Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Stock Antimicrobial Solutions
2.3. Determination of the Minimum Inhibitory Concentration (MIC) and the Minimal Bactericidal Concentration (MCB)
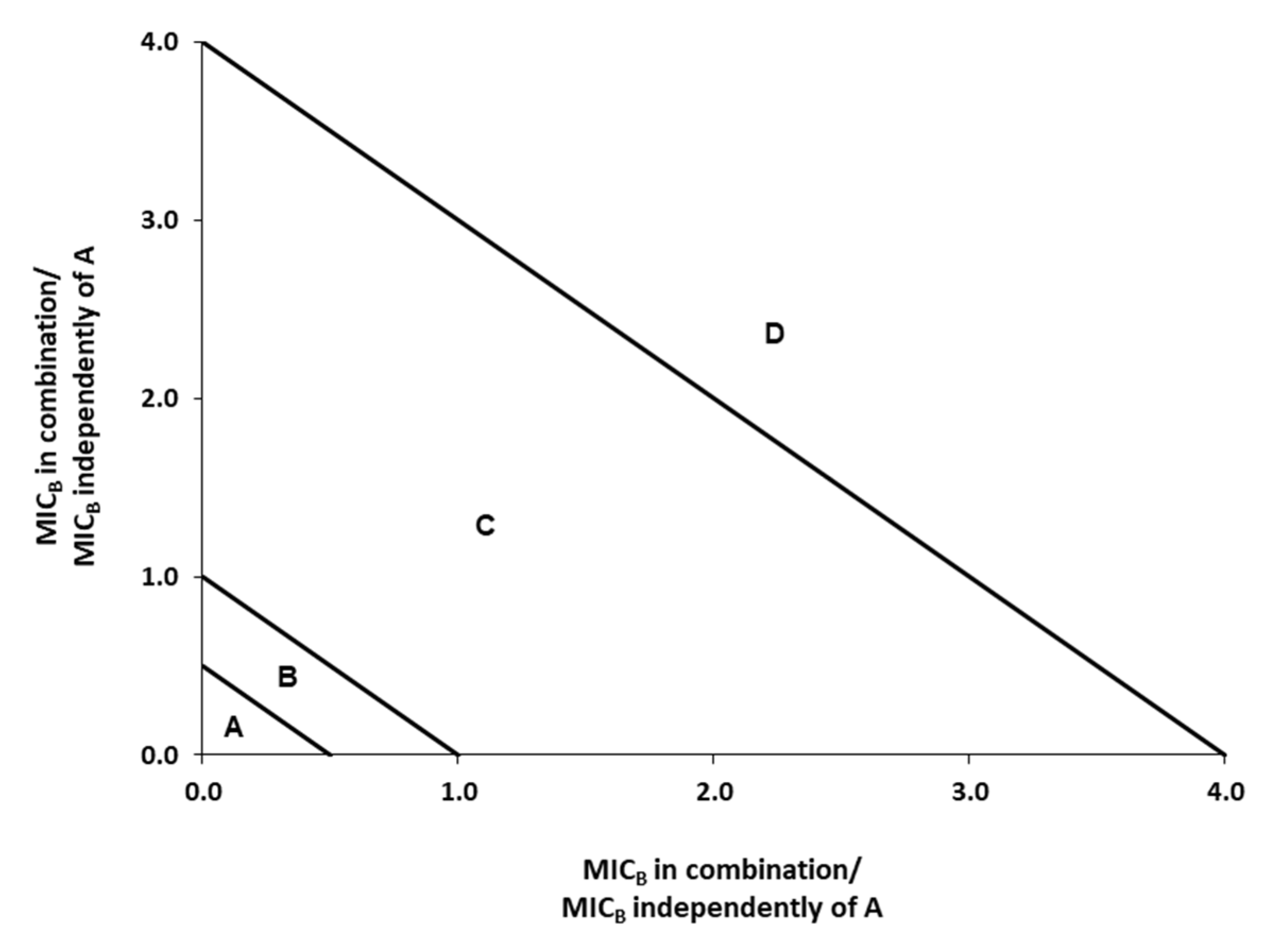
2.4. Evaluation of Interaction between the Antimicrobials Tested
3. Results
3.1. MIC and MBC of EOs and Vanillin
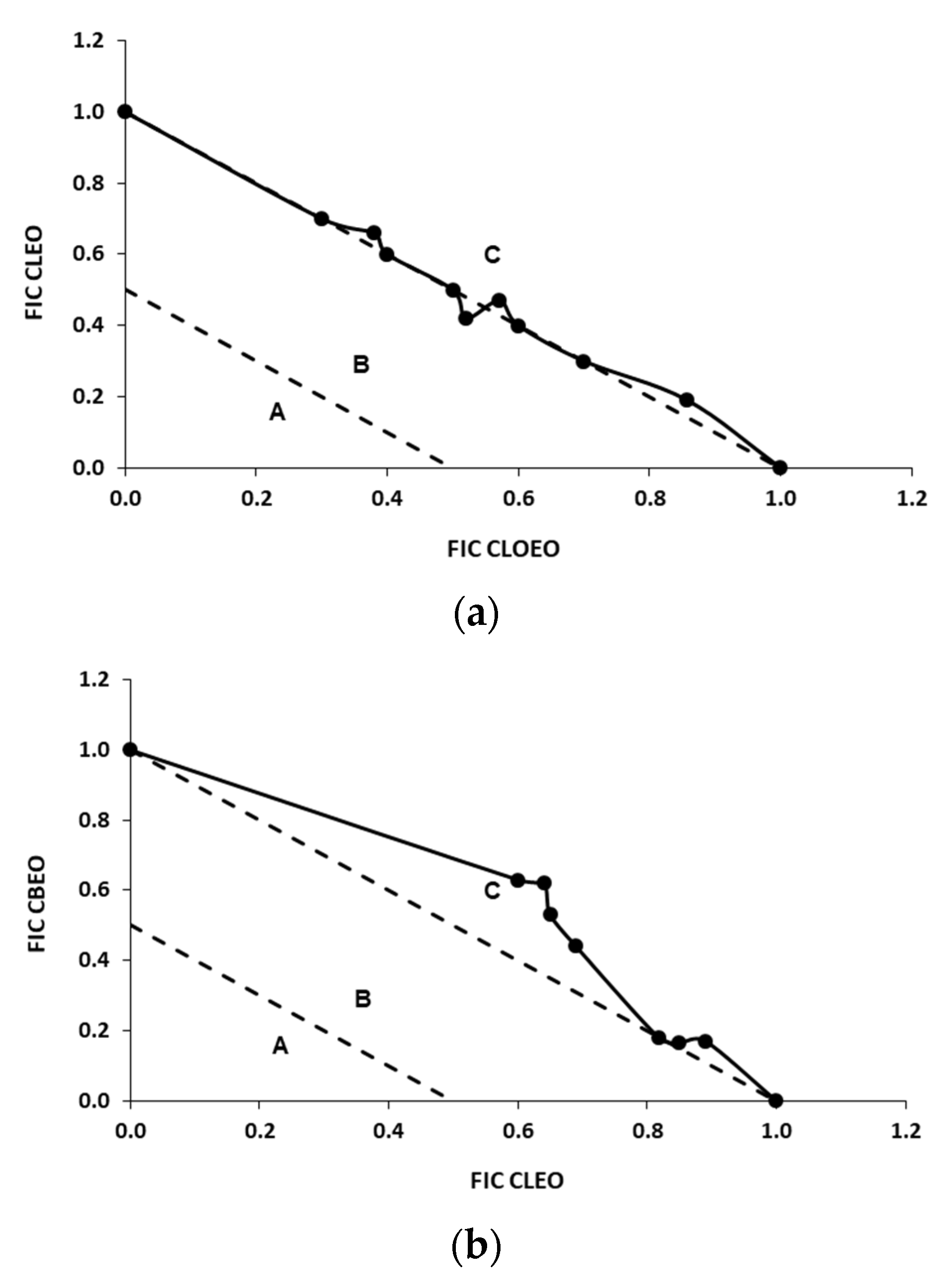
3.2. FICi of Binary Combinations Using Two-Dimensional Checkerboard Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- W.H.O. Food Safety. World Health Organization, Geneva, Switzerland. 30 April 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 10 August 2010).
- Arshad, M.S.; Batool, S.A. Natural Antimicrobials, their Sources and Food Safety. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; IntechOpen: London, UK, 2017; pp. 87–102. [Google Scholar] [CrossRef] [Green Version]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Reddy, D.N. Essential Oils Extracted from Medicinal Plants and Their Applications. In Natural Bio-active Compounds; Akhtar, M.S., Swamy, M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; Volume 1, pp. 237–283. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological Activity of Essential Oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef] [Green Version]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. In Essential Oils—Bioactive Compounds, New Perspectives and Applications; Santana de Oliveira, M., Almeida da Costa, W., Gomes Silva, S., Eds.; IntechOpen: London, UK, 2020; pp. 1–33. [Google Scholar] [CrossRef]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.; Viuda-Martos, M. Introduction to the Special Issue: Application of Essential Oils in Food Systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonam, K.S.; Guleria, S. Synergistic Antioxidant Activity of Natural Products. Ann. Pharmacol. Pharm. 2017, 2, 1–6. [Google Scholar]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- W.H.O. E. coli. World Health Organization, Geneva, Switzerland. 7 February 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/e-coli (accessed on 17 October 2020).
- Control Disease Center. Reports of E. coli Outbreak Investigations from 2020. Control Disease Center. 26 February 2020. Available online: https://www.cdc.gov/ecoli/2020-outbreaks.html (accessed on 17 October 2020).
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial activity of leaf and bark cinnamon essential oils against Listeria monocytogenes and Salmonella typhimurium in broth system and on celery. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT Food Sci. Technol. 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Cava, R.; Nowak, E.; Taboada-Rodríguez, A.; Marin-Iniesta, F. Antimicrobial Activity of Clove and Cinnamon Essential Oils against Listeria monocytogenes in Pasteurized Milk. J. Food Prot. 2007, 70, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Valverde-Franco, M.T.; Marín-Iniesta, F. Antimicrobial Activity of Vanillin and Mixtures with Cinnamon and Clove Essential Oils in Controlling Listeria monocytogenes and Escherichia coli O157:H7 in Milk. Food Bioprocess Technol. 2012, 5, 2120–2131. [Google Scholar] [CrossRef]
- Gilling, D.H.; Ravishankar, S.; Bright, K.R. Antimicrobial efficacy of plant essential oils and extracts against Escherichia coli. J. Environ. Sci. Heath Part A 2019, 54, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mith, H.; Duré, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, S.; Reinders, R. Antibacterial activity of selected plant essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Cava-Roda, R.M.; Taboada-Rodríguez, A.; Palop, A.; López-Gómez, A.; Marín-Iniesta, F. Heat resistance of Listeria monocytogenes in semi-skim milk supplemented with vanillin. Int. J. Food Microbiol. 2012, 157, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-D.; Delaquis, P.; Toivonen, P.; Stanich, K. Effect of vanillin on the fate of Listeria monocytogenes and Escherichia coli O157:H7 in a model apple juice medium and in apple juice. Food Microbiol. 2006, 23, 169–174. [Google Scholar] [CrossRef]
- Corte, F.V.; Fabrizio, S.V.; Salvatori, D.M.; Alzamora, S.M. Survival of Listeria Innocua in Apple Juice as Affected by Vanillin or Potassium Sorbate. J. Food Saf. 2004, 24, 1–15. [Google Scholar] [CrossRef]
- Jonušaite, K.; Venskutonis, P.; Martínez-Hernández, G.; Taboada-Rodríguez, A.; Nieto, G.; López-Gómez, A.; Marín-Iniesta, F. Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers. Foods 2021, 10, 776. [Google Scholar] [CrossRef]
- Sánchez-Rubio, M.; Taboada-Rodríguez, A.; Cava-Roda, R.; Lopez-Gomez, A.; Marín-Iniesta, F. Combined use of thermo-ultrasound and cinnamon leaf essential oil to inactivate Saccharomyces cerevisiae in natural orange and pomegranate juices. LWT Food Sci. Technol. 2016, 73, 140–146. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaquis, P.; Stanich, K.; Toivonen, P. Effect of pH on the Inhibition of Listeria spp. by Vanillin and Vanillic Acid. J. Food Prot. 2005, 68, 1472–1476. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI Document M100-S24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Mann, C.; Markham, J. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef]
- Nikkhah, M.; Hashemi, M.; Najafi, M.B.H.; Farhoosh, R. Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int. J. Food Microbiol. 2017, 257, 285–294. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism and what checkerboard puts between them. J. Antimicrob. Chemother. 2003, 52. [Google Scholar] [CrossRef]
- Suliman, S.; Van Vuuren, S.; Viljoen, A. Validating the in vitro antimicrobial activity of Artemisia afra in polyherbal combinations to treat respiratory infections. S. Afr. J. Bot. 2010, 76, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, S.; Viljoen, A. Plant-Based Antimicrobial Studies—Methods and Approaches to Study the Interaction between Natural Products. Planta Medica 2011, 77, 1168–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, S.G. Antimicrobial Activity of Cinnamic Acid, Citric Acid, Cinnamaldehyde, and Levulinic Acid Against Foodborne Pathogens. Honors Senior Thesis Dissertation, University of Tennessee, Knoxville, TN, USA, 2014. Available online: https://trace.tennessee.edu/utk_chanhonoproj/1701 (accessed on 10 August 2020).
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against Escherichia coli with an Improved Method. J. Food Sci. 2009, 74, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Visvalingam, J.; Holley, R. Temperature-dependent effect of sublethal levels of cinnamaldehyde on viability and morphology of Escherichia coli. J. Appl. Microbiol. 2012, 113, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C.-I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of membrane bound ATPases of Escherichia coli and Listeria monocytogenes by plant oil aromatics. Int. J. Food Microbiol. 2006, 111, 170–174. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol, terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Food Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- Gill, A.; Holley, R.A. Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. monocytogenes and Lactobacillus sakei. Appl. Environ. Microbiol. 2004, 70, 5750–5755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Investigation of antibacterial activity cinnamon bark essential oil (Cinnamomum zeylanicum) in vitro antibacterial activity against five food spoilage bacteria. J. Food Sci. Technol. 2012, 9, 67–76. [Google Scholar]
- Saraf, S.; Mishra, M.S.; Sharma, K. Antibacterial Activity of commercial and wild cinnamon species. J. Phytol. 2011, 3, 102–106. [Google Scholar]
- Gupta, G.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251. [Google Scholar]
- Kim, H.-O.; Park, S.-W.; Park, H.-D. Inactivation of Escherichia coli O157:H7 by cinnamic aldehyde purified from Cinnamomum cassia shoot. Food Microbiol. 2004, 21, 105–110. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of spanish oregano, chinese cinnamon and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Si, W.; Gong, J.R.; Tsao, T.; Zhou, H.; Yu, C.; Poppe, R.; Johnson, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2005, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, G.A.; Lawore, F.M.; Adelowotan, T.; Aibinu, I.E.; Adenipekun, E.; Coker, H.A.B.; Odugbemi, T.O. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). Afr. J. Microbiol. Res. 2008, 2, 162–166. [Google Scholar]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Senhaji, O.; Faid, M.; Kalalou, I. Inactivation of Escherichia coli O157:H7 by essential oil from Cinnamomum zeylanicum. Braz. J. Infect. Dis. 2007, 11, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, D.J.; Stratford, M.; Gasson, M.J.; Narbad, A. Structure−Function Analysis of the Vanillin Molecule and Its Antifungal Properties. J. Agric. Food Chem. 2005, 53, 1769–1775. [Google Scholar] [CrossRef]
- Evrendilek, G.A. Survival of Escherichia coli O157:H7 in yogurt drink, plain yogurt and salted (tuzlu) yogurt: Effects of storage time, temperature, background flora and product characteristics. Int. J. Dairy Technol. 2007, 60, 118–122. [Google Scholar] [CrossRef]
- Fitzgerald, D.; Stratford, M.; Gasson, M.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Ding, Y.; Ding, T. Antibacterial Effect of Cinnamon Oil Combined with Thyme or Clove Oil. J. Integr. Agric. 2011, 10, 1482–1487. [Google Scholar] [CrossRef]
- López-Malo, A.; Palou, E.; Alzamora, S.M. Naturally occurring compounds-plant sources. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press: New York, NY, USA, 2005; pp. 429–451. [Google Scholar]
- Siddiqua, S.; Anusha, B.A.; Ashwini, L.S.; Negi, P.S. Antibacterial activity of cinnamaldehyde and clove oil: Effect on selected foodborne pathogens in model food systems and watermelon juice. J. Food Sci. Technol. 2014, 52, 5834–5841. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.M.; Izadi, M.; Abdollahi, M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Nzeako, B.C.; Al-Kharousi, Z.S.N.; Al-Mahrooqui, Z. Antimicrobial activities of clove and thyme extracts. Sultan Qaboos Univ. Med. J. 2006, 6, 33–39. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiesteban-Lopez, A.; Palou, E.; Lopez-Malo, A. Susceptibility of food-borne bacteria to binary combinations of anti-microbials at selected aw and pH. J. Appl. Microbiol. 2007, 102, 486–497. [Google Scholar] [CrossRef]
- Gill, A.; Delaquis, P.; Russo, P.; Holley, R. Evaluation of antilisterial action of cilantro oil on vacuum packed ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Mourey, A.; Canillac, N. Anti-Listeria monocytogenes activity of essential oils components of conifers. Food Control 2002, 13, 289–292. [Google Scholar] [CrossRef]
- Tamgue, O.; Louis, B.; Nguefack, J.; Do, J.B.L.; Dako, C.D. Synergism and Antagonism of Essential Oil Fractions of Cymbopogon citratus, Ocimum gratissimum and Thymus vulgaris against Penicillium expansum. Int. J. Plant Pathol. 2011, 2, 51–62. [Google Scholar] [CrossRef]





| L. monocytogenes | E. coli O157:H7 | |||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| CLEO | 508 | 1000 | 843 | 1200 |
| CLOEO | 402 | 800 | 778 | 1200 |
| CBEO | 404 | 1200 | 721 | 1500 |
| EU | 562 | 1000 | 766 | 1200 |
| CA | 449 | 1400 | 455 | 1400 |
| VA | 3002 | 8000 | 2795 | 6000 |
| Combinations A/B | MIC(A alone) | MIC(B alone) | MIC(A in the presence of B) | MIC(B in the presence of A) | FICi |
|---|---|---|---|---|---|
| CLOEO/CLEO | 402 | 508 | 121 | 280 | 0.85 |
| CLEO/CBEO | 508 | 404 | 153 | 162 | 0.70 |
| CLOEO/CBEO | 402 | 404 | 121 | 145 | 0.66 |
| CLOEO/VA | 402 | 3003 | 121 | 691 | 0.53 |
| CLEO/VA | 402 | 3003 | 189 | 691 | 0.67 |
| CBEO/VA | 404 | 3003 | 85 | 910 | 0.51 |
| EU/CA | 563 | 449 | 186 | 189 | 0.75 |
| Combinations A/B | MIC(A alone) | MIC(B alone) | MIC(A in the presence of B) | MIC(B in the presence of A) | FICi |
|---|---|---|---|---|---|
| CLOEO/CLEO | 779 | 844 | 335 | 414 | 0.92 |
| CLEO/CBEO | 844 | 721 | 658 | 166 | 1.00 |
| CLOEO/CBEO | 779 | 721 | 218 | 599 | 1.11 |
| CLOEO/VA | 799 | 2796 | 104 | 1006 | 0.49 |
| CLEO/VA | 844 | 2796 | 118 | 979 | 0.49 |
| CBEO/VA | 721 | 2796 | 144 | 784 | 0.48 |
| EU/CA | 767 | 455 | 421 | 45 | 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cava-Roda, R.; Taboada-Rodríguez, A.; López-Gómez, A.; Martínez-Hernández, G.B.; Marín-Iniesta, F. Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves. Foods 2021, 10, 1406. https://doi.org/10.3390/foods10061406
Cava-Roda R, Taboada-Rodríguez A, López-Gómez A, Martínez-Hernández GB, Marín-Iniesta F. Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves. Foods. 2021; 10(6):1406. https://doi.org/10.3390/foods10061406
Chicago/Turabian StyleCava-Roda, Rita, Amaury Taboada-Rodríguez, Antonio López-Gómez, Ginés Benito Martínez-Hernández, and Fulgencio Marín-Iniesta. 2021. "Synergistic Antimicrobial Activities of Combinations of Vanillin and Essential Oils of Cinnamon Bark, Cinnamon Leaves, and Cloves" Foods 10, no. 6: 1406. https://doi.org/10.3390/foods10061406








