eicosanoic acid (C20:0) | 0.22 | 0.23 | 0.17 | 0.2 | 0.64 | 0.82 |
| Linolenic acid (C18:3 n-3) | 1.07 | 0.96 | 1.13 | 0.88 | 30.68 | 1.86 |
| Eicosatrienoic acid (C20:3n3) | 0.44 | 0.37 | 0.56 | 0.47 | 1.5 | 1.37 |
| Lignoceric acid (C24:0) | 0.32 | 0.25 | 0.26 | 0.25 | 0 | 0.73 |
| Docosapentaenoic acid (C22:5 n-6) | 2.42 | 1.92 | 4.7 | 3.78 | 0 | 0 |
| Docosahexaenoic acid (C22:6 n-3) | 6.71 | 5.25 | 13.76 | 10.21 | 0 | 0 |
| Treatment | ||||
|---|---|---|---|---|
| 20 HF | 20 HG | 40 HF | 40 HG | |
| Diet Consumption | ||||
| Alfalfa hay | 1.2 (100) | 0.7 (100) | 1.2 (100) | 0.7 (100) |
| Wheat straw | 0.2 (66) | 0.18 (99) | 0.15 (50) | 0.16 (90) |
| Concentrate mix | 0.97 (97) | 1.29 (99) | 0.84 (84) | 1.09 (84) |
| Schizochytrium spp. g | 19.3 (97) | 19.8 (99) | 33.7 (84) | 33.2 (83) |
| Schizochytrium spp. % of DMI | 0.89 | 1 | 1.68 | 1.86 |
| Forage to Concentrate (F:C) ratio | 1.4:0.97 (59:41) | 0.88:1.29 (40:60) | 1.35:0.84 (61:39) | 0.76:1.09 (41:59) |
| Nutrients Intake | ||||
| Dry Matter | 2161 | 1980 | 2010 | 1788 |
| Ash | 179 | 144 | 173 | 131 |
| Crude Protein | 305 | 309 | 286 | 276 |
| Ether Extract | 79 | 87 | 76 | 83 |
| Ash-free NDF amylase treated | 853 | 709 | 788 | 649 |
| Acid Detergent Fiber | 555 | 398 | 515 | 383 |
| Non Fibrous Carbohydrate | 954 | 920 | 866 | 810 |
| Starch | 460 | 538 | 393 | 459 |
| NDF/Starch | 1.9 | 1.3 | 2.0 | 1.4 |
| Dietary Treatment (D) | Sampling Time (S) | Effect | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forage/Concentrate | Algae Level | Sampling Day | Effect | Interaction Effect | ||||||||||||
| 60/40 | 40/60 | SEM a | 20 g | 40 g | SEM a | 21 | 42 | SEM a | F/C | ALG | S | F/C × A | F/C × S | A × S | F/C × A × S | |
| C14:0 | 0.432 | 0.460 | 0.033 | 0.292 a | 0.599 b | 0.057 | 0.344 a | 0.548 b | 0.052 | NS | *** | ** | NS | NS | *** | NS |
| C16:0 | 15.69 | 16.57 | 0.544 | 15.47 a | 16.79 b | 0.623 | 16.26 | 16.00 | 0.558 | NS | * | NS | NS | ** | NS | NS |
| C16:1 n-7 | 0.423 | 0.617 | 0.072 | 0.351 a | 0.689 b | 0.091 | 0.541 | 0.499 | 0.088 | t | *** | NS | NS | NS | NS | NS |
| C17:0 | 0.949 | 0.799 | 0.075 | 0.842 | 0.906 | 0.087 | 0.832 | 0.916 | 0.091 | NS | NS | NS | NS | NS | NS | NS |
| C18:0 | 20.25 | 15.72 | 2.039 | 22.53 a | 13.45 b | 2.324 | 18.68 | 17.29 | 2.199 | NS | *** | NS | NS | NS | NS | NS |
| C18:1 trans | 1.35 | 1.01 | 0.287 | 1.34 | 1.02 | 0.320 | 1.31 | 1.06 | 0.312 | NS | NS | NS | NS | NS | NS | NS |
| C18:1 trans-11 | 4.40 | 7.56 | 1.152 | 3.73 a | 8.23 b | 1.342 | 5.42 | 6.54 | 1.224 | t | *** | t | * | NS | NS | NS |
| C18:1 cis-9 | 8.66 | 8.71 | 0.390 | 9.19 a | 8.18 b | 0.407 | 9.12 a | 8.25 b | 0.409 | NS | *** | *** | NS | * | NS | * |
| C18:2 n-6 trans | 0.527 | 0.479 | 0.070 | 0.478 | 0.528 | 0.076 | 0.533 | 0.472 | 0.082 | NS | NS | NS | * | NS | * | NS |
| C18:2 n-6 cis | 21.02 | 22.11 | 0.710 | 24.98 a | 18.15 b | 0.859 | 22.11 | 21.03 | 0.906 | NS | *** | NS | * | NS | ** | * |
| C18:3 n-3 | 1.81 a | 1.01 b | 0.166 | 1.44 | 1.39 | 0.178 | 1.43 | 1.39 | 0.173 | ** | NS | NS | NS | NS | NS | NS |
| C20:3 n-6 | 0.424 | 0.544 | 0.071 | 0.279 a | 0.690 b | 0.092 | 0.416 a | 0.553 b | 0.079 | NS | *** | * | NS | NS | NS | NS |
| C20:3 n-3 | 8.63 | 9.06 | 0.333 | 7.43 a | 10.26 b | 0.414 | 8.61 a | 9.09 b | 0.354 | NS | *** | * | * | *** | ** | NS |
| C22:2 n-6 | 4.85 | 5.21 | 0.316 | 3.09 a | 6.98 b | 0.342 | 4.37 a | 5.69 b | 0.322 | NS | *** | *** | * | * | NS | NS |
| C22:5 n-6 | 1.36 | 1.50 | 0.094 | 0.922 a | 1.94 b | 0.130 | 1.29 a | 1.57 b | 0.128 | NS | *** | * | NS | NS | NS | NS |
| C22:6 n-3 | 9.06 | 8.47 | 0.260 | 7.60 a | 9.93 b | 0.317 | 8.61 | 8.92 | 0.296 | NS | *** | NS | NS | NS | ** | ** |
| Dietary Treatment (D) | Sampling Time (S) | Effect | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forage/Concentrate | Algae Level | Sampling Day | Effect | Interaction Effect | ||||||||||||
| 60/40 | 40/60 | SEM a | 20 g | 40 g | SEM a | 21 | 42 | SEM a | F/C | ALG | S | F/C × A | F/C × S | A × S | F/C × A × S | |
| C4:0 | 2.83 | 2.73 | 0.094 | 2.79 | 2.76 | 0.101 | 2.75 | 2.80 | 0.106 | NS | NS | NS | NS | NS | NS | NS |
| C6:0 | 3.25 | 3.58 | 0.268 | 3.21 | 3.63 | 0.291 | 3.55 | 3.28 | 0.300 | NS | NS | NS | NS | NS | NS | NS |
| C8:0 | 3.75 | 3.84 | 0.135 | 3.77 | 3.82 | 0.148 | 3.82 | 3.78 | 0.140 | NS | NS | NS | NS | * | NS | NS |
| C10:0 | 11.71 | 11.77 | 0.367 | 11.73 | 11.75 | 0.445 | 12.04 a | 11.44 b | 0.393 | NS | NS | ** | NS | NS | NS | NS |
| C11:0 | 0.171 | 0.108 | 0.025 | 0.152 | 0.128 | 0.027 | 0.146 | 0.133 | 0.026 | NS | NS | NS | NS | NS | * | NS |
| C12:0 | 4.64 | 4.56 | 0.250 | 4.65 | 4.54 | 0.289 | 4.78 | 4.41 | 0.259 | NS | NS | *** | NS | NS | NS | NS |
| C13:0 | 0.029 | 0.014 | 0.011 | 0.030 a | 0.014 b | 0.012 | 0.026 | 0.018 | 0.013 | NS | * | NS | * | NS | NS | NS |
| C14:0 | 10.26 | 9.67 | 0.304 | 9.97 | 9.95 | 0.335 | 10.08 | 9.85 | 0.318 | NS | NS | NS | NS | NS | NS | NS |
| C14:1 | 0.304 | 0.275 | 0.015 | 0.293 | 0.287 | 0.017 | 0.304 a | 0.277 b | 0.017 | NS | NS | * | NS | NS | NS | NS |
| C15:0 | 0.969 a | 0.767 b | 0.037 | 0.853 | 0.883 | 0.041 | 0.888 a | 0.848 b | 0.037 | *** | NS | *** | NS | NS | * | NS |
| C15:1 | 0.227 | 0.208 | 0.022 | 0.257 a | 0.178 b | 0.026 | 0.232 a | 0.203 b | 0.024 | NS | *** | * | NS | NS | NS | NS |
| C16:0 | 29.19 | 27.99 | 0.670 | 27.89 a | 29.29 b | 0.730 | 28.57 | 28.61 | 0.693 | NS | ** | NS | NS | NS | NS | NS |
| C16:1 n-7 | 0.497 | 0.455 | 0.023 | 0.405 a | 0.547 b | 0.028 | 0.403 a | 0.549 b | 0.029 | NS | *** | *** | NS | NS | *** | NS |
| C17:1 | 0.097 a | 0.028 b | 0.013 | 0.090 a | 0.034 b | 0.018 | 0.078 a | 0.046 b | 0.015 | ** | ** | * | NS | NS | NS | NS |
| C18:0 | 7.07 | 5.67 | 0.912 | 7.98 a | 4.76 b | 1.100 | 6.33 | 6.41 | 0.952 | NS | ** | NS | NS | NS | NS | NS |
| C18:1 trans | 5.92 a | 9.94 b | 1.189 | 6.45 a | 9.42 b | 1.364 | 7.66 | 8.20 | 1.219 | * | ** | NS | NS | NS | * | NS |
| C18:1 cis-9 | 12.52 | 11.36 | 0.747 | 13.48 a | 10.40 b | 0.931 | 11.86 | 12.03 | 0.784 | NS | ** | NS | NS | NS | NS | NS |
| C18:2 n-6 trans | 0.410 | 0.371 | 0.031 | 0.407 | 0.375 | 0.036 | 0.400 | 0.382 | 0.032 | NS | NS | NS | NS | NS | NS | *** |
| C18:2 n-6 cis | 2.03 | 2.24 | 0.088 | 2.39 a | 1.88 b | 0.101 | 2.080 | 2.193 | 0.109 | NS | *** | NS | * | NS | NS | NS |
| C18:3 n-3 | 0.443 a | 0.250 b | 0.034 | 0.331 | 0.361 | 0.039 | 0.347 | 0.346 | 0.035 | *** | NS | NS | NS | NS | NS | NS |
| C20:0 | 0.122 | 0.109 | 0.006 | 0.124 a | 0.107 b | 0.008 | 0.114 | 0.116 | 0.007 | NS | * | NS | NS | NS | ** | NS |
| C18:2 cis-9,trans-11 | 1.21 | 1.26 | 0.175 | 0.879 a | 1.59 b | 0.219 | 1.16 | 1.31 | 0.184 | NS | ** | NS | NS | NS | NS | NS |
| C18:2 trans-10, cis-12 | 0.037 a | 0.089 b | 0.017 | 0.044 a | 0.081 b | 0.021 | 0.069 | 0.057 | 0.019 | * | * | NS | NS | NS | * | NS |
| C22:0 | 0.052 | 0.029 | 0.012 | 0.059 a | 0.023 b | 0.016 | 0.052 a | 0.030 b | 0.013 | NS | * | * | NS | NS | NS | NS |
| C20:3 n-3 | 0.320 a | 0.393 b | 0.021 | 0.302 a | 0.410 b | 0.021 | 0.339 a | 0.374 b | 0.021 | * | *** | *** | *** | *** | *** | *** |
| C20:4 n-6 | 0.380 | 0.450 | 0.036 | 0.190 a | 0.641 b | 0.039 | 0.328 a | 0.502 b | 0.039 | NS | *** | *** | ** | NS | ** | * |
| C20:5 n-3 | 0.009 a | 0.054 b | 0.013 | 0.025 | 0.038 | 0.015 | 0.043 a | 0.020 b | 0.014 | * | NS | * | NS | NS | NS | NS |
| C24:1 n-9 | 0.283 a | 0.357 b | 0.018 | 0.203 a | 0.437 b | 0.021 | 0.292 a | 0.348 b | 0.019 | ** | *** | *** | ** | ** | *** | *** |
| C22:5 n-6 | 0.215 a | 0.263 b | 0.016 | 0.176 a | 0.302 b | 0.019 | 0.213 a | 0.265 b | 0.019 | * | *** | ** | NS | * | NS | NS |
| C22:6 n-3 | 1.05 | 1.17 | 0.055 | 0.865 a | 1.35 b | 0.059 | 1.05 a | 1.17 b | 0.060 | NS | *** | ** | *** | * | *** | ** |
| Grouped Fatty Acids | ||||||||||||||||
| SCFA | 21.71 | 22.03 | 0.661 | 21.65 | 22.09 | 0.740 | 22.30 a | 21.44 b | 0.691 | NS | NS | * | NS | NS | NS | NS |
| ΜCFA | 45.08 | 42.99 | 0.741 | 43.39 | 44.68 | 0.881 | 44.35 | 43.73 | 0.789 | t | t | NS | NS | NS | NS | NS |
| LCFA | 7.25 | 5.81 | 0.924 | 8.17 a | 4.89 b | 1.116 | 6.50 | 6.56 | 0.965 | NS | ** | NS | NS | NS | NS | NS |
| ΜUFA | 19.86 a | 22.63 b | 0.833 | 21.18 | 21.30 | 0.923 | 20.83 a | 21.65 b | 0.871 | * | NS | * | NS | NS | NS | NS |
| PUFA | 5.69 | 6.17 | 0.205 | 5.21 a | 6.65 b | 0.235 | 5.62 a | 6.23 b | 0.245 | NS | *** | ** | * | NS | ** | ** |
| SFA | 74.04 a | 70.83 b | 0.959 | 73.21 a | 71.67 b | 1.047 | 73.15 a | 71.73 b | 1.029 | * | * | * | NS | NS | NS | NS |
| UFA | 25.55 a | 28.79 b | 0.965 | 26.38 a | 27.96 b | 1.054 | 26.45 a | 27.89 b | 1.035 | * | * | * | NS | NS | NS | NS |
| SFA/UFA | 2.99 a | 2.52 b | 0.139 | 2.84 a | 2.67 b | 0.150 | 2.84 a | 2.67 b | 0.147 | * | * | * | * | NS | NS | NS |
| ω6 | 3.07 a | 3.41 b | 0.113 | 3.21 | 3.28 | 0.127 | 3.09 a | 3.40 b | 0.134 | * | NS | ** | NS | NS | NS | NS |
| ω3 | 1.82 | 1.87 | 0.074 | 1.52 a | 2.16 b | 0.079 | 1.78 a | 1.91 b | 0.079 | NS | *** | ** | *** | * | *** | ** |
| ω6/ω3 | 1.72 | 2.05 | 0.129 | 2.23 a | 1.54 b | 0.144 | 1.79 | 1.98 | 0.144 | NS | *** | NS | * | NS | NS | NS |
| Fatty Acids Health Indices | ||||||||||||||||
| AI | 2.37 a | 1.79 b | 0.171 | 1.41 a | 2.74 b | 0.188 | 1.46 a | 2.69 b | 0.184 | * | *** | *** | NS | NS | *** | * |
| ΤΙ | 1.55 | 1.49 | 0.032 | 1.62 a | 1.41 b | 0.039 | 1.52 | 1.51 | 0.034 | NS | *** | NS | * | NS | ** | NS |
| HΡΙ | 0.669 | 0.679 | 0.006 | 0.666 a | 0.683 b | 0.007 | 0.670 a | 0.679 b | 0.007 | NS | *** | * | NS | NS | * | NS |
| Δ−9 Desaturase Indices | ||||||||||||||||
| C14:1/C14:0 | 0.030 | 0.029 | 0.002 | 0.030 | 0.029 | 0.002 | 0.030 | 0.028 | 0.002 | NS | NS | NS | NS | NS | NS | NS |
| C16:1/C16:0 | 0.017 | 0.016 | 0.001 | 0.015 a | 0.019 b | 0.001 | 0.014 a | 0.019 b | 0.001 | NS | *** | *** | NS | NS | *** | NS |
| C18:1/C18:0 | 2.29 | 2.81 | 0.250 | 2.11 a | 2.99 b | 0.313 | 2.60 | 2.50 | 0.261 | NS | ** | NS | NS | NS | NS | NS |
| Dietary Treatment (D) | Sampling Time (S) | Effect | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Forage/Concentrate | Algae Level | Sampling Day | Effect | Interaction Effect | ||||||||||||
| 60/40 | 40/60 | SEM a | 20 g | 40 g | SEM a | 21 | 42 | SEM a | F/C | ALG | S | F/C × A | F/C × S | A × S | F/C × A × S | |
| Blood Plasma | ||||||||||||||||
| CAT units/mL | 5.54 | 7.38 | 0.841 | 7.39 a | 5.53 b | 0.979 | 6.01 | 6.91 | 0.937 | 0.065 | * | NS | NS | *** | NS | NS |
| GSH-Px units/mL | 0.103 | 0.107 | 0.005 | 0.106 | 0.104 | 0.006 | 0.102 | 0.108 | 0.005 | NS | NS | 0.063 | NS | *** | NS | NS |
| GR units/mL | 0.065 | 0.059 | 0.004 | 0.061 | 0.063 | 0.005 | 0.060 | 0.065 | 0.004 | NS | NS | NS | NS | NS | NS | NS |
| GSTs units/mL | 0.248 | 0.266 | 0.012 | 0.246 | 0.268 | 0.016 | 0.241 a | 0.273 b | 0.013 | NS | NS | ** | NS | NS | NS | NS |
| SOD units/mL | 13.65 | 14.37 | 0.314 | 12.96 a | 15.06 b | 0.368 | 13.16 a | 14.85 b | 0.341 | NS | *** | *** | NS | NS | NS | *** |
| ABTS % | 40.88 | 40.14 | 0.492 | 39.98 | 41.04 | 0.670 | 41.41 a | 39.61 b | 0.631 | NS | NS | ** | ** | NS | *** | NS |
| FRAP μΜ | 0.984 | 1.11 | 0.053 | 1.14 a | 0.955 b | 0.070 | 1.02 | 1.07 | 0.063 | NS | * | NS | NS | NS | NS | ** |
| CP nmol/ml | 3.74 | 3.89 | 0.104 | 3.42 a | 4.21 b | 0.134 | 3.50 a | 4.13 b | 0.146 | NS | *** | *** | NS | NS | NS | NS |
| MDA μΜ | 1.33 a | 1.84 b | 0.119 | 1.23 a | 1.94 b | 0.137 | 1.57 | 1.60 | 0.127 | ** | *** | NS | NS | NS | NS | NS |
| Milk | ||||||||||||||||
| LPO units/mL | 0.804 | 0.737 | 0.042 | 0.768 | 0.772 | 0.044 | 0.765 | 0.775 | 0.046 | NS | NS | NS | NS | NS | NS | NS |
| SOD units/mL | 33.28 | 31.96 | 1.473 | 32.01 | 33.22 | 1.703 | 36.39 a | 28.85 b | 1.959 | NS | NS | *** | ** | NS | NS | * |
| ABTS % | 13.59 | 13.98 | 0.477 | 15.56 a | 12.00 b | 0.615 | 14.27 | 13.29 | 0.676 | NS | *** | NS | * | * | *** | * |
| FRAP μΜ | 1.44 | 1.39 | 0.062 | 1.46 | 1.37 | 0.073 | 1.65 a | 1.18 b | 0.078 | NS | NS | *** | * | NS | *** | ** |
| CP nmol/mL | 2.87 a | 3.29 b | 0.119 | 2.99 | 3.16 | 0.153 | 2.91 a | 3.24 b | 0.142 | * | NS | ** | NS | NS | ** | *** |
| MDA μΜ | 0.597 | 0.603 | 0.049 | 0.600 | 0.600 | 0.056 | 0.421 a | 0.779 b | 0.053 | NS | NS | *** | ** | NS | NS | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrommatis, A.; Sotirakoglou, K.; Kamilaris, C.; Tsiplakou, E. Effects of Inclusion of Schizochytrium spp. and Forage-to-Concentrate Ratios on Goats’ Milk Quality and Oxidative Status. Foods 2021, 10, 1322. https://doi.org/10.3390/foods10061322
Mavrommatis A, Sotirakoglou K, Kamilaris C, Tsiplakou E. Effects of Inclusion of Schizochytrium spp. and Forage-to-Concentrate Ratios on Goats’ Milk Quality and Oxidative Status. Foods. 2021; 10(6):1322. https://doi.org/10.3390/foods10061322
Chicago/Turabian StyleMavrommatis, Alexandros, Kyriaki Sotirakoglou, Charalampos Kamilaris, and Eleni Tsiplakou. 2021. "Effects of Inclusion of Schizochytrium spp. and Forage-to-Concentrate Ratios on Goats’ Milk Quality and Oxidative Status" Foods 10, no. 6: 1322. https://doi.org/10.3390/foods10061322
APA StyleMavrommatis, A., Sotirakoglou, K., Kamilaris, C., & Tsiplakou, E. (2021). Effects of Inclusion of Schizochytrium spp. and Forage-to-Concentrate Ratios on Goats’ Milk Quality and Oxidative Status. Foods, 10(6), 1322. https://doi.org/10.3390/foods10061322








