Biological Activity of Extracts from Aromatic Plants as Control Agents against Spoilage Molds Isolated from Sheep Cheese
Abstract
:1. Introduction
2. Materials and Methods
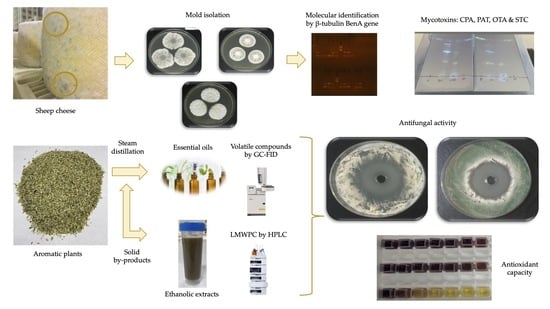
2.1. Cheese Samples and Mold Isolation
2.2. Molecular Identification of Molds by DNA Barcoding
2.3. Analysis of Mycotoxins Production
2.4. Obtainment of Essential Oils and Ethanolic Extracts from Aromatic Plants
2.5. Chemical Composition of Essential Oils and Ethanolic Extracts
2.6. Screening of Antifungal Activity from Aromatic Plant Extracts
2.7. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
2.8. Antioxidant Capacity of Extracts from Aromatic Plants
2.8.1. Total Phenolic Content (TPC)
2.8.2. Scavenging Activity of DPPH Radicals
2.9. Statistical Analysis
3. Results
3.1. Identification of Molds Isolated from Sheep Cheese
3.2. Mycotoxin Production
3.3. Chemical Composition of Aromatic Plant Extracts
3.4. Antifungal Activity of Aromatic Plant Extracts
3.5. Antioxidant Capacity of Essential Oils and Ethanolic Extracts from Aromatic Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Zalacain, A.; Carmona, M. Mycotoxicogenic fungal inhibition by innovative cheese cover with aromatic plants. J. Sci. Food Agric. 2013, 93, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B. Significance of Mycotoxins to Food Safety and Human Health. J. Food Prot. 1979, 42, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Alldrick, A.J.; van Egmond, H.P.; Solfrizzo, M. Mycotoxins: Food safety management implications. Qual. Assur. Saf. Crop. Foods 2009, 1, 153–159. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozturkoglu-Budak, S.; De Vries, R.P. Mold-ripened and raw milk cheeses: Production, risks, and benefits to human health. In Dairy in Human Health and Disease across the Lifespan; Ross Watson, R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 353–361. ISBN 978-0-12-809868-4. [Google Scholar]
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.; Barbier, G.; Coton, E. Filamentous fungi and mycotoxins in Cheese: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456. [Google Scholar] [CrossRef]
- Barrios, M.J.; Medina, L.M.; Lopez, M.C.; Jordano, R. Fungal biota isolated from Spanish cheeses. J. Food Saf. 1998, 18, 151–157. [Google Scholar] [CrossRef]
- Bullerman, L.B. Public Health Significance of Molds and Mycotoxins in Fermented Dairy Products. J. Dairy Sci. 1981, 64, 2439–2452. [Google Scholar] [CrossRef]
- Sengun, I.; Yaman, D.; Gonul, S. Mycotoxins and mould contamination in cheese: A review. World Mycotoxin J. 2008, 1, 291–298. [Google Scholar] [CrossRef]
- Ramos-Pereira, J.; Mareze, J.; Patrinou, E.; Santos, J.A.; López-Díaz, T.M. Polyphasic identification of Penicillium spp. isolated from Spanish semi-hard ripened cheeses. Food Microbiol. 2019, 84, 103253. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int. J. Food Microbiol. 2001, 68, 125–133. [Google Scholar] [CrossRef]
- Ciardo, D.E.; Schär, G.; Altwegg, M.; Böttger, E.C.; Bosshard, P.P. Identification of moulds in the diagnostic laboratory-an algorithm implementing molecular and phenotypic methods. Diagn. Microbiol. Infect. Dis. 2007, 59, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Susca, A. Penicillium Species and Their Associated Mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols, Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1542, pp. 107–119. ISBN 978-1-4939-6707-0. [Google Scholar]
- Leblois, R.; Fre, L. Four years of DNA barcoding: Current advances and prospects. Infect. Genet. Evol. 2008, 8, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bearth, A.; Cousin, M.; Siegrist, M. The consumer’s perception of artificial food additives: Influences on acceptance, risk and benefit perceptions. Food Qual. Prefer. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of fi ve spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Paster, N.; Menasherov, M.; Ravid, U.; Juven, B. Antifungal activity of oregano and thyme essential oils applied as fumigants against fungi attacking stored grain. J. Food Prot. 1995, 58, 70–75. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Antifungal activities of thyme, clove and oregano essential oils. J. Food Saf. 2006, 27, 91–101. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; de los Mozos-Pascual, M.; Tarantilis, P.; Herraiz-Peñalver, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crop. Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Polyphenol composition, antioxidant and bioplaguicide activities of the solid residue from hydrodistillation of Rosmarinus officinalis L. Ind. Crop. Prod. 2014, 59, 125–134. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Jurjević, Ž.; Peterson, S.W.; Solfrizzo, M.; Peraica, M. Sterigmatocystin production by nine newly described Aspergillus species in section Versicolores grown on two different media. Mycotoxin Res. 2013, 29, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; ISBN 978-90-70351-82-3. [Google Scholar]
- Gqaleni, N.; Smith, J.E.; Lacey, J.; Gettinby, G. Production of the Mycotoxin Cyclopiazonic Acid by Penicillium commune on Solid Agar Media: Effects of Water Activity, Temperature, and Incubation Time. J. Food Process. Preserv. 1996, 59, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Filtenborg, O.; Thrane, U. Analysis and screening for mycotoxins and other secondary metabolites in fungal cultures by thin-layer chromatography and high-performance liquid chromatography. Arch. Environ. Contam. Toxicol. 1989, 18, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, M.G.; Lafont, P. Determination of Mycophenolic Acid, Penicillic Acid, Patulin, Sterigmatocystin, and Aflatoxins in Cheese. J. Dairy Sci. 1979, 62, 1145–1148. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Ortiz De Elguea-Culebras, G.; Sánchez-Vioque, R.; Santana Méridas, O. Identification of a hybrid species of sage (Salvia officinalis L. x S. lavandulifolia subsp. lavandulifolia) through the study of the essential oil. J. Essent. Oil Res. 2015, 27, 363–372. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with vine-shoots. Modulating the composition of wines by using their own resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- CLSI. M38-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard—Second Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Espinel-Ingroff, A.; Chaturvedi, V.; Fothergill, A.; Rinaldi, M.G. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 2002, 40, 3776–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolic with phosphomolybdic-phosphotungestic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Giraud, F.; Giraud, T.; Aguileta, G.; Fournier, E.; Samson, R.; Cruaud, C.; Lacoste, S.; Ropars, J.; Tellier, A.; Dupont, J. Microsatellite loci to recognize species for the cheese starter and contaminating strains associated with cheese manufacturing. Int. J. Food Microbiol. 2010, 137, 204–213. [Google Scholar] [CrossRef]
- De Santi, M.; Sisti, M.; Barbieri, E.; Piccoli, G.; Brandi, G.; Stocchi, V. A combined morphologic and molecular approach for characterizing fungal microflora from a traditional Italian cheese (Fossa cheese). Int. Dairy J. 2010, 20, 465–471. [Google Scholar] [CrossRef]
- Montagna, M.T.; Santacroce, M.P.; Spilotros, G.; Napoli, C.; Minervini, F.; Papa, A.; Dragoni, I. Investigation of fungal contamination in sheep and goat cheeses in southern Italy. Mycopathologia 2004, 158, 245–249. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Kirbag, S. Microbial quality and presence of moulds in Kuflu cheese. Int. J. Food Microbiol. 2007, 115, 376–380. [Google Scholar] [CrossRef]
- Kandasamy, S.; Park, W.S.; Yoo, J.; Yun, J.; Seol, K.H.; Oh, M.H.; Ham, J.S. Characterisation of fungal contamination sources for use in quality management of cheese production farms in Korea. Asian-Australas. J. Anim. Sci. 2019, 33, 1002–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decontardi, S.; Soares, C.; Lima, N.; Battilani, P. Polyphasic identification of Penicillia and Aspergilli isolated from Italian grana cheese. Food Microbiol. 2018, 73, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Decontardi, S.; Mauro, A.; Lima, N.; Battilani, P. Survey of Penicillia associated with Italian grana cheese. Int. J. Food Microbiol. 2017, 246, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houbraken, J.; Samson, R.A. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud. Mycol. 2011, 70, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3th ed.; Springer: New York, NY, USA, 2009; Volume 53, ISBN 978-0-387-92206-5. [Google Scholar]
- Serrano Martinez, C.E. Estudio de la micoflora del queso manchego con denominacion de origen. Ph.D. Thesis, Universidad de Córdoba, Córdoba, Spain, 1996. [Google Scholar]
- Kure, C.F.; Skaar, I.; Brendehaug, J. Mould contamination in production of semi-hard cheese. Int. J. Food Microbiol. 2004, 93, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Anelli, P.; Haidukowski, M.; Epifani, F.; Cimmarusti, M.T.; Moretti, A.; Logrieco, A.; Susca, A. Fungal mycobiota and mycotoxin risk for traditional artisan Italian cave cheese. Food Microbiol. 2019, 78, 62–72. [Google Scholar] [CrossRef]
- Ropars, J.; Didiot, E.; Rodríguez de la Vega, R.C.; Bennetot, B.; Coton, M.; Poirier, E.; Coton, E.; Snirc, A.; Le Prieur, S.; Giraud, T. Domestication of the Emblematic White Cheese-Making Fungus Penicillium camemberti and Its Diversification into Two Varieties. Curr. Biol. 2020, 30, 4441–4453. [Google Scholar] [CrossRef]
- Rundberget, T.; Skaar, I.; Flåøyen, A. The presence of Penicillium and Penicillium mycotoxins in food wastes. Int. J. Food Microbiol. 2004, 90, 181–188. [Google Scholar] [CrossRef]
- Ropars, J.; Cruaud, C.; Lacoste, S.; Dupont, J. A taxonomic and ecological overview of cheese fungi. Int. J. Food Microbiol. 2012, 155, 199–210. [Google Scholar] [CrossRef]
- Jurjevic, Z.; Peterson, S.W.; Horn, B.W. Aspergillus section Versicolores: Nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 2012, 3, 59–79. [Google Scholar] [CrossRef]
- Finoli, C.; Vecchio, A.; Galli, A.; Franzetti, L. Production of cyclopiazonic acid by molds isolated from Taleggio cheese. J. Food Prot. 1999, 62, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Flamm, W.G. Review article: Safety assessment of the mycotoxin cyclopiazonic acid. Int. J. Toxicol. 2000, 19, 195–218. [Google Scholar] [CrossRef]
- Lund, F.; Filtenborg, O.; Frisvad, J.C. Associated mycoflora of cheese. Food Microbiol. 1995, 12, 173–180. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 1–81. [Google Scholar] [CrossRef]
- Rubio, R.; Licón, C.C.; Berruga, M.I.; Molina, M.P.; Molina, A. Short communication: Occurrence of aflatoxin M1 in the Manchego cheese supply chain. J. Dairy Sci. 2011, 94, 2775–2778. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- García Vallejo, M.C.; Guijarro Herraiz, J.; Pérez-Alonso, M.; Velasco-Negueruela, A. Volatile oil of hyssopus officinalis L. From Spain. J. Essent. Oil Res. 1995, 7, 567–568. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Peñalver, D.; González-Coloma, A.; Andrés, M.F.; Santana-Méridas, O. Biocidal Potential and Chemical Composition of Industrial Essential Oils from Hyssopus officinalis, Lavandula × intermedia var. Super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, e1700313. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Godevac, D.; Dimkić, I.; Stanković, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crop. Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Lopez-Reyes, J.G.; Spadaro, D.; Gullinoa, M.L.; Garibaldia, A. Efficacy of plant essential oils on postharvest control of rot caused by fungi on four cultivars of apples in vivo. Flavour Fragr. J. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [Green Version]
- Sobieszczańska, N.; Myszka, K.; Szwengiel, A.; Majcher, M.; Grygier, A.; Wolko, Ł. Tarragon essential oil as a source of bioactive compounds with anti-quorum sensing and anti-proteolytic activity against Pseudomonas spp. isolated from fish—In vitro, in silico and in situ approaches. Int. J. Food Microbiol. 2020, 331, 108732. [Google Scholar] [CrossRef] [PubMed]
- Hassiotis, C.N. Chemical compounds and essential oil release through decomposition process from Lavandula stoechas in Mediterranean region. Biochem. Syst. Ecol. 2010, 38, 493–501. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant activity and phenolic composition of lavandin (Lavandula x intermedia Emeric ex Loiseleur) waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [Green Version]
- Potapovich, M.V.; Kurchenko, V.P.; Metelitza, D.I.; Shadyro, O.I. Antioxidant activity of oxygen-containing aromatic compounds. Appl. Biochem. Microbiol. 2011, 47, 346–355. [Google Scholar] [CrossRef]
- Carmo, E.S.; De Oliveira Lima, E.; De Souza, E.L. The potential of origanum vulgare L. (Lamiaceae) essential oil in inhibiting the growth of some food-related aspergillus species. Braz. J. Microbiol. 2008, 39, 362–367. [Google Scholar] [CrossRef] [Green Version]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.S.; Lee, D.W.; Kim, J.; Choi, Y.W. Identification of antifungal constituents of essential oils extracted from Boesenbergia pulcherrima against Fusarium wilt (Fusarium oxysporum). Appl. Biol. Chem. 2020, 63, 34. [Google Scholar] [CrossRef]
- Kurita, N.; Miyaji, M.; Kurane, R.; Takahara, Y. Antifungal activity of components of essential oils. Agric. Biol. Chem. 1981, 45, 945–952. [Google Scholar] [CrossRef]
- Camiletti, B.X.; Asensio, C.M.; Giménez de la Pecci, M.D.; Lucini, E.I. Natural Control of Corn Postharvest Fungi Aspergillus flavus and Penicillium sp. Using Essential Oils from Plants Grown in Argentina. J. Food Sci. 2014, 79, M2499–M2506. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Ortiz-de Elguea-Culebras, G.; Berruga, M.I.; Santana-Méridas, O.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Chemical Composition and Antioxidant Capacities of Four Mediterranean Industrial Essential Oils and Their Resultant Distilled Solid By-Products. Eur. J. Lipid Sci. Technol. 2017, 119, 1–10. [Google Scholar] [CrossRef]
- Julianus Sohilait, H.; Kainama, H. Free Radical Scavenging Activity of Essential Oil of Eugenia caryophylata from Amboina Island and Derivatives of Eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic plants: Antioxidant capacity and polyphenol characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [Green Version]

| Mold Code | Farm Code | Type of Cheese Milk | Fungal Strain |
|---|---|---|---|
| 1A01 | 1A | Raw | Penicillium crustosum |
| 1A02 | 1A | Raw | Penicillium crustosum |
| 1A04 | 1A | Raw | Penicillium commune/biforme |
| 1A05 | 1A | Raw | Aspergillus puulaauensis |
| 1A06 | 1A | Raw | Penicillium commune/biforme |
| 1B01 | 1B | Raw | Penicillium crustosum |
| 1B02 | 1B | Raw | Penicillium crustosum |
| 203 | 2 | Pasteurized | Penicillium commune/biforme |
| 301 | 3 | Raw | Penicillium commune/biforme |
| 401 | 4 | Raw | Penicillium crustosum |
| 402 | 4 | Raw | Penicillium crustosum |
| 403 | 4 | Raw | Penicillium crustosum |
| 501 | 5 | Raw | Aspergillus jensenii |
| 601 | 6 | Raw | Penicillium commune/biforme |
| Compound | RT1 (min) | Artemisia dracunculus (Tarragon) (%) | Hyssopus officinalis (Hyssop) (%) | Lavandula stoechas (Spanish Lavender) (%) | Origanum vulgare (Oregano) (%) | Satureja montana (Savory) (%) |
|---|---|---|---|---|---|---|
| tricyclene | 11.00 | - | 0.19 | 0.07 | 0.41 | - |
| α -thujene | 11.06 | - | - | 0.11 | - | - |
| α-pinene | 11.36 | - | 2.67 | 12.11 | 0.21 | 0.08 |
| camphene | 11.98 | - | 0.08 | 0.50 | - | 0.07 |
| 1-octen-3-ol | 12.63 | 0.25 | 3.32 | 0.97 | 0.24 | 0.87 |
| sabinene | 12.82 | - | - | 0.08 | 0.09 | - |
| ß-pinene + mircene | 12.95 | - | 16.36 | 1.15 | 0.69 | 0.26 |
| delta-3-carene | 13.71 | - | 0.078 | 0.09 | 0.06 | - |
| 3-carene | 13.91 | - | - | 4.20 | - | - |
| α -terpinene | 14.15 | - | 0.08 | 0.33 | 0.40 | 0.38 |
| p-cymene | 14.44 | - | 1.25 | 0.81 | 2.43 | 6.62 |
| limonene | 14.65 | - | 6.02 | 3.73 | 0.09 | 0.06 |
| 1,8-cineole | 14.93 | - | 48.23 | 20.59 | - | 0.28 |
| ß-e-ocimene | 15.00 | - | 1.97 | 0.06 | - | - |
| γ-terpinene | 15.61 | - | 0.27 | 0.18 | 1.82 | 1.28 |
| cis-sabinene hydrate | 16.15 | - | 0.24 | 0.29 | 0.09 | 0.26 |
| terpinolene | 16.66 | - | 0.13 | 0.18 | 0.06 | 0.06 |
| linalool | 16.98 | 0.25 | 0.17 | 33.79 | 0.11 | 1.23 |
| α-thujone | 17.53 | - | - | 0.05 | - | - |
| ß-thujone | 18.01 | - | 0.07 | 0.30 | - | 0.05 |
| camphor | 19.31 | - | 0.75 | 9.56 | - | - |
| pinocamphone | 19.70 | - | 3.42 | - | - | - |
| pinocarvone | 19.77 | - | 3.16 | - | - | - |
| ß-pinene oxide | 19.90 | - | 0.28 | 0.47 | - | - |
| isoborneol | 20.11 | - | - | - | - | 2.23 |
| borneol | 20.21 | 0.17 | 0.18 | 0.61 | 0.26 | 0.12 |
| isopinocamphone | 20.31 | - | 4.38 | - | - | - |
| terpinen-4-ol | 20.31 | 2.08 | - | 0.42 | 0.70 | 0.95 |
| α-terpineol | 20.80 | 0.37 | 1.87 | 0.37 | 0.08 | 0.14 |
| myrtenol | 20.96 | 0.15 | 0.20 | 0.16 | 0.08 | - |
| verbonene | 21.34 | - | - | 0.35 | 0.06 | - |
| trans-carveol | 21.61 | 0.45 | - | 0.12 | - | - |
| nerol | 22.11 | - | - | - | - | 0.05 |
| linalyl acetate | 22.32 | 0.13 | - | 0.21 | - | - |
| geraniol | 22.58 | 0.31 | - | 0.12 | - | - |
| geranial | 23.08 | - | - | 0.08 | - | - |
| bornyl acetate | 23.94 | 0.72 | - | 0.17 | - | 0.44 |
| thymol | 24.15 | - | 0.05 | - | 4.98 | - |
| carvacrol | 24.55 | - | - | - | 82.42 | 77.59 |
| terpin-4-ol acetate | 24.98 | - | - | 0.09 | - | - |
| α-terpinyl acetate | 25.91 | - | 0.14 | - | - | - |
| α-cubebene | 26.20 | 0.27 | - | - | - | 0.053 |
| geranyl acetate | 26.68 | 0.55 | - | - | - | - |
| α-copaene | 27.09 | - | - | 0.09 | - | 0.08 |
| ß-elemene | 27.42 | - | 0.38 | - | - | 0.06 |
| ß-cubebene | 27.56 | - | 0.08 | - | - | 0.46 |
| methyl eugenol | 27.74 | 38.72 | - | - | - | - |
| α-gurjunene | 28.19 | - | 0.08 | - | - | - |
| α-trans-bergamotene | 28.65 | - | 0.32 | - | - | 1.01 |
| trans-caryophyllene | 28.68 | - | - | - | 2.70 | - |
| z-ß-farnesene | 29.06 | - | - | - | 0.14 | 0.14 |
| aromadendrene | 29.25 | - | 0.21 | - | - | 0.11 |
| e-ß-farnesene | 29.80 | - | 0.07 | 0.05 | 0.22 | 0.06 |
| ar-curcumene | 30.23 | - | - | - | - | 0.20 |
| valencene | 30.56 | - | 1.25 | 0.22 | - | 0.17 |
| cis-methyl-isoeugenol | 30.66 | 2.87 | - | - | - | - |
| γ-muurolene | 31.01 | - | 0.22 | - | 0.49 | 0.53 |
| cubebol | 31.52 | - | - | - | - | 0.27 |
| delta-cadinene | 31.69 | - | - | - | - | 0.07 |
| elimicin | 32.25 | 26.12 | - | - | - | - |
| ß-sesquiphellandrene | 32.46 | 0.29 | 0.20 | - | - | 0.55 |
| germacrene d | 33.54 | - | 0.10 | - | - | 0.27 |
| spathulenol | 33.58 | 1.44 | - | - | - | - |
| cariophyllene oxide | 33.83 | 0.29 | 0.06 | - | 0.57 | 0.52 |
| viridiflorol | 34.11 | - | 0.07 | - | - | - |
| humulene epóxido ii | 35.02 | - | - | - | - | 0.05 |
| (z)-isoelemicin | 35.19 | 20.24 | - | - | - | - |
| cubenol | 35.28 | - | - | - | - | 0.06 |
| ß-eudesmol | 35.93 | 0.14 | - | - | - | - |
| α-eudesmol | 36.13 | - | - | - | - | 0.08 |
| geranyl tiglate | 36.81 | 0.12 | - | - | - | - |
| Total | 96.31 | 98.86 | 93.47 | 99.40 | 97.77 |
| Compound | Artemisia dracunculus (Tarragon) (mg/L) | Hyssopus officinalis (Hyssop) (mg/L) | Lavandula stoechas (Spanish Lavender) (mg/L) | Origanum vulgare (Oregano) (mg/L) | Satureja montana (Savory) (mg/L) |
|---|---|---|---|---|---|
| Protocatechuic acid | 2.07 ± 0.04 | 1.36 ± 0.01 | 3.48 ± 0.09 | 52.26 ± 0.20 | 2.80 ± 0.03 |
| Syringic acid | - | 3.04 ± 0.11 | 1.76 ± 0.01 | - | 6.74 ± 0.18 |
| Caffeic Acid | 29.07 ± 0.34 | 14.23 ± 0.71 | 14.76 ± 0.05 | 13.68 ± 0.14 | 22.84 ± 0.20 |
| Sinapaldehyde | - | - | - | 15.68 ± 0.36 | 12.40 ± 0.66 |
| Code | Strain | WD (mm) 1 | % Inhibition | ||||
|---|---|---|---|---|---|---|---|
| Artemisia dracunculus (Tarragon) | Origanum vulgare (Oregano) | Satureja montana (Savory) | Artemisia dracunculus (Tarragon) | Origanum vulgare (Oregano) | Satureja montana (Savory) | ||
| 1A01 | P. crustosum | 26.45 ± 0.55 a | 30.21 ± 2.81 ab | 33.68 ± 1.62 b | 29.39 ± 0.61 a | 33.56 ± 3.12 ab | 37.42 ± 1.79 b |
| 1A02 | P. crustosum | 31.05 ± 0.51 | 30.24 ± 1.69 | 31.86 ± 0.70 | 34.50 ± 0.56 | 33.60 ± 1.88 | 35.39 ± 0.78 |
| 1A04 | P. commune * | 30.17 ± 2.80 a | 38.63 ± 0.47 b | 41.89 ± 0.94 c | 33.53 ± 3.11 a | 42.93 ± 0.53 b | 46.54 ± 1.04 c |
| 1A05 | A. puulaauensis | 32.56 ± 0.75 a | 43.16 ± 1.19 b | 51.32 ± 1.80 c | 36.18 ± 0.84 a | 47.96 ± 1.32 b | 57.02 ± 2.00 c |
| 1A06 | P. commune * | 26.27 ± 0.41 a | 37.88 ± 0.71 c | 35.36 ± 0.53 b | 29.19 ± 0.45 a | 42.09 ± 0.79 c | 39.28 ± 0.59 b |
| 1B01 | P. crustosum | 25.24 ± 1.59 a | 28.40 ± 2.07 b | 32.93 ± 0.91 c | 28.05 ± 1.77 a | 31.55 ± 2.30 b | 36.58 ± 1.01 c |
| 1B02 | P. crustosum | 27.78 ± 1.10 a | 42.87 ± 1.95 c | 36.85 ± 0.69 b | 30.87 ± 1.23 a | 47.63 ± 2.16 c | 40.95 ± 0.77 b |
| 203 | P. commune * | 28.77 ± 1.93 a | 37.26 ± 1.81 b | 39.07 ± 1.56 b | 31.97 ± 2.15 a | 41.40 ± 2.02 b | 43.41 ± 1.73 b |
| 301 | P. commune * | 31.67 ± 1.46 a | 40.75 ± 0.31 b | 38.78 ± 0.45 b | 35.19 ± 1.62 a | 45.28 ± 0.34 b | 43.08 ± 0.50 b |
| 401 | P. crustosum | 39.93 ± 0.72 a | 45.47 ± 1.06 b | 41.66 ± 1.36 a | 44.37 ± 0.80 a | 50.52 ± 1.17 b | 46.29 ± 1.51 a |
| 402 | P. crustosum | 27.87 ± 1.13 a | 33.26 ± 1.37 b | 34.91 ± 1.26 b | 30.97 ± 1.26 a | 36.95 ± 1.52 b | 38.78 ± 1.40 b |
| 403 | P. crustosum | 24.25 ± 0.67 a | 38.63 ± 0.57 c | 33.76 ± 0.71 b | 26.94 ± 0.74 a | 42.93 ± 0.64 c | 37.51 ± 0.79 b |
| 501 | A. jensenii | 39.16 ± 0.80 a | 42.41 ± 1.25 b | 42.08 ± 0.40 b | 43.51 ± 0.89 a | 47.12 ± 1.39 b | 46.76 ± 0.44 b |
| 601 | P. commune * | 30.47 ± 0.88 a | 40.71 ± 1.16 b | 40.71 ± 0.78 b | 33.86 ± 0.98 a | 45.23 ± 1.29 b | 45.23 ± 0.86 b |
| Code | Strain | Artemisia dracunculus (Tarragon) | Origanum vulgare (Oregano) | Satureja montana (Savory) | |||
|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | ||
| 1A01 | P. crustosum | 0.63 | 0.63 | 0.63 | 0.63 | 1.25 | 1.25 |
| 1A02 | P. crustosum | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| 1A04 | P. commune * | 0.63 | 2.5 | 0.16 | 0.63 | 0.31 | 0.31 |
| 1A05 | A. puulaauensis | 0.63 | 0.63 | 0.16 | 0.63 | 0.31 | 0.31 |
| 1A06 | P. commune * | 1.25 | 1.25 | 0.63 | 0.63 | 0.63 | 0.63 |
| 1B01 | P. crustosum | 1.25 | 1.25 | 0.63 | 0.63 | 0.63 | 0.63 |
| 1B02 | P. crustosum | 1.25 | 1.25 | 0.31 | 0.31 | 0.31 | 0.31 |
| 203 | P. commune * | 0.31 | 0.31 | 0.08 | 0.31 | 0.08 | 0.08 |
| 301 | P. commune * | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| 401 | P. crustosum | 1.25 | 1.25 | 0.63 | 0.63 | 0.31 | 0.31 |
| 402 | P. crustosum | 1.25 | 1.25 | 0.16 | 0.63 | 0.31 | 0.31 |
| 403 | P. crustosum | 0.31 | 0.31 | 0.63 | 0.63 | 0.63 | 0.63 |
| 501 | A. jensenii | 1.25 | 1.25 | 0.16 | 0.63 | 0.63 | 0.63 |
| 601 | P. commune * | 0.63 | 2.5 | 0.63 | 0.63 | 0.63 | 0.63 |
| Aromatic Plant | TPC 3 | IC50 | ||
|---|---|---|---|---|
| EO 4 | EE 5 | EO 4 | EE 5 | |
| Artemisia dracunculus | 23.77 ± 1.60 | 91.19 ± 1.61 | 1.18 ± 0.18 | 4.86 ± 0.15 |
| Hyssopus officinalis | 10.67 ± 0.96 | 68.94 ± 1.61 | 29.91 ± 1.75 | 9.24 ± 0.69 |
| Lavandula stoechas | 9.31 ± 0.96 | 143.42 ± 1.61 | 14.80 ± 1.52 | 2.97 ± 0.28 |
| Satureja montana | 74.31 ± 0.34 | 87.33 ± 1.93 | 0.09 ± 0.04 | 2.32 ± 0.18 |
| Origanum vulgare | 100.87 ± 0.36 | 341.19 ± 1.28 | 0.07 ± 0.01 | 0.12 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Tebar, N.; González-Navarro, E.J.; López-Díaz, T.M.; Santos, J.A.; Elguea-Culebras, G.O.d.; García-Martínez, M.M.; Molina, A.; Carmona, M.; Berruga, M.I. Biological Activity of Extracts from Aromatic Plants as Control Agents against Spoilage Molds Isolated from Sheep Cheese. Foods 2021, 10, 1576. https://doi.org/10.3390/foods10071576
Muñoz-Tebar N, González-Navarro EJ, López-Díaz TM, Santos JA, Elguea-Culebras GOd, García-Martínez MM, Molina A, Carmona M, Berruga MI. Biological Activity of Extracts from Aromatic Plants as Control Agents against Spoilage Molds Isolated from Sheep Cheese. Foods. 2021; 10(7):1576. https://doi.org/10.3390/foods10071576
Chicago/Turabian StyleMuñoz-Tebar, Nuria, Emilio J. González-Navarro, Teresa María López-Díaz, Jesús A. Santos, Gonzalo Ortiz de Elguea-Culebras, M. Mercedes García-Martínez, Ana Molina, Manuel Carmona, and María Isabel Berruga. 2021. "Biological Activity of Extracts from Aromatic Plants as Control Agents against Spoilage Molds Isolated from Sheep Cheese" Foods 10, no. 7: 1576. https://doi.org/10.3390/foods10071576
APA StyleMuñoz-Tebar, N., González-Navarro, E. J., López-Díaz, T. M., Santos, J. A., Elguea-Culebras, G. O. d., García-Martínez, M. M., Molina, A., Carmona, M., & Berruga, M. I. (2021). Biological Activity of Extracts from Aromatic Plants as Control Agents against Spoilage Molds Isolated from Sheep Cheese. Foods, 10(7), 1576. https://doi.org/10.3390/foods10071576