In Vitro Activity of Selected Phenolic Compounds against Planktonic and Biofilm Cells of Food-Contaminating Yeasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Phenolic Compounds
2.3. Growth Inhibition Activity and Calculation of Minimum Inhibitory Concentrations (MICs)
2.4. Biofilm Formation and Treatment
2.5. Adhesion Inhibition Assay
2.6. Crystal Violet Staining
2.7. Fluorescence Microscopy Studies
2.8. Statistical Analysis
3. Results
3.1. Influence of Phenolic Compounds on Yeast Growth
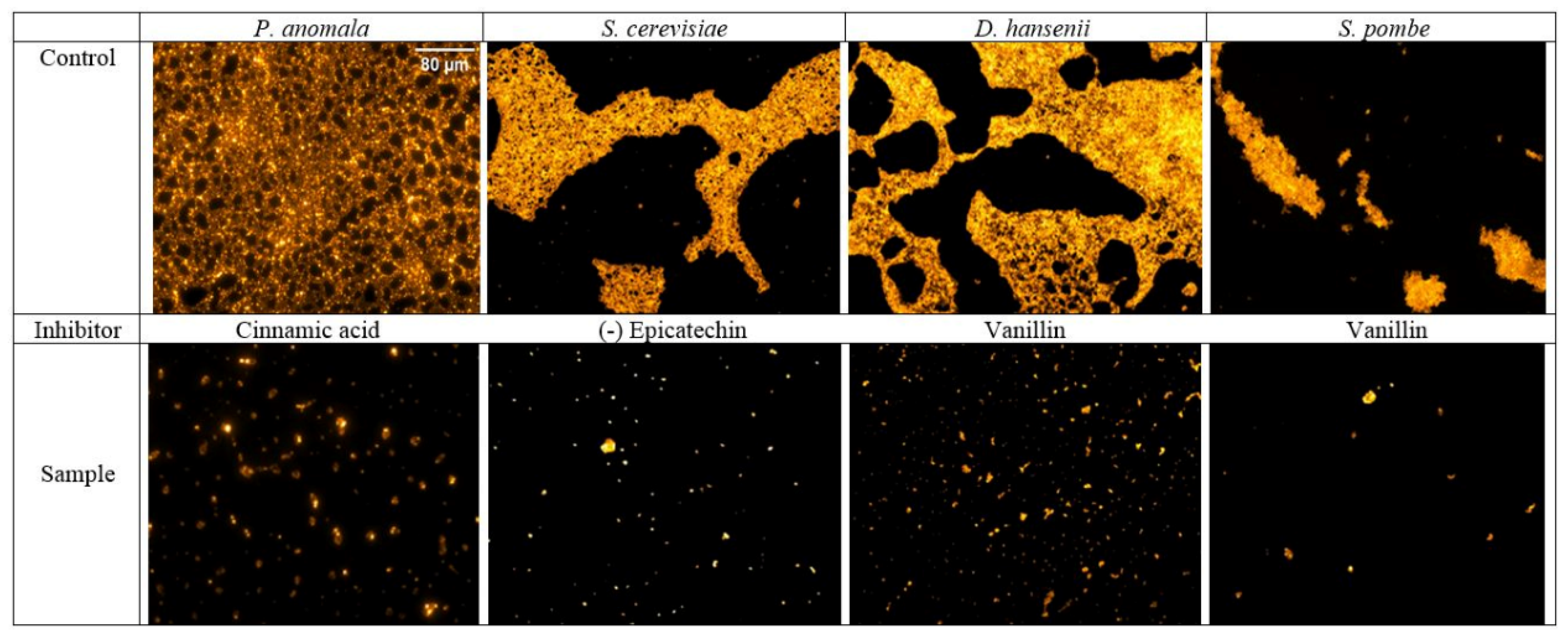
3.2. Influence of Phenolic Compounds on the Formation of Yeast Biofilms
3.3. Influence of Phenolic Compounds on Yeast Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawat, S. Food spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Krisch, J.; Chandrasekaran, M.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C. Latest about spoilage by yeasts: Focus on the deterioration of beverages and other plant-derived products. J. Food Prot. 2016, 79, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Shwaiki, L.N.; Arendt, E.K.; Lynch, K.M.; Thery, T.L. Inhibitory effect of four novel synthetic peptides on food spoilage yeasts. Int. J. Food Microbiol. 2019, 300, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kuanyshev, N.; Adamo, G.M.; Porro, D.; Branduardi, P. The spoilage yeast Zygosaccharomyces bailii: Foe or friend? Yeast 2017, 34, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhi, Y.; Wu, Q.; Du, R.; Xu, Y. Zygosaccharomyces bailii is a potential producer of various flavor compounds in Chinese Maotai-flavor liquor fermentation. Front. Microbiol. 2017, 8, 2609. [Google Scholar] [CrossRef]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.J.; Villalobos, M.C.; Martín, A.; Córdoba, M.G. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef]
- Zara, G.; Budroni, M.; Mannazzu, I.; Fancello, F.; Zara, S. Yeast biofilm in food realms: Occurrence and control. World J. Microbiol. Biotechnol. 2020, 36, 134. [Google Scholar] [CrossRef]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Phenylpropanoids of plant origin as inhibitors of biofilm formation by Candida albicans. J. Microbiol. Biotechnol. 2014, 24, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Huang, X.; Chen, J.; Han, B. Formation of a mixed-species biofilm is a survival strategy for unculturable lactic acid bacteria and Saccharomyces cerevisiae in Daqu, a Chinese traditional fermentation starter. Front. Microbiol. 2020, 11, 138. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evensen, N.A.; Braun, P.C. The effects of tea polyphenols on Candida albicans: Inhibition of biofilm formation and proteasome inactivation. Can. J. Microbiol. 2009, 55, 1033–1039. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Rahman, R.T.; Lou, Z.; Zhang, J.; Chen, W.; Azam, S.; Hossen, M.J. Biofilm inhibitor and its inhibition mechanism: Plant derived agents. Eur. Acad. Res. 2015, 3, 7748–7769. [Google Scholar]
- Zhong, Y.; Wu, L.; Chen, X.; Huang, Z.; Hu, W. Effects of food-additive-information on consumers’ willingness to accept food with additives. Int. J. Environ. Res. Public Health 2018, 15, 2394. [Google Scholar] [CrossRef] [Green Version]
- Babbel, J.; Ramos, C.; Wangberg, H.; Luskin, K.; Simon, R. Adverse reactions to food additives. J. Food Allergy 2021, 3, 8–23. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, A. Phenolics in foods: Extraction, analysis and measurements. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.D.R., Eds.; IntechOpen: London, UK, 2017; pp. 61–88. [Google Scholar]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Khubber, S.; Remize, F.; Tomasevic, I.; Roselló-Soto, E.; Barba, F.J. Obtaining antioxidants and natural preservatives from food by-products through fermentation: A review. Fermentation 2021, 7, 106. [Google Scholar] [CrossRef]
- Macé, S.; Hansen, L.T.; Rupasinghe, H.P.V. Anti-bacterial activity of phenolic compounds against Streptococcus pyogenes. Medicines 2017, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, C.; Kerekes, E.B.; Kotogán, A.; Papp, T.; Vágvölgyi, C.; Krisch, J.; Takó, M. Antimicrobial activity of grape, apple and pitahaya residue extracts after carbohydrase treatment against food-related bacteria. LWT Food Sci. Technol. 2019, 100, 416–425. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Narbad, A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food Microbiol. 2003, 86, 113–122. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska, L. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of phenolic antioxidants from grape, apple and pitahaya residues via solid state fungal fermentation and carbohydrase treatment. LWT Food Sci. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Park, C.; Imlay, J.A.; Park, W. 4-Hydroxybenzaldehyde sensitizes Acinetobacter baumannii to amphenicols. Appl. Microbiol. Biotechnol. 2018, 102, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Shinde, R.B.; Chauhan, N.M.; Karuppayil, S.M. Terpenoids of plant origin inhibit morphogenesis, adhesion, and biofilm formation by Candida albicans. Biofouling 2013, 29, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2009; pp. 357–382. [Google Scholar]
- Stratford, M.; Plumridge, A.; Archer, D.B. Decarboxylation of sorbic acid by spoilage yeasts is associated with the PAD1 gene. Appl. Environ. Microbiol. 2007, 73, 6534–6542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeboye, P.T.; Bettiga, M.; Olsson, L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 2014, 4, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.-P.; Olsson, L. Physiological responses to acid stress by Saccharomyces cerevisiae when applying high initial cell density. FEMS Yeast Res. 2016, 16, fow072. [Google Scholar] [CrossRef] [Green Version]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP 53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Kristan, K.; Bratkovic, T.; Sova, M.; Gobec, S.; Prezelj, A.; Urleb, U. Novel inhibitors of β-ketoacyl-ACP reductase from Escherichia coli. Chem. Biol. Interact. 2009, 178, 310–316. [Google Scholar] [CrossRef]
- Vilaça, R.; Mendes, V.; Mendes, M.V.; Carreto, L.; Amorim, M.A.; De Freitas, V.; Moradas-Ferreira, P.; Mateus, N.; Costa, V. Quercetin protects Saccharomyces cerevisiae against oxidative stress by inducing trehalose biosynthesis and the cell wall integrity pathway. PLoS ONE 2012, 7, e45494. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Arora, N.; Chatrath, A.; Gangwar, R.; Pruthi, V.; Poluri, K.M.; Prasad, R. Delineating the biofilm inhibition mechanisms of phenolic and aldehydic terpenes against Cryptococcus neoformans. ACS Omega 2019, 4, 17634–17648. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castillo, Y.; Lima, T.C.; Ferreira, A.R.; Silva, C.R.; Campos, R.S.; Neto, J.B.A.; Magalhães, H.I.F.; Cavalcanti, B.C.; Júnior, H.V.N.; de Sousa, D.P. Bioactivity and molecular docking studies of derivatives from cinnamic and benzoic acids. BioMed Res. Int. 2020, 2020, 6345429. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.D.C.O.; Gullo, F.P.; Freires, I.A.; de Souza Pitangui, N.; Segalla, M.P.; Fusco-Almeida, A.M.; Rosalen, P.L.; Regasini, L.O.; Mendes-Giannini, M.J.S. Synthesis, antifungal activity of caffeic acid derivative esters, and their synergism with fluconazole and nystatin against Candida spp. Diagn. Microbiol. Infect. Dis. 2016, 86, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.-M.; Abe, T.; Komiyama, T.; Wang, W.; Hattori, M.; Daneshtalab, M. Synthesis, anti-fungal and 1,3-β-d-glucan synthase inhibitory activities of caffeic and quinic acid derivatives. Bioorg. Med. Chem. 2010, 18, 7009–7014. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.; Baetz, K. Multi-faceted systems biology approaches present a cellular landscape of phenolic compound inhibition in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 539902. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Klis, F.M. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 2006, 60, 5–15. [Google Scholar] [CrossRef]
- Dranginis, A.M.; Rauceo, J.M.; Coronado, J.E.; Lipke, P.N. A biochemical guide to yeast adhesins: Glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 2007, 71, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Willaert, R.G. Adhesins of yeasts: Protein structure and interactions. J. Fungi 2018, 4, 119. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.; Kavanagh, K.; Velasco-Torrijos, T. Targeting adhesion in fungal pathogen Candida albicans. Future Med. Chem. 2021, 13, 313–334. [Google Scholar] [CrossRef]
- Glasenapp, Y.; Lucas, C.; Wöltje, T.; Fohrer, J.; Papenbrock, J. Anti-adhesion activity of tannins isolated from the Mangrove Laguncularia racemosa. Chem. Biodivers. 2019, 16, e1800632. [Google Scholar] [CrossRef]
- Martins, C.V.B.; Da Silva, D.L.; Neres, A.T.M.; Magalhaes, T.F.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; De Fátima, A.; De Resende, M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Chemical Group | Compound | Chemical Structure |
|---|---|---|
| Hydroxybenzoic acids | Vanillic acid | 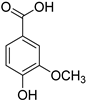 |
| Syringic acid |  | |
| Gallic acid | 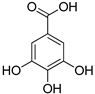 | |
| Protocatechuic acid | 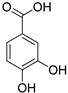 | |
| 4-Hydroxybenzoic acid | 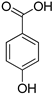 | |
| Hydroxycinnamic acids | Cinnamic acid |  |
| p-Coumaric acid |  | |
| Caffeic acid |  | |
| Ferulic acid | 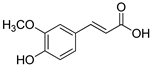 | |
| Stilbenes | Resveratrol | 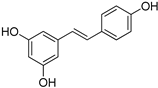 |
| Polydatin | 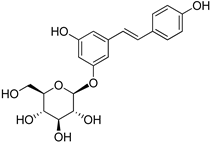 | |
| Flavonoids | (−)-Epicatechin | 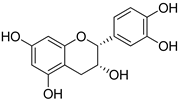 |
| Quercetin |  | |
| Phenolic aldehydes | Vanillin |  |
| 4-Hydroxybenzaldehyde | 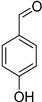 |
| Phenolic Compounds | MIC (mg/mL) | |||
|---|---|---|---|---|
| S. cerevisiae | S. pombe | D. hansenii | P. anomala | |
| Vanillic acid | >2 | >2 | >2 | >2 |
| Syringic acid | >2 | >2 | >2 | >2 |
| Gallic acid | >2 | >2 | >2 | >2 |
| Protocatechuic acid | >2 | >2 | >2 | >2 |
| 4-Hydroxybenzoic acid | >2 | >2 | >2 | >2 |
| Cinnamic acid | 0.5 | 0.5 | 0.5 | 0.5 |
| p-Coumaric acid | 2 | 2 | >2 | >2 |
| Caffeic acid | >2 | >2 | >2 | >2 |
| Ferulic acid | 2 | 2 | >2 | >2 |
| Resveratrol | >2 | >2 | 2 | >2 |
| Polydatin | >2 | >2 | >2 | >2 |
| (−)-Epicatechin | >2 | 2 | >2 | >2 |
| Vanillin | 2 | 2 | 1 | 1 |
| 4-Hydroxybenzaldehyde | >2 | >2 | >2 | 2 |
| Quercetin | 2 | >2 | >2 | >2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimani, B.G.; Kerekes, E.B.; Szebenyi, C.; Krisch, J.; Vágvölgyi, C.; Papp, T.; Takó, M. In Vitro Activity of Selected Phenolic Compounds against Planktonic and Biofilm Cells of Food-Contaminating Yeasts. Foods 2021, 10, 1652. https://doi.org/10.3390/foods10071652
Kimani BG, Kerekes EB, Szebenyi C, Krisch J, Vágvölgyi C, Papp T, Takó M. In Vitro Activity of Selected Phenolic Compounds against Planktonic and Biofilm Cells of Food-Contaminating Yeasts. Foods. 2021; 10(7):1652. https://doi.org/10.3390/foods10071652
Chicago/Turabian StyleKimani, Bernard Gitura, Erika Beáta Kerekes, Csilla Szebenyi, Judit Krisch, Csaba Vágvölgyi, Tamás Papp, and Miklós Takó. 2021. "In Vitro Activity of Selected Phenolic Compounds against Planktonic and Biofilm Cells of Food-Contaminating Yeasts" Foods 10, no. 7: 1652. https://doi.org/10.3390/foods10071652
APA StyleKimani, B. G., Kerekes, E. B., Szebenyi, C., Krisch, J., Vágvölgyi, C., Papp, T., & Takó, M. (2021). In Vitro Activity of Selected Phenolic Compounds against Planktonic and Biofilm Cells of Food-Contaminating Yeasts. Foods, 10(7), 1652. https://doi.org/10.3390/foods10071652








