Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparatiom of ECE
2.3. Cell Culture
2.4. Prostaglandin E2 (PGE2)
2.5. ROS Analysis
2.6. Western Blotting Analyses
2.7. Animals
2.8. Induction of Ligature-Induced Periodontitis
2.9. Micro-Computerized Tomography (Micro-CT)
2.10. Quantitative Real-Time PCR (qRT-PCR)
2.11. Statistical Analysis
3. Results and Discussion
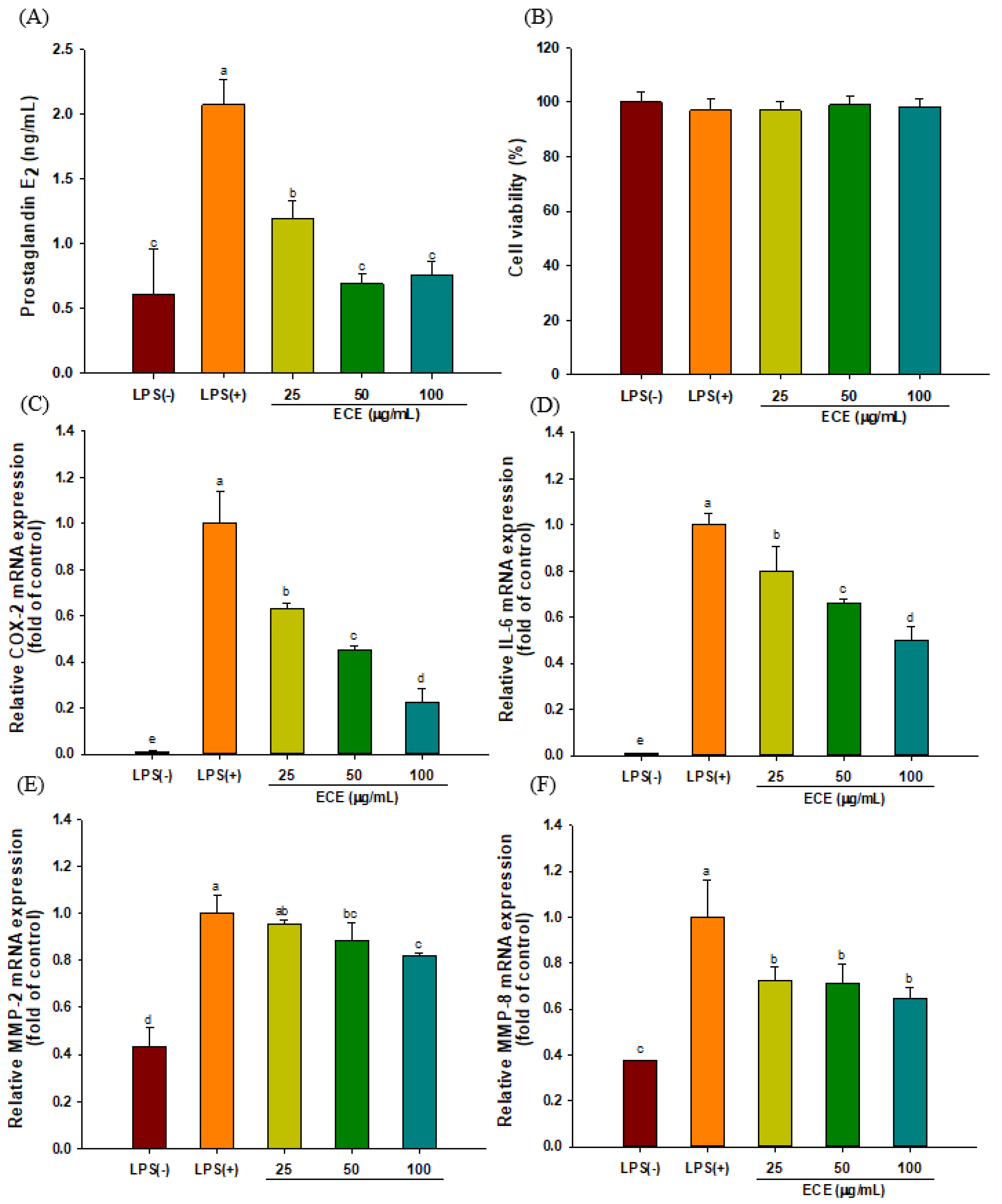
3.1. ECE Decreases PGE2 Production and Pro-inflammatory Enzyme Expression in LPS-Stimulated HGF-1 Cells
3.2. ECE Lowers Pro-Inflammatory Chemokine Gene Expressions in LPS-stimulated HGF-1 Cells
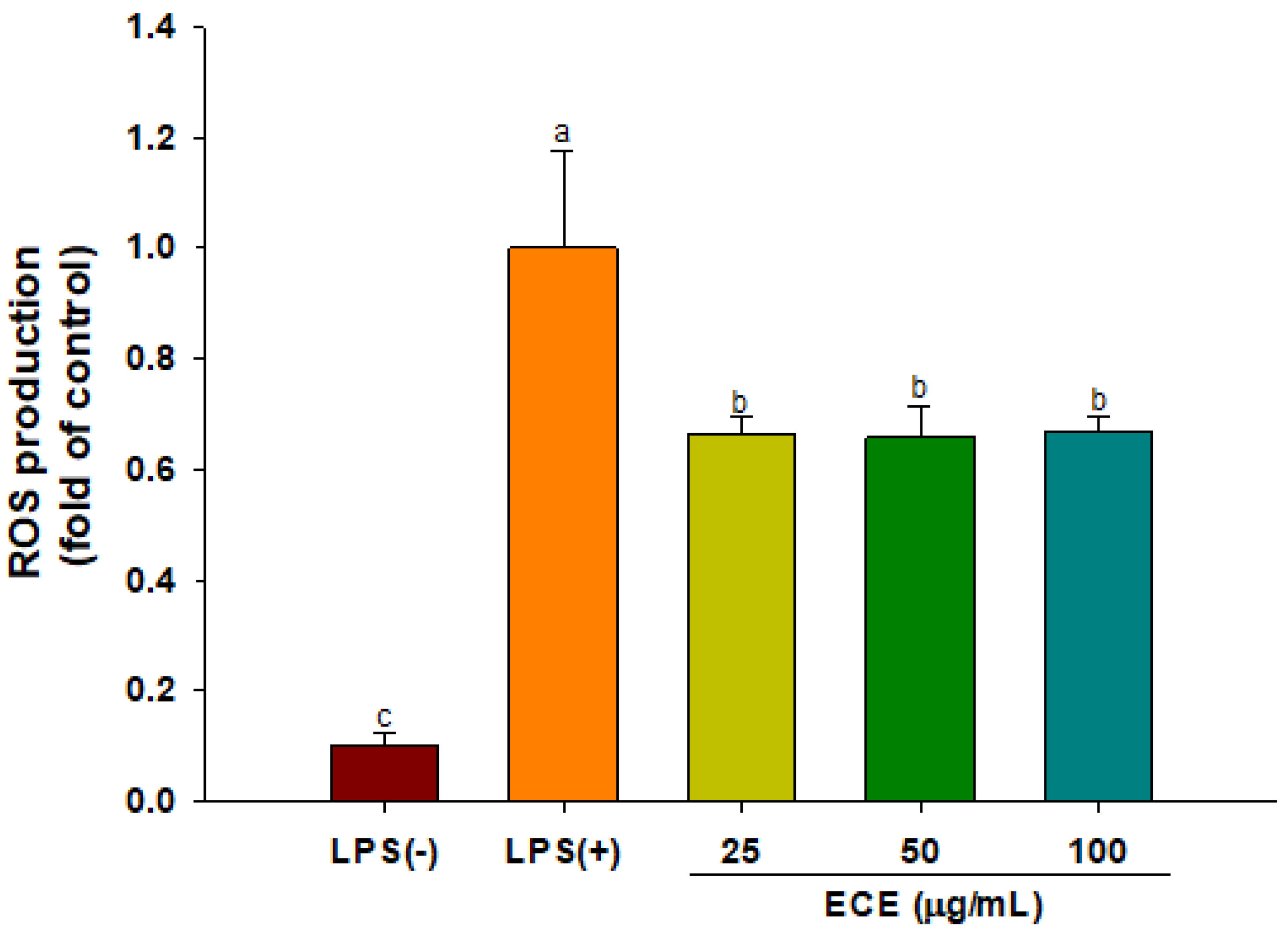
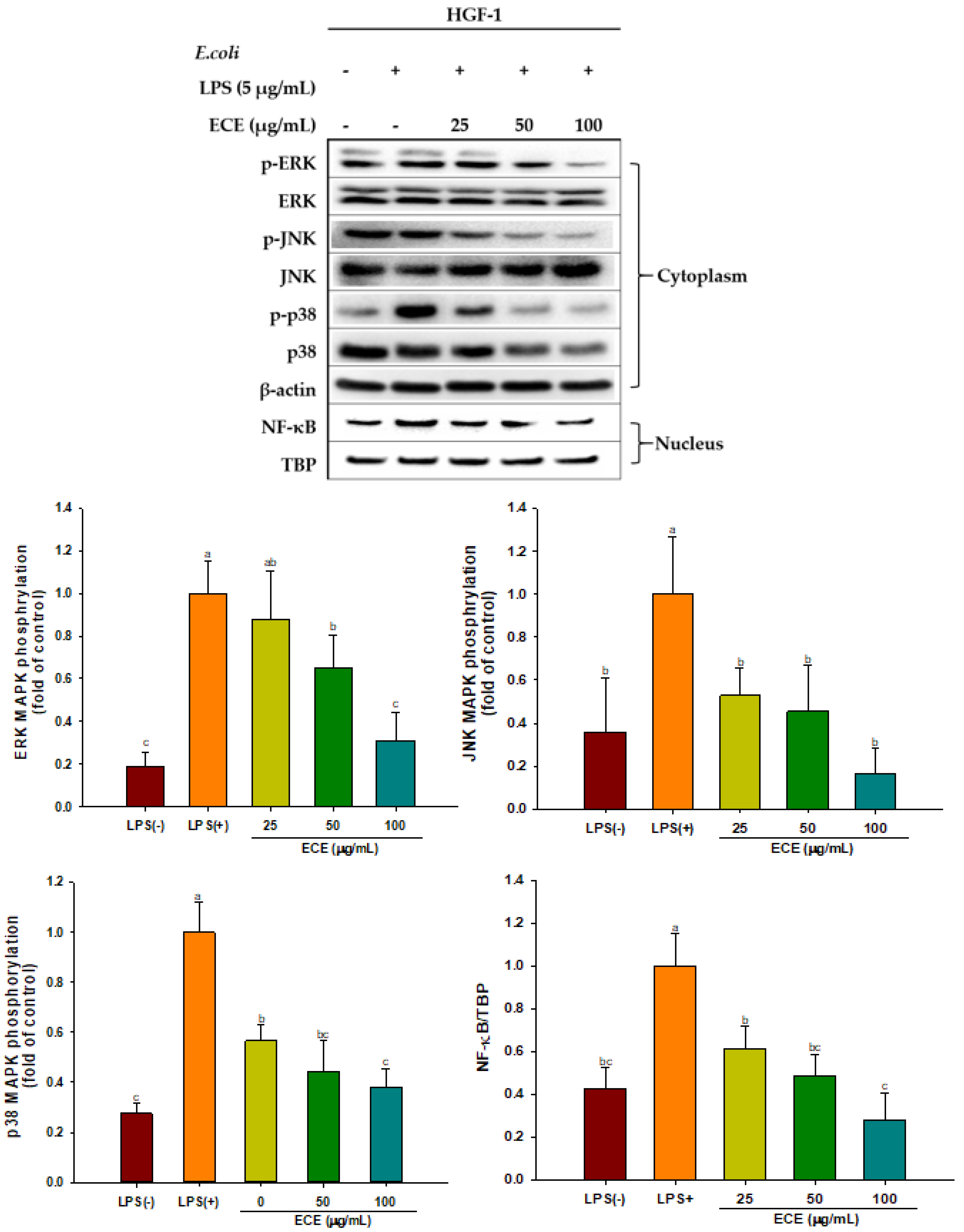
3.3. ECE Suppresses ROS Production and MAPK Signaling in LPS-Stimulated HGF-1 Cells
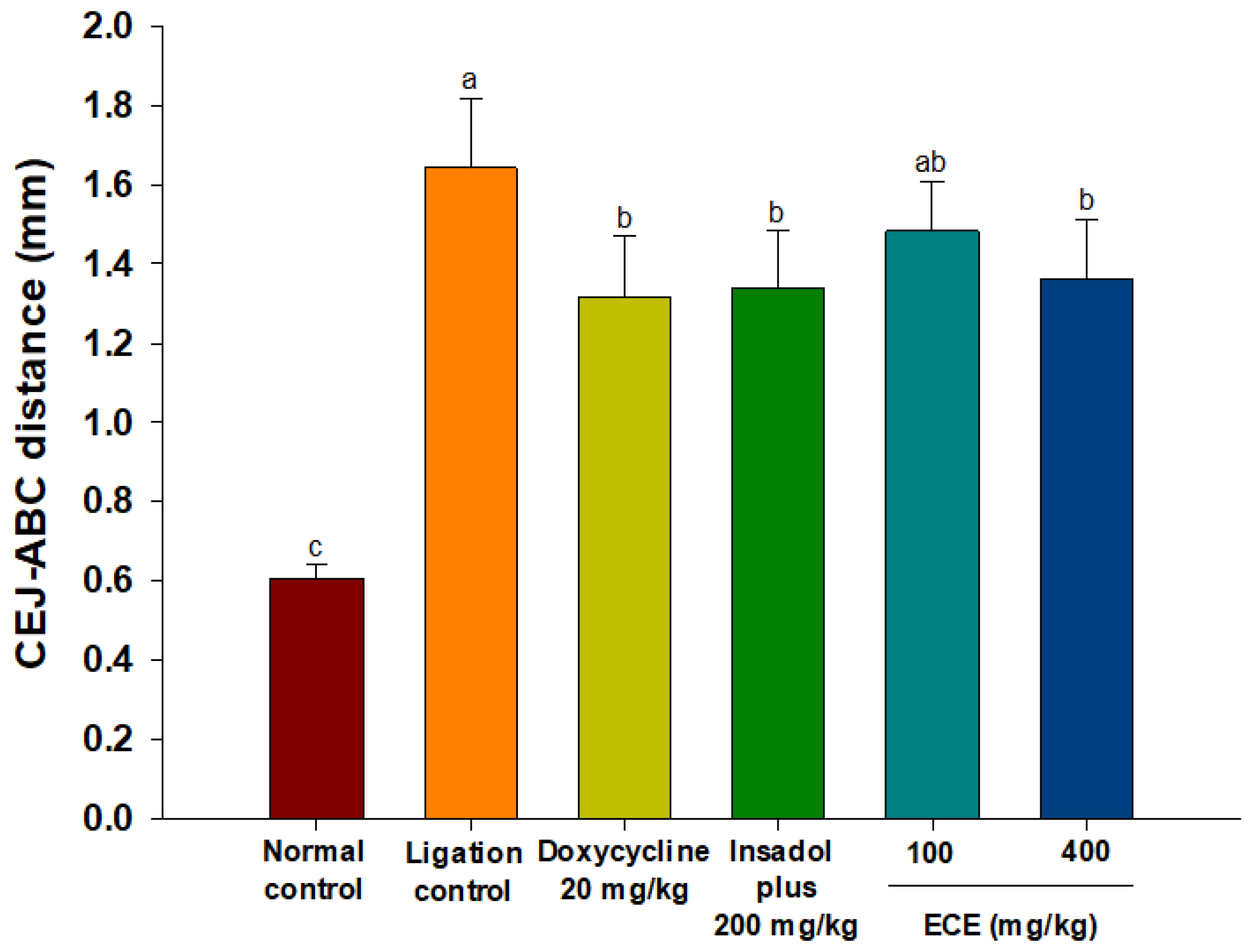
3.4. ECE Improves Alveolar Bone Loss in Ligatured-induced Periodontitis in Rats
3.5. ECE Lowers Elevated RANKL/OPG Gene Expressions in Gingival Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontology 2000, 69, 7–17. [Google Scholar] [CrossRef]
- Shaddox, L.M.; Gonçalves, P.F.; Vovk, A.; Allin, N.; Huang, H.; Hou, W.; Wallet, S.M. LPS-induced inflammatory response after therapy of aggressive periodontitis. J. Dent. Res. 2013, 92, 702–708. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Shu, R.; Li, C.L.; Zhang, M.Z. Gram-negative periodontal bacteria induce the activation of toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J. Periodontol. 2010, 81, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Akira, S. Pillars. Toll-like receptor 4 (TLR4)-deficient mice are hyperesponsive to lipopolysaccharide: Evidence for TLR4 as the lps gene product. J. Immunol. 2016, 197, 2563–2566. [Google Scholar] [PubMed]
- Yucel-Lindberg, T.; Bage, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2000, 84, 45–68. [Google Scholar] [CrossRef]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Wang, P.L. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef]
- Bartold, P.M.; Haynes, D.R. Interleukin—6 production by human gingival fibroblasts. J. Periodontal Res. 1991, 26, 339–345. [Google Scholar] [CrossRef]
- Wang, P.L.; Ohura, K. Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts–CD14 and Toll-like receptors. Crit. Rev. Oral Biol. Med. 2002, 13, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Soares, D.J.; Walker, J.; Pignitter, M.; Walker, J.M.; Imboeck, J.M.; Ehrnhoefer-Ressler, M.M.; Brasil, I.M.; Somoza, V. Pitanga (Eugenia uniflora L.) fruit juice and two major constituents thereof exhibit anti-inflammatory properties in human gingival and oral gum epithelial cells. Food Funct. 2014, 5, 2981–2988. [Google Scholar] [CrossRef]
- Chowdhury, M.T.H.; Bangoura, I.; Kang, J.-Y.; Park, N.G.; Ahn, D.-H.; Hong, Y.-K. Distribution of phlorotannins in the brown Alga Ecklonia cava and comparison of pretreatments for extraction. Fish. Aquat. Sci. 2011, 14, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Kang, M.-C.; Ahn, G.; Yang, X.; Kim, K.-N.; Kang, S.-M.; Lee, S.-H.; Ko, S.-C.; Ko, J.-Y.; Kim, D.; Kim, Y.-T.; et al. Hepatoprotective effects of dieckol-rich phlorotannins from Ecklonia cava, a brown seaweed, against ethanol induced liver damage in BALB/c mice. Food Chem. Toxicol. 2012, 50, 1986–1991. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.S.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Ecklonia cava extract containing dieckol suppresses RANKL-induced osteoclastogenesis via MAP kinase/NF-κB pathway inhibition and heme oxygenase-1 induction. J. Microbiol. Biotechnol. 2019, 29, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Choi, S.I.; Kim, G.H.; Imm, J.Y. Anti-inflammatory effect of Ecklonia cava extract on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages and a periodontitis rat model. Nutrients 2019, 11, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.-I.; Baek, S.-M.; Nguyen, T.H.; Kim, J.W.; Kang, C.-H.; Kim, S.; Imm, J.-Y. Effects of probiotic culture supernatant in cariogenic biofilm formation and RANKL-induced osteoclastogenesis in RAW 264.7 macrophages. Molecules 2021, 26, 733. [Google Scholar] [CrossRef]
- Kim, J.G.; Lim, D.W.; Cho, S.; Han, D.; Kim, Y.T. The edible brown seaweed Ecklonia cava reduces hypersensitivity in postoperative and neuropathic pain models in rats. Molecules 2014, 19, 7669–7678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neatea, M.G.; van Deuren, M.; Kullberg, B.J.; Cavaillon, J.M.; Van der Meer, J.W. Does the shape of lipid a determine the interaction of LPS with toll-like receptors? Trends Immunol. 2002, 23, 135–139. [Google Scholar] [CrossRef]
- Ara, T.; Fujinami, Y.; Imamura, Y.; Wang, P.-L. Lipopolysaccharide-treated human gingival fibroblasts continuously produce PGE2. J. Hard Tissue Biol. 2008, 17, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, K.; Ishikawa, I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontology 2000, 43, 85–101. [Google Scholar] [CrossRef]
- Campi, P.; Herrera, B.S.; de Jesus, F.N.; Napolitano, M.; Teixeira, S.A.; Maia-Dantas, A.; Spolidorio, L.C.; Akamine, E.H.; Mayer, M.P.A.; de Carvalho, M.H.C.; et al. Endothelial dysfunction in rats with ligature-induced periodontitis; participation of nitric oxide and cyclooxygenase-2-derived products. Arch. Oral Biol. 2016, 63, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, C.M., Jr.; Pacheco, C.M.F.; Maltos, K.L.M.; Caliari, M.V.; Duarte, D.G.; Francischi, J.N. Role of systemic and local administration of selective inhibitors of cyclooxygenase 1 and 2 in an experimental model of periodontal disease in rats. J. Periodontal Res. 2009, 44, 133–160. [Google Scholar]
- Ha, J.W.; Song, H.; Hong, S.S.; Boo, Y.C. Marine alga Ecklonia cava extract and dieckol attenuate prostaglandin E2 production in HaCaT keratinocytes exposed to airborne particulate matter. Antioxidants 2019, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Irwin, C.R.; Myrillas, T.T. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998, 4, 43–47. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.M.; Kim, S.K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Kähäri, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Tervahartiala, T.; Pirila, E.; Ceponis, A.; Maisi, P.; Salo, T.; Tuter, G.; Kallio, P.; Tornwall, J.; Srinivas, R.; Konttinen, Y.T.; et al. The in vivo expressionof the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 2000, 79, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix metalloproteinases as regulators of periodontal inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.A.; Garlet, G.P.; Fukada, S.Y.; Silva, J.S.; Cunha, F.Q. Chemokines in oral inflammatory diseases: Apical periodontitis and periodontal disease. J. Dent. Res. 2007, 86, 306–319. [Google Scholar] [CrossRef]
- Tsubaki, M.; Kato, C.; Manno, M.; Ogaki, M.; Satou, T.; Itoh, T.; Kusunoki, T.; Tanimori, Y.; Fujiwara, K.; Matsuoka, H.; et al. Macrophage inflammatory protein-1α (MIP-1α) enhances a recptor activator of nuclear factor κB ligand (RANKL) expression in mouse bone marrow stromal cells and osteoblasts through MAPK and PI3K/Akt pathways. Mol. Cell Biochem. 2007, 304, 53–60. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, D.; Chen, O.; Zhang, Z.; Xue, J.; Huang, S.; Wang, Y. Upregulation of CCL3/MIP-1alpha regulated by MAPKs and NF-kappaB mediates microglial inflammatory response in LPS-induced brain injury. Acta Neurobiol. Exp. 2016, 76, 304–317. [Google Scholar] [CrossRef] [Green Version]
- Werner, L.; Guzner-Gur, H.; Dotan, I. Involvement of CXCR4/CXCR7/CXCL12 Interactions in Inflammatory bowel disease. Theranostics 2013, 3, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Zhu, Y.Q.; Du, R.; Gu, Y.X.; Xia, L.; Qin, F.; Ritchie, H.H. The expression and role of stromal cell–derived factor-1α–CXCR4 axis in human dental pulp. J. Endod. 2008, 34, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, A. CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted therapy. Immunol. Lett. 2020, 217, 91–115. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, P.; Cui, N.; Song, S.; Liang, H.; Ji, A. Sulfated polysaccharide isolated from the sea cucumber Stichopus japonicas promotes the SDF-1α/CXCR4 axis-induced NSC migration via the PI3K/Akt/FOXO3a, ERK/MAPK, and NF-κB signaling pathways. Neurosci. Lett. 2016, 616, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; Cristino, S.; Toneguzzi, S.; Piacentini, A.; Facchino, A.; Lisgnoli, G. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL 12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J. Cell. Physiol. 2004, 199, 244–251. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Zhu, Z.; Xiao, A.; Yu, H.; Gan, X. Blockade of cyclophilin D rescues dexamethasone-induced oxidative stress in gingival tissue. PLoS ONE 2017, 12, e0173270. [Google Scholar] [CrossRef]
- Ko, S.C.; Cha, S.H.; Heo, S.J.; Lee, S.H.; Kang, S.M.; Jeon, Y.J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Lipopolysaccharide-regulated production of bone sialoprotein and interleukin-8 in human periodontal ligament fibroblasts: The role of toll-like receptors 2 and 4 and the MAPK pathway. J. Periodontal Res. 2015, 50, 141–151. [Google Scholar] [CrossRef]
- Yang, G.; Chang, C.-C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.T.; Li, S. Resveratrol alleviates rheumatoid artritis via reducing ROS and inflammation, inhibiting MAPK signaling pathways, and suppressing angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef]
- Lee, S.; Youn, K.; Kim, D.H.; Ahn, M.R.; Yoon, E.; Kim, O.Y.; Jun, M. Anti-neuroinflammatory property of phlorotannins from Ecklonia cava on Aβ25-35-induced damage in PC12 cells. Mar. Drugs 2019, 17, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.R.; Park, H.Y.; Kim, Y.; Lee, K.W. Methylglyoxal-derived advanced glycation end products induce matrix metalloproteinases through activation of ERK/JNK/NF-κB pathway in kidney proximal epithelial cells. Food Sci. Biotechnol. 2020, 29, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S., Jr. The NF-κB and I kappaB proteins: New discoveries and insights. Annu. Rev. Immunol. 1996, 14, 649–683. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, I.-S.; More, S.V.; Kim, B.-W.; Choi, D.-K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef]
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, I.W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- Brunetti, G.; Rosa, G.D.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan maintenance and prevention of Parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [Green Version]
- Kanner, J. Polyphenols by generating H2O2 affect cell redox signaling, inhibit PTPs and activate Nrf2axis for adaptation and cell surviving: In vitro, in vivo and human health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef]
- Graves, D.T.; Fine, D.; Teng, Y.T.A.; Van Dyke, T.E.; Hajishengallis, G. The use of rodent models to investigate host–bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 2013, 394, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Investig. 2014, 44, 1000–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.; Shim, M.; Son, M.; Jang, J.T.; Son, K.H.; Byun, K. Attenuating effects of dieckol on endothelial cell dysfunction via modulation of Th17/Treg balance in the intestine and aorta of spontaneously hypertensive rats. Antioxidants 2021, 10, 298. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N. The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 2012, 39, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.T.A.; Nguyen, H.; Gao, X.; Kong, Y.Y.; Gorczynski, R.M.; Singh, B.; Penninger, J.M. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 2000, 106, R59–R67. [Google Scholar] [CrossRef] [Green Version]
- Wara-aswapati, N.; Surarit, R.; Chayasadom, A.; Boch, J.A.; Pitiphat, W. RANKL upregulation associated with periodontitis and Porphyromonas gingivalis. J. Peridontol. 2007, 78, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.-S.; Sun, L.-J.; Qu, H.-L.; Chen, F.; Tian, B.-M.; Chen, F.-M. The relationship between T-helper cell polarization and the RANKL/OPG ratio in gingival tissues from chronic periodontitis. Clin. Exp. Dent. Res. 2019, 5, 377–388. [Google Scholar] [PubMed] [Green Version]
- Ribeiro, F.V.; Pino, D.S.; Franck, F.C.; Benatti, B.B.; Tenenbaum, H.; Davies, J.E.; Pimentel, S.P.; Casarin, R.C.; Cirano, F.R.; Casati, M.Z. Resveratrol inhibits periodontitis related bone loss in rats subjected to cigarette smoke inhalation. J. Periodontol. 2017, 88, 788–798. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.-I.; Kim, S.; Baek, S.-M.; Choi, S.-I.; Kim, G.-H.; Imm, J.-Y. Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines. Foods 2021, 10, 1656. https://doi.org/10.3390/foods10071656
Jung J-I, Kim S, Baek S-M, Choi S-I, Kim G-H, Imm J-Y. Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines. Foods. 2021; 10(7):1656. https://doi.org/10.3390/foods10071656
Chicago/Turabian StyleJung, Jae-In, Seonyoung Kim, Seung-Min Baek, Soo-Im Choi, Gun-Hee Kim, and Jee-Young Imm. 2021. "Ecklonia cava Extract Exerts Anti-Inflammatory Effect in Human Gingival Fibroblasts and Chronic Periodontitis Animal Model by Suppression of Pro-Inflammatory Cytokines and Chemokines" Foods 10, no. 7: 1656. https://doi.org/10.3390/foods10071656





