Chemical Composition and Functional Properties of Dietary Fibre Concentrates from Winemaking By-Products: Skins, Stems and Lees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical Composition
2.2.1. Moisture and Ash
2.2.2. Crude Protein and Total Fat
2.2.3. Determination of Soluble Sugars
2.2.4. Determination of Total, Soluble, and Insoluble Dietary Fibre
2.3. Characterisation of Insoluble Fibre: Cellulose, Hemicellulose, and Lignin
2.4. Characterisation of Soluble Fibre: Neutral Sugar and Pectins
2.5. Functional Properties of the Fibre
2.5.1. Swelling, Water Retention Capacity and Fat Retention Capacity
2.5.2. Non-Extractable Polyphenols and Antioxidant Activity
2.5.3. In Vitro Prebiotic Capacity
2.6. Statistical Analysis
3. Results and Discussion
3.1. General Chemical Composition
3.1.1. Total Soluble Sugars
3.1.2. Total, Insoluble and Soluble Dietary Fibre
3.2. Fibre Constituents: Cellulose, Hemicellulose, Lignin, Neutral Sugars, and Pectins
3.3. Functional Properties of the Fibre
3.3.1. Swelling, Water Retention Capacity and Oil Retention Capacity
3.3.2. Non-Extractable Polyphenols and Antioxidant Activity
3.3.3. In Vitro Prebiotic Capacity
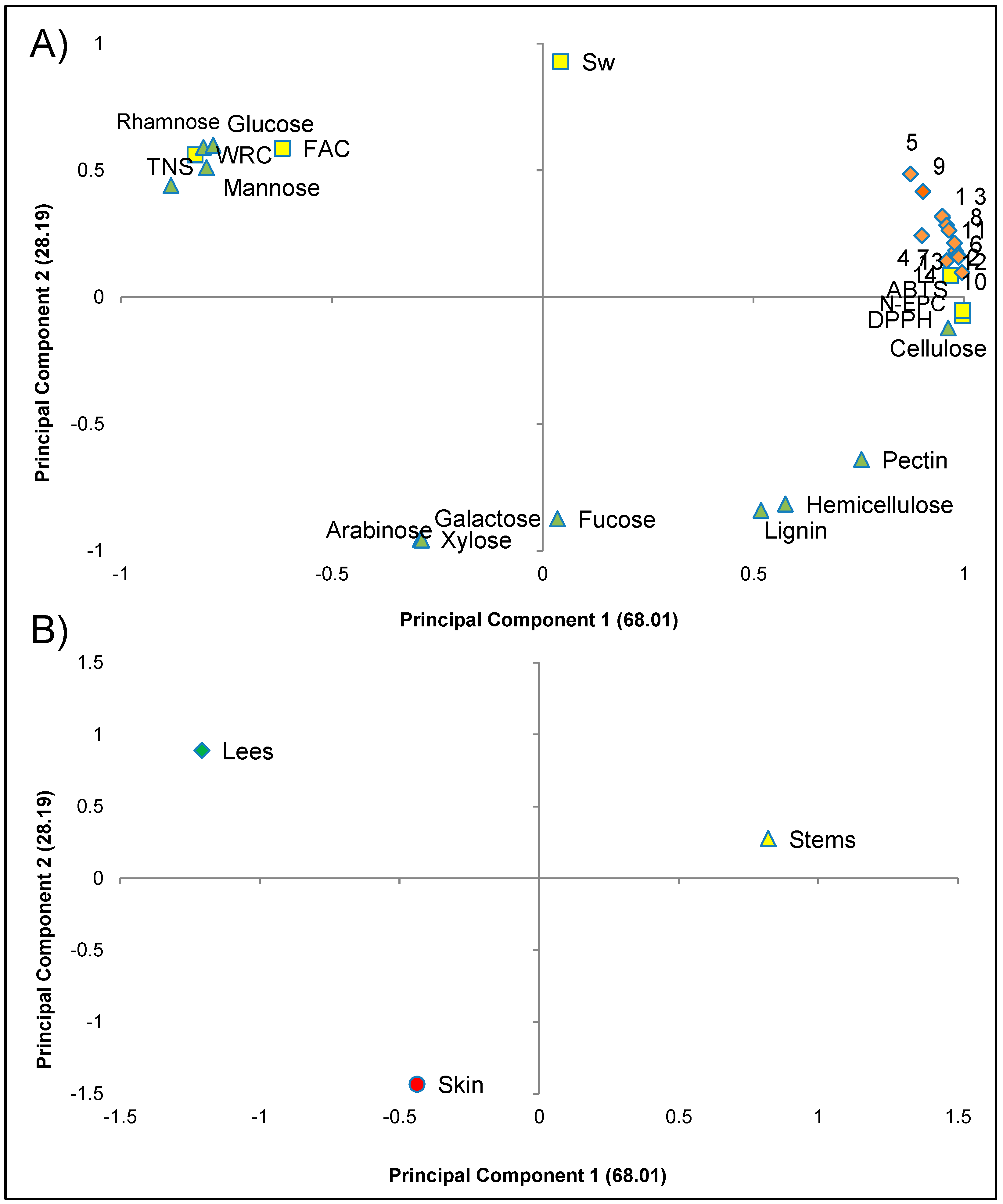
3.4. Multivariate Analysis of the Parameters Related to Dietary Fibre Extracted from Skin, Stems and Lees
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization Corporate Statistical Database (2017). FAO Database. Available online: http://faostat.fao.org (accessed on 26 May 2021).
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coïsson, J.D. Spent grape pomace as a still potential by-product. Int. J. Food Sci. Technol. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- González-Paramás, A.M.; Esteban-Ruano, S.; Santos-Buelga, C.; de Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Flavanol Content and Antioxidant Activity in Winery Byproducts. J. Agric. Food Chem. 2004, 52, 234–238. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.; Romani, A.; Lampe, A.; Nicoli, S.F.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- González-Ballesteros, N.; Rodríguez-González, J.; Rodríguez-Argüelles, M. Harnessing the wine dregs: An approach towards a more sustainable synthesis of gold and silver nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 178, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Basanta, R.; Delgado, M.A.G.; Martínez, J.E.C.; Vázquez, H.M.; Vázquez, G.B. Sustainability of waste recycling from the sugar agribusiness: A review. Cienc. Tecnol. Aliment. 2007, 5, 293–305. [Google Scholar] [CrossRef]
- Bonamente, E.; Scrucca, F.; Asdrubali, F.; Cotana, F.; Presciutti, A. The Water Footprint of the Wine Industry: Implementation of an Assessment Methodology and Application to a Case Study. Sustainability 2015, 7, 12190–12208. [Google Scholar] [CrossRef] [Green Version]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of winemaking by-products for innovative food applications—A review. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B. Polysaccharide Profile and Content during the Vinification and Aging of Tempranillo Red Wines. J. Agric. Food Chem. 2007, 55, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonso, I.; Borderias, A.J. Technological effect of red grape antioxidant dietary fibre added to minced fish muscle. Int. J. Food Sci. Technol. 2008, 43, 1009–1018. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.; Brenes, A.; Goñi, I. Effect of grape antioxidant dietary fiber on the lipid oxidation of raw and cooked chicken hamburgers. LWT 2009, 42, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- García-Gasca, T.; Castro, V.C.; García-Gasca, T.; Cruz, F.C.M.D.L. Nutraceutical Characterization and Sensory Analysis of Red Grape Bagasse Tea. 2008. Available online: www.uaq.mx/investigacion/difusion/veranos/memorias-2008/10VeranoRegionCentro/33UAZChavezCastroMunozdelaCruz.pdf (accessed on 23 January 2021).
- Pérez-Jiménez, J.; Sayago-Ayerdi, S.G. Grape antioxidant dietary fibre: A natural food additive and a dietary supplement. Agro-Food-Ind. Hi Tech 2009, 20, 17–19. [Google Scholar]
- Rivas, M.Á.; Casquete, R.; Córdoba, M.D.G.; Benito, M.J.; Hernández, A.; Ruiz-Moyano, S.; Martín, A. Functional properties of extracts and residual dietary fibre from pomegranate (Punica granatum L.) peel obtained with different supercritical fluid conditions. LWT 2021, 145, 111305. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2012, 48, 221–237. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Borderías, J.; Larsson, K.; Undeland, I. Inhibition of Hemoglobin-Mediated Oxidation of Regular and Lipid-Fortified Washed Cod Mince by a White Grape Dietary Fiber. J. Agric. Food Chem. 2007, 55, 5299–5305. [Google Scholar] [CrossRef]
- Özvural, E.B.; Vural, H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of Insoluble Fibers in the Fining of Wine Phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.E.; Sánchez, P.G. La fibra dietética. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Grigelmo-Miguel, N.; Martín-Belloso, O. Characterization of dietary fiber from orange juice extraction. Food Res. Int. 1998, 31, 355–361. [Google Scholar] [CrossRef]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R. Novel trends in development of dietary fiber rich meat products—A critical review. J. Food Sci. Technol. 2013, 52, 633–647. [Google Scholar] [CrossRef] [Green Version]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary fibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT 2020, 134, 110140. [Google Scholar] [CrossRef]
- López-Oliva, M.E.; Torres, A.A.; Goñi, I.; Muñoz-Martínez, E. Grape antioxidant dietary fibre reduced apoptosis and induced a pro-reducing shift in the glutathione redox state of the rat proximal colonic mucosa. Br. J. Nutr. 2009, 103, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Pozuelo, M.J.; Torres, A.A.; Hervert-Hernández, D.; López-Oliva, M.E.; Muñoz-Martínez, E.; Rotger, R.; Goñi, I. Grape Antioxidant Dietary Fiber Stimulates Lactobacillus Growth in Rat Cecum. J. Food Sci. 2012, 77, H59–H62. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Ma, N.; Pan, T.; Du, C.-L.; Sun, J.-Y. Punicagranine, a new pyrrolizine alkaloid with anti-inflammatory activity from the peels of Punica granatum. Tetrahedron Lett. 2019, 60, 1231–1233. [Google Scholar] [CrossRef]
- Sánchez-Tena, S.; Lizárraga, D.; Miranda, A.; Vinardell, M.P.; Garcia-Garcia, F.; Dopazo, J.; Torres, J.; Saura-Calixto, F.; Capella, G.; Cascante, M. Grape antioxidant dietary fiber inhibits intestinal polyposis in Apc Min/+ mice: Relation to cell cycle and immune response. Carcinogenesis 2013, 34, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Yusefi, M.; Shameli, K.; Ali, R.R.; Pang, S.-W.; Teow, S.-Y. Evaluating Anticancer Activity of Plant-Mediated Synthesized Iron Oxide Nanoparticles Using Punica granatum Fruit Peel Extract. J. Mol. Struct. 2020, 1204, 127539. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis 925.40, 19th ed.; AOAC International: Rockville, MD, USA, 2005. [Google Scholar]
- AOAC International. Official Methods of Analysi 950.40s, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- International Organization for Standardization. ISO 1444; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- Albalasmeh, A.; Berhe, A.A.; Ghezzehei, T. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces pastorianus cell wall polysaccharides with brewing process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC International. Official Methods of Analysis 935.53, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Femenia, A.; García-Pascual, P.; Simal, S.; Rosselló, C. Effects of heat treatment and dehydration on bioactive polysaccharide acemannan and cell wall polymers from Aloe barbadensis Miller. Carbohydr. Polym. 2003, 51, 397–405. [Google Scholar] [CrossRef]
- Van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High Contents of Nonextractable Polyphenols in Fruits Suggest That Polyphenol Contents of Plant Foods Have Been Underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Teixeira, D.M.; Canelas, V.C.; Canto, A.M.D.; Teixeira, J.M.G.; Dias, C.B. HPLC-DAD Quantification of Phenolic Compounds Contributing to the Antioxidant Activity of Maclura pomifera, Ficus carica and Ficus elastica Extracts. Anal. Lett. 2009, 42, 2986–3003. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; dos Santos, M.T.P.G.; Galván, A.I.; Merchán, A.V.; González, E.; Córdoba, M.D.G.; Benito, M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: Probiotic characteristics and prebiotic metabolism. LWT 2019, 114, 108388. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Casquete, R.; Serradilla, M.J.; Córdoba, M.D.G. Safety and functional aspects of pre-selected lactobacilli for probiotic use in Iberian dry-fermented sausages. Meat Sci. 2009, 83, 460–467. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Martín, A.; Benito, M.J.; Aranda, E.; Casquete, R.; Córdoba, M.D.G. Safety and Functional Aspects of Preselected Enterococci for Probiotic Use in Iberian Dry-Fermented Sausages. J. Food Sci. 2009, 74, M398–M404. [Google Scholar] [CrossRef]
- Rubio, E.; Carmona, Y.; Igartuburu, J.M.; García, C.; Macías, D.F.A.; García, M.D.V. Study of the Composition of Winemaking Residues for Food Purposes. Available online: http://hdl.handle.net/10498/16312 (accessed on 26 January 2021).
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- González-Centeno, M.; Rosselló, C.; Simal, S.; Garau, M.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Bordiga, M. Valorization of Wine Making By-Products; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Charpentier, C.; Feuillat, M. Élevage des vins rouges sur lies. Incidence de l’additiond’uneβ-glucanase sur la composition en polysaccharides et leurs interactions avec les polyphénols. Rev. Oenol. 2008, 35, 31–35. [Google Scholar]
- Llobera, A.; Cañellas, J. Antioxidant activity and dietary fibre of Prensal Blanc white grape (Vitis vinifera) by-products. Int. J. Food Sci. Technol. 2008, 43, 1953–1959. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Costa, E.L.; Morari, J.; Velloso, L.A.; Leal, R.; Rodrigues, E.; Bochi, V.C.; et al. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: A major role for dietary fiber and fiber-bound polyphenols. Food Res. Int. 2019, 123, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.; Oliveira, R.; Domínguez, J.M. Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT 2020, 117, 108652. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins extraction from grape pomace: Characterization and utilization as wood adhesives for wood particleboard. Ind. Crops Prod. 2011, 34, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Effect of Different Drying Methods and Storage Time on the Retention of Bioactive Compounds and Antibacterial Activity of Wine Grape Pomace (Pinot Noir and Merlot). J. Food Sci. 2012, 77, H192–H201. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Quantitative Prediction of Cell Wall Polysaccharide Composition in Grape (Vitis vinifera L.) and Apple (Malus domestica) Skins from Acid Hydrolysis Monosaccharide Profiles. J. Agric. Food Chem. 2009, 57, 3611–3619. [Google Scholar] [CrossRef]
- Igartuburu, J.; Pando, E.; Luis, F.R.; Gil-Serrano, A. An acidic xyloglucan from grape skins. Phytochemistry 1997, 46, 1307–1312. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ros-García, J.; López-Roca, J.; Gómez-Plaza, E. The effect of a commercial pectolytic enzyme on grape skin cell wall degradation and colour evolution during the maceration process. Food Chem. 2012, 130, 626–631. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2007, 227, 223–231. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.-C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayestarán, B.; Guadalupe, Z.; León, D. Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process. Anal. Chim. Acta 2004, 513, 29–39. [Google Scholar] [CrossRef]
- Fernández, O.; Martínez, O.; Hernández, Z.; Guadalupe, Z.; Ayestarán, B. Effect of the presence of lysated lees on polysaccharides, color and main phenolic compounds of red wine during barrel ageing. Food Res. Int. 2011, 44, 84–91. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Chau, C.-F.; Wang, Y.-T.; Wen, Y.-L. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007, 100, 1402–1408. [Google Scholar] [CrossRef]
- Lattimer, J.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrient 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Q.; Fang, D.; Zhuang, W.; Chen, C.; Jiang, W.; Zheng, Y. Modification of insoluble dietary fibers from bamboo shoot shell: Structural characterization and functional properties. Int. J. Biol. Macromol. 2018, 120, 1461–1467. [Google Scholar] [CrossRef]
- Putrino, F.M.; Tedesco, M.; Bodini, R.B.; de Oliveira, A.L. Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose. Bioresour. Technol. 2020, 309, 123387. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotechnol. 2016, 33, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landete, J.M.; Rodríguez, H.; Rivas, B.D.L.; Muñoz, R. High-Added-Value Antioxidants Obtained from the Degradation of Wine Phenolics by Lactobacillus plantarum. J. Food Prot. 2007, 70, 2670–2675. [Google Scholar] [CrossRef]
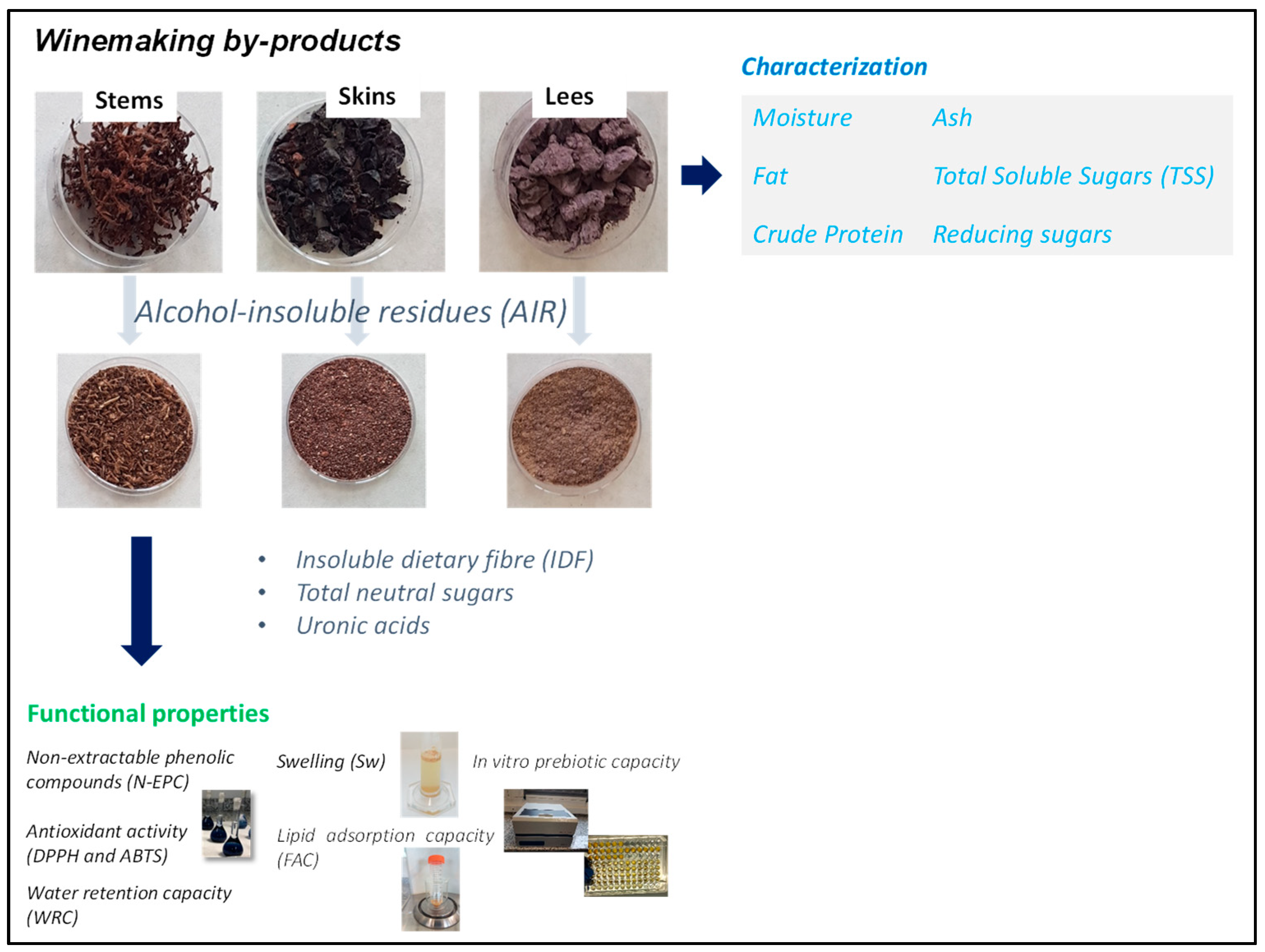
| Parameters | Skins | Stems | Lees |
|---|---|---|---|
| Mean SD 1 | Mean SD | Mean SD | |
| Moisture | 7.66 ± 0.15 a | 16.26 ± 0.16 c | 9.09 ± 0.27 b |
| Ash | 8.12 ± 1.40 a | 6.08 ± 1.18 a | 13.18 ± 0.92 b |
| Protein | 12.24 ± 0.88 b | 7.94 ± 0.35 a | 20.32 ± 0.75 c |
| Fat | 4.24 ± 0.93 | 1.95 ± 0.39 | 4.85 ± 1.15 |
| TSS | 3.65 ± 0.35 a | 22.01 ± 1.56 b | 1.63 ± 0.09 a |
| Reducing sugars | 1.78 ± 0.08 a | 19.60 ± 2.37 b | 0.49 ± 0.05 a |
| Glucose | 0.90 ± 0.73 a | 10.62 ± 11.22 b | <0.5 *a |
| Fructose | 0.79 ± 0.54 a | 11.71 ± 12.01 b | <0.5 *a |
| TDF | 82.30 ± 2.71 b | 71.39 ± 1.01 a | 82.32 ± 1.69 b |
| IDF | 78.18 ± 2.91 b | 67.68 ± 0.95 a | 78.43 ± 1.32 b |
| SDF | 4.13 ± 0.20 | 3.71 ± 0.06 | 3.90 ± 0.37 |
| Parameters | Skins | Stems | Lees |
|---|---|---|---|
| Mean SD 1 | Mean SD | Mean SD | |
| IDF (g/100 g) | |||
| Hemicellulose | 22.57 ± 2.16 | 17.18 ± 0.22 | 28.79 ± 9.25 |
| Cellulose | 7.24 ± 0.04 a | 14.55 ± 3.59 a | 30.03 ± 0.54 b |
| Lignin | 47.31 ± 3.61 b | 29.83 ± 3.27 a | 44.41 ± 5.91 b |
| Total neutral sugars of AIR (mg/g) | 114.71 ± 19.04 b | 84.82 ± 16.76 a | 156.04 ± 8.82 c |
| Rhamnose | <0.5 *a | <0.5 *a | 50.33 ± 0.95 b |
| Fucose | 32.37 ± 2.51 b | 12.40 ± 11.31 ab | 2.32 ± 0.47 a |
| Xylose | 11.76 ± 1.15 b | <0.5 *a | <0.5 *a |
| Mannose | 19.61 ± 0.77 a | 17.94 ± 4.42 a | 35.63 ± 1.32 b |
| Glucose | 26.07 ± 1.06 a | 28.07 ± 5.30 a | 78.30 ± 1.45 b |
| Galactose | 7.56 ± 1.97 b | <0.5 *a | <0.5 *a |
| Arabinose | 17.90 ± 0.66 b | 4.08 ± 0.57 a | 5.30 ± 1.76 a |
| Uronic acids of AIR (mg/g) | |||
| Uronic acids (pectin) | 31.37 ± 1.47 b | 31.09 ± 3.17 b | 12.37 ± 0.53 a |
| Parameters | Skins | Stems | Lees |
|---|---|---|---|
| Mean SD 1 | Mean SD | Mean SD | |
| Sw (mL water/g) | 6.55 ± 0.03 a | 7.76 ± 0.54 ab | 8.43 ± 0.47 b |
| WRC (g water/g) | 4.57 ± 0.21 | 4.61 ± 0.34 | 9.11 ± 2.39 |
| FAC (g oil/g) | 3.76 ± 0.27 | 5.48 ± 0.51 | 5.48 ± 0.88 |
| N-EPC (mg GAE/100 g) | 92.83 ± 15.76 b | 138.63 ± 28.68 c | 44.64 ± 4.35 a |
| DPPH (mg Trolox/100 g) | 3823.68 ± 63.65 b | 6093.01 ± 376.40 c | 2049.23 ± 33.73 a |
| ABTS (mg Trolox/100 g) | 4205.82 ± 307.11 b | 5682.74 ± 308.97 c | 2395.50 ± 671.69 a |
| Microorganisms | Skins | Stems | Lees |
|---|---|---|---|
| Mean SD * | Mean SD | Mean SD | |
| Lactobacillus curvatus CECT 904 | 11.04 ± 0.86 a1,2 | 25.83 ± 0.77 b1 | 11.04 ± 0.86 a1,2 |
| Lactococcus lactis CECT 188 | 9.28 ± 0.76 b1 | 26.74 ± 0.06 c1 | 6.00 ± 0.34 a1 |
| Lactobacillus sakei CECT 5765 | 28.74 ± 2.59 a4,5 | 67.11 ± 0.85 b3,4 | 27.11 ± 0.05 a4 |
| L. sakei CECT 980 | 20.08 ± 1.08 b2,3,4 | 44.61 ± 1.58 c2 | 14.08 ± 0.57 a2,3 |
| Lactobacillus brevis CECT 815 | 9.42 ± 0.64 a1 | 29.90 ± 0.58 c1 | 14.12 ± 0.43 b2,3 |
| Lactobacillus plantarum G1LB5 | 10.58 ± 0.89 a1,2 | 33.50 ± 8.62 b1,2 | 8.17 ± 0.54 a1,2 |
| Lactobacillus casei HL 245 | 10.30 ± 0.42 a1,2 | 39.65 ± 3.51 b1,2 | 12.19 ± 4.08 a1,2,3 |
| L. casei HL 233 | 15.52 ± 6.92 a1,2,3 | 47.39 ± 0.08 b2 | 13.28 ± 4.65 a1,2,3 |
| Enterococcus faecium SE 906 | 30.46 ± 4.58 a5 | 76.79 ± 0.48 b4 | 23.02 ± 3.79 a4 |
| E. faecium SE 920 | 22.49 ± 0.68 b3,4,5 | 62.46 ± 3.11 c3 | 12.11 ± 0.17 a1,2,3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas, M.Á.; Casquete, R.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J.; Pérez-Nevado, F.; Martín, A. Chemical Composition and Functional Properties of Dietary Fibre Concentrates from Winemaking By-Products: Skins, Stems and Lees. Foods 2021, 10, 1510. https://doi.org/10.3390/foods10071510
Rivas MÁ, Casquete R, Córdoba MdG, Ruíz-Moyano S, Benito MJ, Pérez-Nevado F, Martín A. Chemical Composition and Functional Properties of Dietary Fibre Concentrates from Winemaking By-Products: Skins, Stems and Lees. Foods. 2021; 10(7):1510. https://doi.org/10.3390/foods10071510
Chicago/Turabian StyleRivas, María Ángeles, Rocío Casquete, María de Guía Córdoba, Santiago Ruíz-Moyano, María José Benito, Francisco Pérez-Nevado, and Alberto Martín. 2021. "Chemical Composition and Functional Properties of Dietary Fibre Concentrates from Winemaking By-Products: Skins, Stems and Lees" Foods 10, no. 7: 1510. https://doi.org/10.3390/foods10071510
APA StyleRivas, M. Á., Casquete, R., Córdoba, M. d. G., Ruíz-Moyano, S., Benito, M. J., Pérez-Nevado, F., & Martín, A. (2021). Chemical Composition and Functional Properties of Dietary Fibre Concentrates from Winemaking By-Products: Skins, Stems and Lees. Foods, 10(7), 1510. https://doi.org/10.3390/foods10071510








