Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
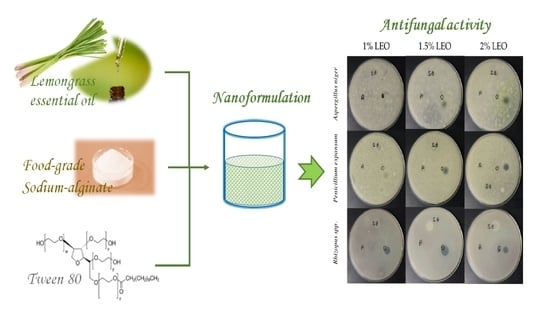
2.2. Preparation of Nanoformulations
2.3. Nanoformulations Characterization
2.4. Antifungal Activity
2.4.1. Microorganisms
2.4.2. Antifungal Activity of Nanoformulations
3. Results and Discussion
3.1. Characterization of Alginate-LEO Based Nanoformulations
3.2. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Sandner, G.; Heckmann, M.; Weghuber, J. Immunomodulatory activities of selected essential oils. Biomolecules 2020, 10, 1139. [Google Scholar] [CrossRef]
- De Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; De Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araújo, M.T.; Coutinho, H.D.M. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Development of flexible antimicrobial films using essential oils as active agents. J. Agric. Food Chem. 2007, 55, 8814–8824. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, F.; Perugini, L.; Marconi, E.; Messia, M.C.; Lopez, F. Enhanced curcumin bioavailability through nonionic surfactant/caseinate mixed nanoemulsions. J. Food Sci. 2019, 84, 2584–2591. [Google Scholar] [CrossRef]
- Mosca, M.; Diantom, A.; Lopez, F.; Ambrosone, L.; Ceglie, A. Impact of antioxidants dispersions on the stability and oxidation of water-in-olive-oil emulsions. Eur. Food Res. Technol. 2013, 236, 319–328. [Google Scholar] [CrossRef]
- Cuomo, F.; Cinelli, G.; Chirascu, C.; Marconi, E.; Lopez, F. Antioxidant effect of vitamins in olive oil emulsion. Colloids Interfaces 2020, 4, 23. [Google Scholar] [CrossRef]
- Cuomo, F.; Iacovino, S.; Messia, M.C.; Sacco, P.; Lopez, F. Protective action of lemongrass essential oil on mucilage from chia (Salvia hispanica) seeds. Food Hydrocoll. 2020, 105, 105860. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Doost, A.S.; Van Camp, J.; Dewettinck, K.; Van der Meeren, P. Production of thymol nanoemulsions stabilized using Quillaja Saponin as a biosurfactant: Antioxidant activity enhancement. Food Chem. 2019, 293, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Cofelice, M.; Lopez, F.; Cuomo, F. Rheological Properties of Alginate–Essential Oil Nanodispersions Colloids Interfaces. Colloids Interfaces 2018, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Farrés, I.F.; Norton, I. Formation kinetics and rheology of alginate fluid gels produced by in-situ calcium release. Food Hydrocoll. 2014, 40, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef]
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Colloids Interfaces 2019, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Davidson, P.M.; Zhong, Q. Nanodispersed eugenol has improved antimicrobial activity against Escherichia coli O157: H7 and Listeria monocytogenes in bovine milk. Int. J. Food Microbiol. 2013, 161, 53–59. [Google Scholar] [CrossRef]
- Xue, J.; Davidson, P.M.; Zhong, Q. Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. Int. J. Food Microbiol. 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Hu, W.; Feng, K.; Xiu, Z.; Jiang, A.; Lao, Y. Efficacy of thyme oil-alginate-based coating in reducing foodborne pathogens on fresh-cut apples. Int. J. Food Sci. Technol. 2019, 54, 3128–3137. [Google Scholar]
- Kim, I.-H.; Oh, Y.A.; Lee, H.; Song, K.B.; Min, S.C. Grape berry coatings of lemongrass oil-incorporating nanoemulsion. LWT Food Sci. Technol. 2014, 58, 1–10. [Google Scholar] [CrossRef]
- Bhargava, K.; Conti, D.S.; da Rocha, S.R.; Zhang, Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 2015, 47, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Morales, H.; Sanchis, V.; Usall, J.; Ramos, A.J.; Marín, S. Effect of biocontrol agents Candida sake and Pantoea agglomerans on Penicillium expansum growth and patulin accumulation in apples. Int. J. Food Microbiol. 2008, 122, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Reale, A.; Tremonte, P.; Maiuro, L.; Succi, M.; Tipaldi, L.; Di Renzo, T.; Pannella, G.; Coppola, R. Lactobacillus plantarum 29 Inhibits Penicillium spp. Involved in the Spoilage of Black Truffles (Tuber aestivum). J. Food Sci. 2013, 78, M1188–M1194. [Google Scholar]
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chitosan polymer as bioactive coating and film against Aspergillus niger contamination. J. Food Sci. 2005, 70, M100–M104. [Google Scholar] [CrossRef]
- Saleh, I.; Al-Thani, R. Fungal food spoilage of supermarkets’ displayed fruits. Vet. World 2019, 12, 1877. [Google Scholar] [CrossRef]
- Snyder, A.B.; Worobo, R.W. Risk mitigation for immunocompromised consumers of mucormycete spoiled and fermented foods: Germane guidance and remaining needs. Microorganisms 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Kawada, H.; Kume, T.; Matsunaga, T.; Iwai, H.; Sano, T.; Shibayama, M. Structure and rheology of a self-standing nanoemulsion. Langmuir 2010, 26, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, H.; Liu, W.; Zou, L.; McClements, D.J. A review of the rheological properties of dilute and concentrated food emulsions. J. Texture Stud. 2020, 51, 45–55. [Google Scholar] [CrossRef]
- Alarcón-Moyano, J.; Matiacevich, S. Active emulsions based on alginate and lemongrass/citral essential oils: Effect of encapsulating agents on physical and antimicrobial properties. Int. J. Food Prop. 2019, 22, 1952–1965. [Google Scholar] [CrossRef]
- Ma, J.; Lin, Y.; Chen, X.; Zhao, B.; Zhang, J. Flow behavior, thixotropy and dynamical viscoelasticity of sodium alginate aqueous solutions. Food Hydrocoll. 2014, 38, 119–128. [Google Scholar] [CrossRef]
- Rodríguez-Abreu, C.; Lazzari, M. Emulsions with structured continuous phases. Curr. Opin. Colloid Interface Sci. 2008, 13, 198–205. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Decker, E.A.; McClements, D.J. Influence of an anionic polysaccharide on the physical and oxidative stability of omega-3 nanoemulsions: Antioxidant effects of alginate. Food Hydrocoll. 2016, 52, 690–698. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Wang, L.; Hu, Y.; Chen, S.; Liu, D.; Ye, X. Edible coating from citrus essential oil-loaded nanoemulsions: Physicochemical characterization and preservation performance. RSC Adv. 2016, 6, 20892–20900. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Campos, V.E.B.; Senna, J.P.; Coutinho, C.d.S.C.; Tebaldi, B.S.; e Silva, K.G.d.H.; Mansur, C.R.E. Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 214–221. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Coupland, J.N.; Friberg, S.; Larsson, K. Orthokinetic stability of food emulsions. Food Emuls. 2004, 327e352. [Google Scholar] [CrossRef]
- de Billerbeck, V.G.; Roques, C.G.; Bessière, J.-M.; Fonvieille, J.-L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mahalwal, V.S.; Ali, M. Volatile constituents of Cymbopogon nardus (Linn.) Rendle. Flavour Fragr. J. 2003, 18, 73–76. [Google Scholar] [CrossRef]
- Aguiar, R.W.d.S.; Ootani, M.A.; Ascencio, S.D.; Ferreira, T.P.; Santos, M.M.d.; Santos, G.R.d. Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, M.K.; Jamil, S.; Ibnouf, E.O.; Shakeel, F. Enhanced antibacterial effects of clove essential oil by nanoemulsion. J. Oleo Sci. 2014, 63, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Serna, C.M.; Dacanal, G.C.; Fernandes, A.M.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef]





| LEO 0% | LEO 1% | LEO 1.5% | LEO 2% | |
|---|---|---|---|---|
| k | 0.96 ± 0.06 | 0.90 ± 0.06 | 0.86 ± 0.06 | 0.88 ± 0.06 |
| n | 0.65 ± 0.01 | 0.66 ± 0.01 | 0.66 ± 0.01 | 0.66 ± 0.01 |
| r2 | 0.997 | 0.997 | 0.997 | 0.997 |
| After 3 Days of Incubation | After 10 Days of Incubation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LEO (%) | LEO (%) | |||||||||||
| 0.1 | 0.3 | 0.7 | 1 | 1.5 | 2 | 0.1 | 0.3 | 0.7 | 1 | 1.5 | 2 | |
| Aspergillus niger | − | − | − | + | + | + | − | − | − | − | + | + |
| Penicillium expansum | − | − | − | + | + | + | − | − | − | +/− | + | + |
| Rhizopus spp. | − | − | − | + | + | + | − | − | − | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofelice, M.; Cinelli, G.; Lopez, F.; Di Renzo, T.; Coppola, R.; Reale, A. Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity. Foods 2021, 10, 1528. https://doi.org/10.3390/foods10071528
Cofelice M, Cinelli G, Lopez F, Di Renzo T, Coppola R, Reale A. Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity. Foods. 2021; 10(7):1528. https://doi.org/10.3390/foods10071528
Chicago/Turabian StyleCofelice, Martina, Giuseppe Cinelli, Francesco Lopez, Tiziana Di Renzo, Raffaele Coppola, and Anna Reale. 2021. "Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity" Foods 10, no. 7: 1528. https://doi.org/10.3390/foods10071528
APA StyleCofelice, M., Cinelli, G., Lopez, F., Di Renzo, T., Coppola, R., & Reale, A. (2021). Alginate-Assisted Lemongrass (Cymbopogon nardus) Essential Oil Dispersions for Antifungal Activity. Foods, 10(7), 1528. https://doi.org/10.3390/foods10071528