Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.1.1. Sample Preparation
2.1.2. Conventional Solid–Liquid Extraction (CSLE)
2.1.3. Ultrasound-Assisted Extraction (UAE)
2.1.4. Total Phenolic Compounds (TPC) Determination
2.1.5. The Total Flavonoid Content (TFC) Determination
2.1.6. The Condensed Tannin Content (CTC) Determination
2.1.7. Kinetic Modeling for the Extraction Methods
- All particles were sphere-shaped with a uniform size.
- The solute component was uniformly distributed in the matrix.
- The diffusion coefficient of the solute components was constant.
- Solid particles were well distributed in the extracting solvent.
2.1.8. Characterization of the Extracts by UHPLC-ESI/QTOF Mass Spectrometry
2.2. Statistical Analysis
3. Results and Discussion
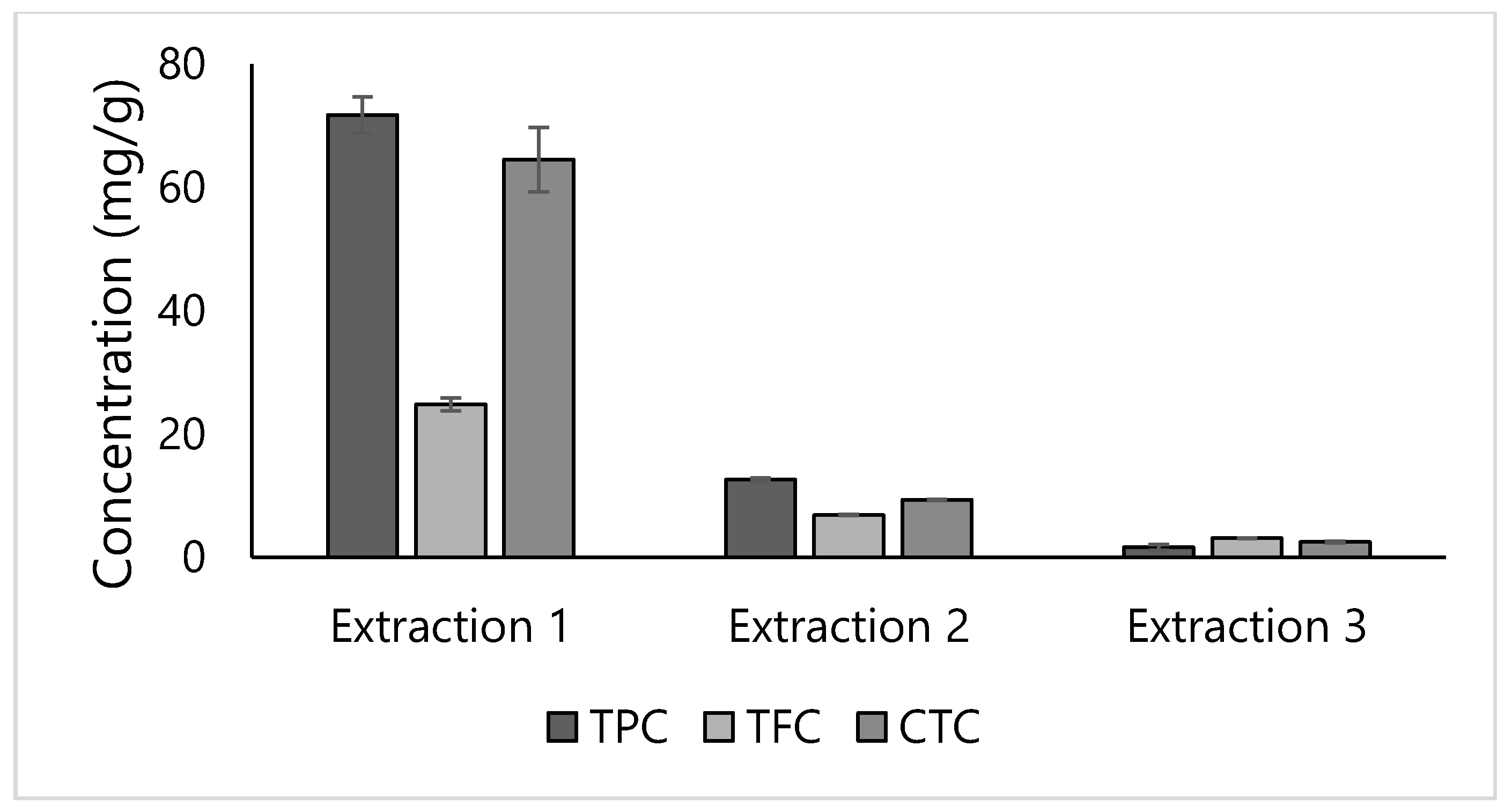
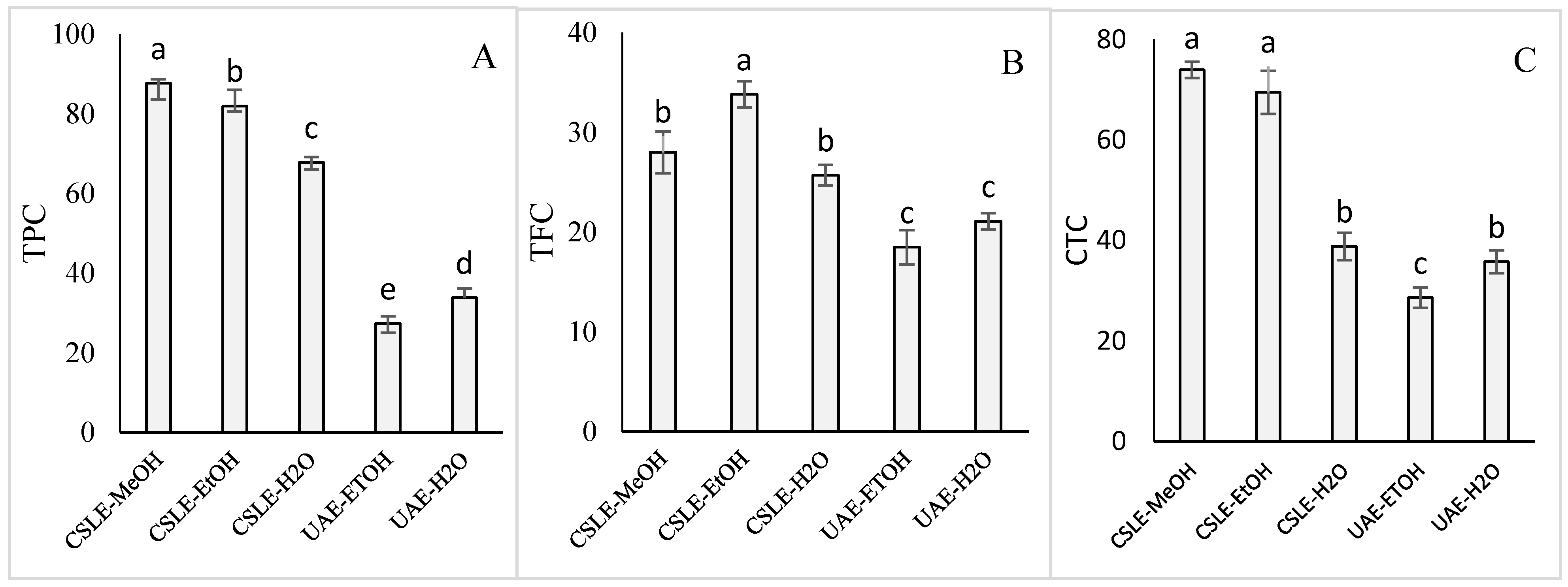
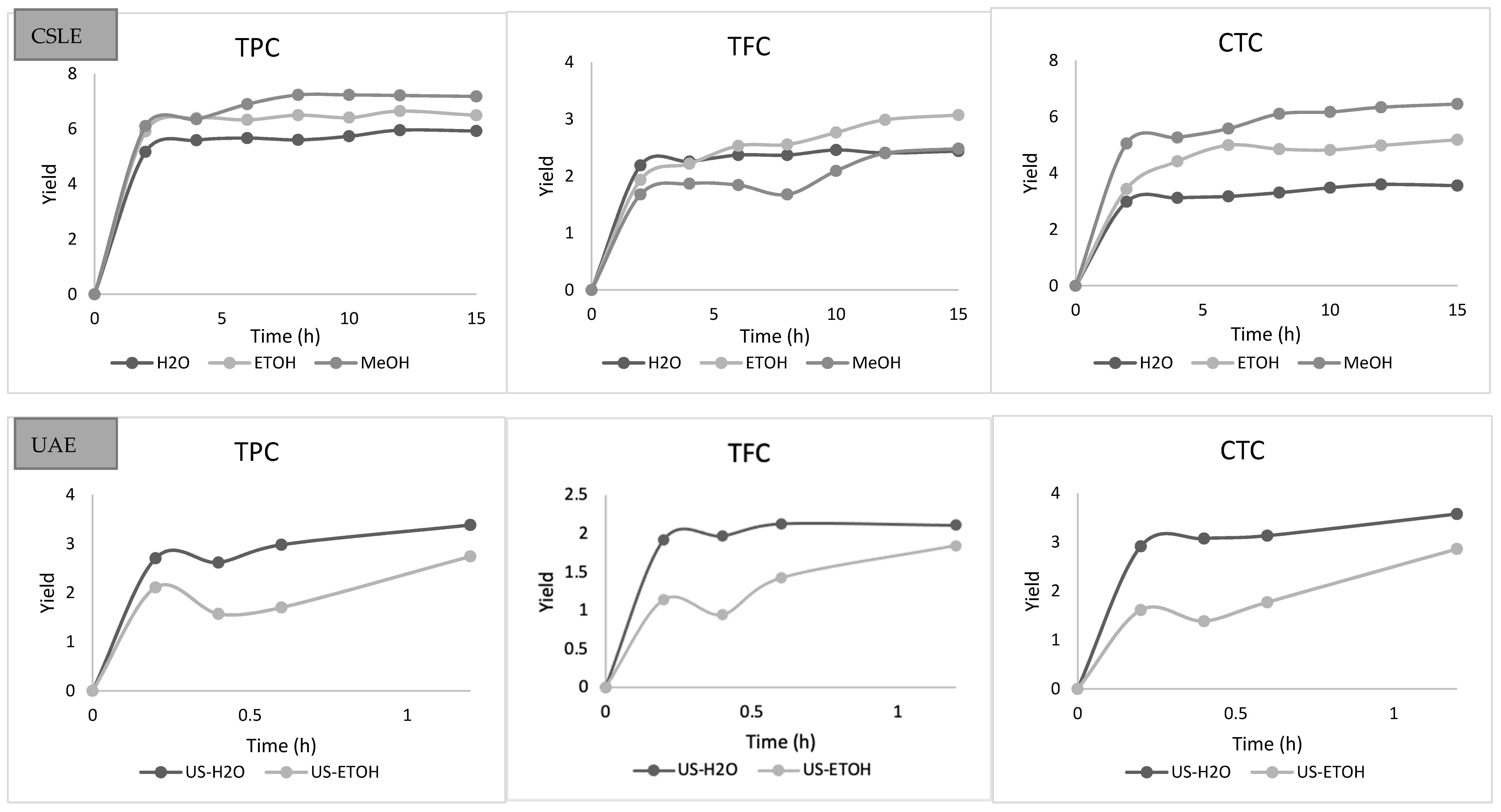
3.1. Conventional Solid-Liquid Extraction and Ultrasound-Assisted Extraction
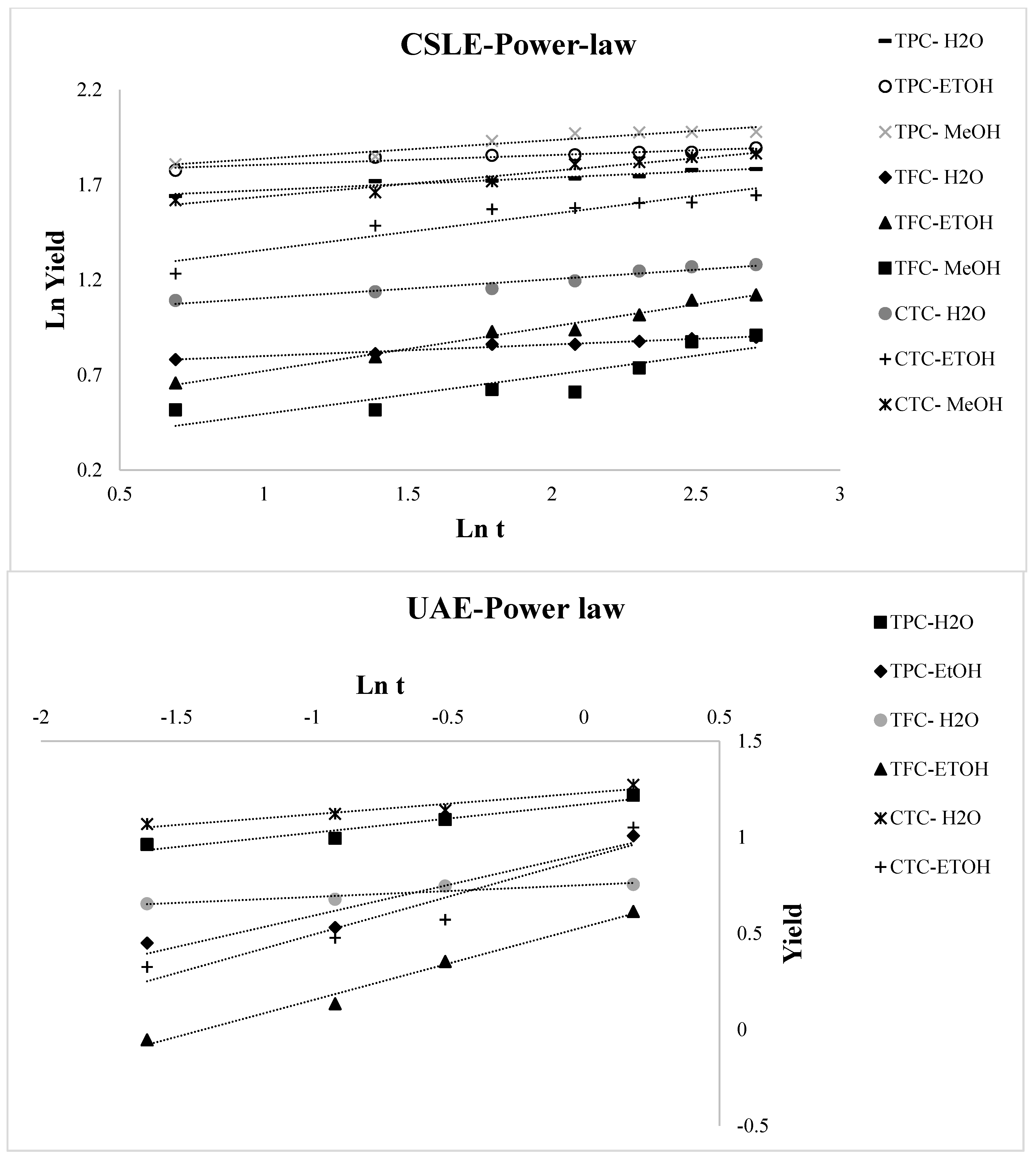
3.2. Kinetic Modeling of the Extraction Process
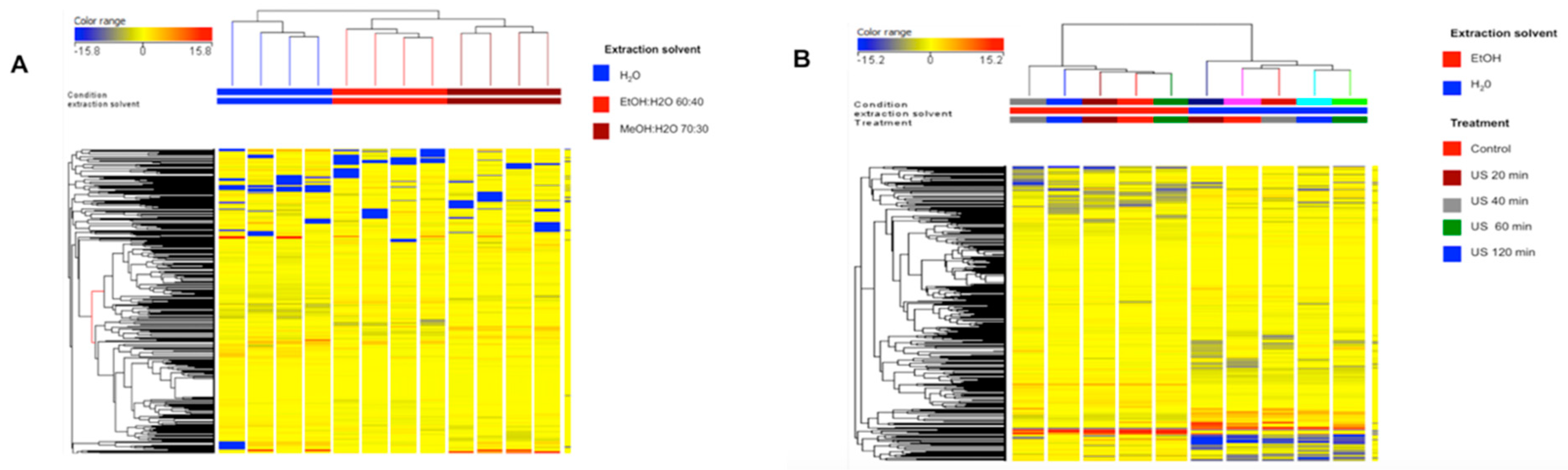
3.3. Characterization of the Extracts by UHPLC-ESI/QTOF Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional Quality of Legumes, and Their Role in Cardiometabolic Risk Prevention: A Review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.; Panozzo, J. Phytochemicals for health, the role of pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, D.M.; Vandenberg, A.; Glahn, R.P. Seed Coat Removal Improves Iron Bioavailability in Cooked Lentils: Studies Using an in Vitro Digestion/Caco-2 Cell Culture Model. J. Agric. Food Chem. 2013, 61, 8084–8089. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I. Phenolic composition of the cotyledon and the seed coat of lentils (Lens culinaris L.). Eur. Food Res. Technol. 2002, 215, 478–483. [Google Scholar] [CrossRef]
- Oomah, B.D.; Caspar, F.; Malcolmson, L.J.; Bellido, A.-S. Phenolics and antioxidant activity of lentil and pea hulls. Food Res. Int. 2011, 44, 436–441. [Google Scholar] [CrossRef]
- Yeo, J.; Shahidi, F. Identification and quantification of soluble and insoluble-bound phenolics in lentil hulls using HPLC-ESI-MS/MS and their antioxidant potential. Food Chem. 2020, 315, 126202. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Roy, D.; Wahab, M.F.; Talebi, M.; Armstrong, D.W. Replacing methanol with azeotropic ethanol as the co-solvent for improved chiral separations with supercritical fluid chromatography (SFC). Green Chem. 2020, 22, 1249–1257. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018, 258, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Gharibzahedi, S.M.T. Mathematical Modeling of Mucilage Extraction Kinetic from the Waste Hydrolysates of Fruiting Bodies of Zizyphus jujuba Mill. J. Food Process. Preserv. 2017, 41, e13064. [Google Scholar] [CrossRef]
- Gani, A.; Prasad, K.; Ahmad, M.; Gani, A. Time-dependent extraction kinetics of infused components of different Indian black tea types using UV spectroscopy. Cogent Food Agric. 2016, 2, 1137157. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Agu, C.M.; Udeigwe, T.K. Kinetic and parametric studies for the extractive synthesis of oil from Terminalia catappa L. kernel. React. Kinet. Mech. Catal. 2016, 120, 129–147. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water ultrasound-assisted extraction of polyphenol compounds from brewer’s spent grain: Kinetic study, extract characterization, and concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Peleg, M. An Empirical Model for the Description of Moisture Sorption Curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Piwowarska, N.; González-Alvarez, J. Extraction of antioxidants from forestry biomass: Kinetics and optimization of extraction conditions. Biomass Bioenergy 2012, 43, 42–51. [Google Scholar] [CrossRef]
- Jokic, S.; Velic, D.; Bilic, M.; Bucic-Kojic, A.; Planinic, M.; Tomas, S. Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech J. Food Sci. UZEI 2010, 208, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Mourtzinos, I.; Makris, D.P. Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Int. J. Ind. Chem. 2015, 6, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Simeonov, E.; Koleva, V. Solid-liquid extraction of tannins from Geranium sanguineum L.—Experimental kinetics and modelling. Chem. Biochem. Eng. Q. 2012, 26, 249–255. [Google Scholar]
- Putnik, P.; Bursać Kovačević, D.; Radojčin, M.; Dragović-Uzelac, V. Influence of acidity and extraction time on the recovery of flavonoids from grape skin pomace optimized by response surface methodology. Chem. Biochem. Eng. Q. 2016, 30, 455–464. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Caruso, M.C.; Galgano, F.; Grippo, A.; Condelli, N.; Di Cairano, M.; Tolve, R. Assay of healthful properties of wild blackberry and elderberry fruits grown in Mediterranean area. J. Food Meas. Charact. 2019, 13, 1591–1598. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Gallo, A.; Masoero, F.; Trevisan, M. Phenolic profile and fermentation patterns of different commercial gluten-free pasta during in vitro large intestine fermentation. Food Res. Int. 2017, 97, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, R.; Broadhurst, D.; Smilde, A.K.; Kristal, B.S.; Baker, J.D.; Beger, R.; Bessant, C.; Connor, S.; Capuani, G.; Craig, A.; et al. Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 2007, 3, 231–241. [Google Scholar] [CrossRef]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Martinez-Correa, H.A.; Cabral, F.; Magalhães, P.M.; Queiroga, C.L.; Godoy, A.T.; Sánchez-Camargo, A.P.; Paviani, L.C. Extracts from the leaves of Baccharis dracunculifolia obtained by a combination of extraction processes with supercritical CO2, ethanol and water. J. Supercrit. Fluids 2012, 63, 31–39. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Cabral, F. Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2015, 99, 68–75. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Tan, S.P.; Parks, S.E.; Stathopoulos, C.E.; Roach, P.D. Extraction of Flavonoids from Bitter Melon. Food Nutr. Sci. 2014, 05, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of ultrasound-assisted extraction conditions for recovery of phenolic compounds and antioxidant capacity from banana (Musa cavendish) peel. J. Food Process. Preserv. 2017, 41, e13148. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacogn. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, D.; Bao, C.; You, J.; Wang, Z.; Shi, Y.; Zhang, H. Ultrasonic extraction and separation of anthraquinones from Rheum palmatum L. Ultrason. Sonochemistry 2008, 15, 738–746. [Google Scholar] [CrossRef]
- Menkiti, M.; Agu, C.M.; Agu, I.E. Extraction Kinetics and Physicochemical Studies of Terminalia catappa L. Kernel Oil Utilization Potential. Iran. J. Chem. Chem. Eng. 2019, 38, 223–243. [Google Scholar]
- Kriaa, K.; Hadrich, B. A New Modeling Approach of Ultrasonic Extraction of Polyphenols from Carob. J. Pharm. Innov. 2018, 14, 141–151. [Google Scholar] [CrossRef]
- Paunovic, D.Đ.; Mitić, S.S.; Kostic, D.A.; Mitić, M.M.; Stojanović, B.T.; Pavlović, J.L. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols from barley. Savrem. Teh. 2014, 3, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Tušek, A.J.; Benković, M.; Cvitanović, A.B.; Valinger, D.; Jurina, T.; Kljusurić, J.G. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind. Crop. Prod. 2016, 91, 205–214. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process. Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochemistry 2016, 32, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kitanović, S.; Milenović, D.; Veljković, V.B. Empirical kinetic models for the resinoid extraction from aerial parts of St. John’s wort (Hypericum perforatum L.). Biochem. Eng. J. 2008, 41, 1–11. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic Extracts from Wild Olive Leaves and Their Potential as Edible Oils Antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Sinclair, G.W.; Peppas, N.A. Analysis of non-fickian transport in polymers using simplified exponential expressions. J. Membr. Sci. 1984, 17, 329–331. [Google Scholar] [CrossRef]
- Kashaninejad, M.; Sanz, M.; Blanco, B.; Beltrán, S.; Niknam, S. Freeze dried extract from olive leaves: Valorisation, extraction kinetics and extract characterization. Food Bioprod. Process. 2020, 124, 196–207. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Foods 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Yanık, D.K.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a potential source of phenolic compounds: Evaluation of ethanol–water binary solvent extraction on antioxidant activity and phenolic content of pistachio hull extracts. Waste Biomass Valoriz. 2020, 11, 2101–2110. [Google Scholar] [CrossRef]
- Aguilar-Hernández, G.; García-Magaña, M.D.L.; Vivar-Vera, M.D.L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A.; Morales-Castro, J.; Anaya-Esparza, L.M.; González, E.M. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds from Annona muricata by-Products and Pulp. Molecules 2019, 24, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]

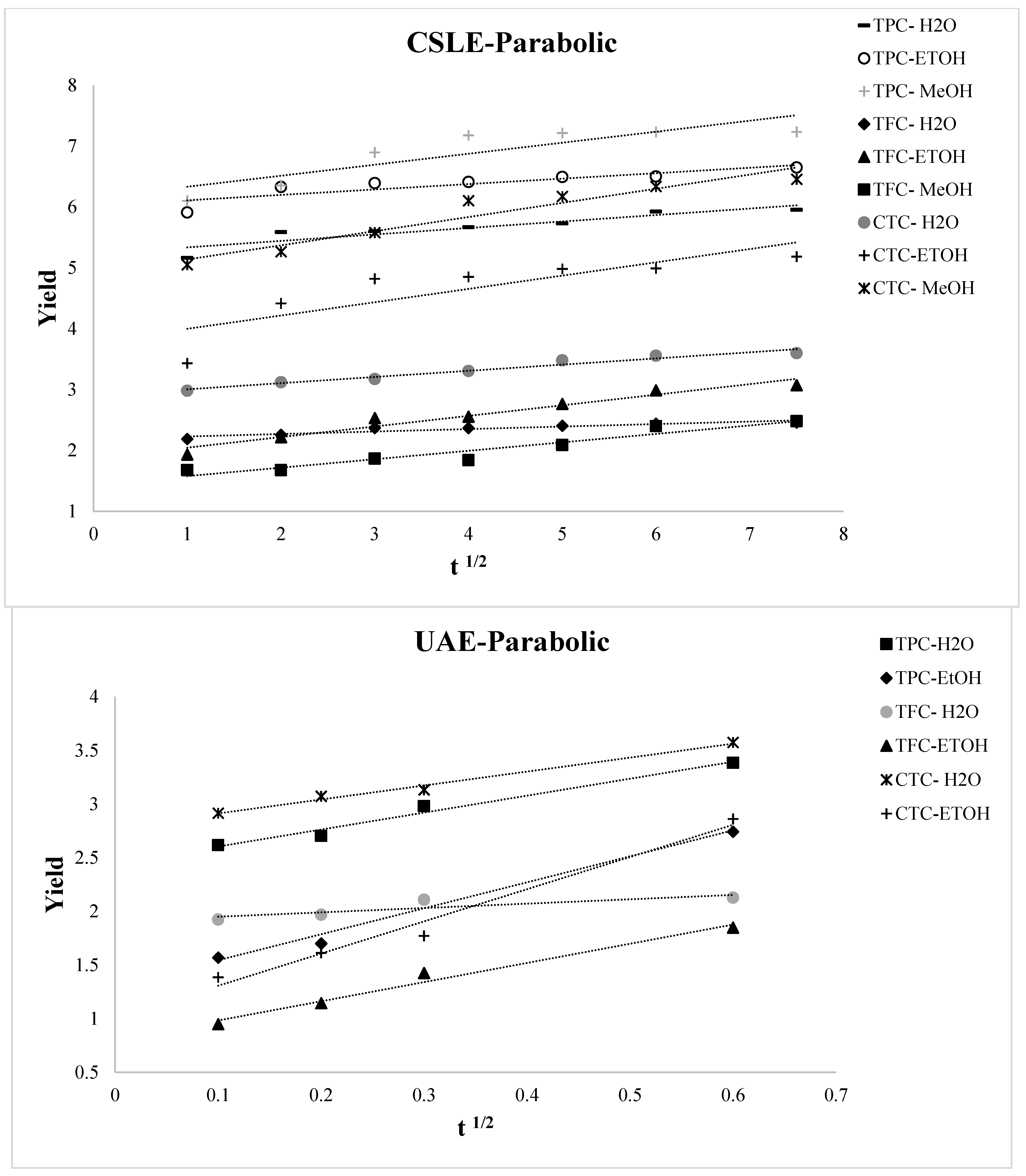


| Kinetic Model | Non-Linear Equation | Linearized Equation |
|---|---|---|
| Parabolic diffusion | = A0 + A1t1/2 | = A0 + A1t1/2 |
| Power law | = Btn | ln = ln B + n ln t |
| Hyperbolic (Peleg’s) | = | = × + |
| Elovich’s | = E0 + E1 ln t | = E0 + E1 ln t |
| Model | Parabolic | Power-Law | Hyperbolic | Elovich’s | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | R2 | SEE | RMS | A0 | A1 | R2 | SEE | RMS | B | n | R2 | SEE | RMS | C1 | C2 | R2 | SEE | RMS | E0 | E1 |
| CSLE-H2O | 0.848 | 0.085 | 4.663 | 5.244 | 0.103 | 0.931 | 0.047 | 4.663 | 5.008 | 0.064 | 0.907 | 0.05 | 4.663 | 18.544 | 3.096 | 0.933 | 0.046 | 4.663 | 4.979 | 0.356 |
| CSLE-EtOH | 0.77 | 0.139 | 5.383 | 6.07 | 0.077 | 1 | 0.16 | 5.389 | 3.375 | 0.344 | 0.953 | 0.046 | 5.383 | 27.589 | 4.142 | 0.915 | 0.077 | 5.383 | 5.67 | 0.634 |
| CSLE-MeOH | 0.766 | 0.389 | 5.888 | 6.179 | 0.174 | 0.909 | 0.174 | 5.389 | 5.751 | 0.344 | 0.903 | 0.154 | 5.887 | 14.703 | 1.959 | 0.916 | 0.077 | 5.888 | 5.67 | 0.634 |
| UAE-H2O | 0.979 | 0.034 | 1.921 | 2.466 | 1.519 | 0.935 | 0.07 | 1.92 | 3.222 | 0.144 | 0.775 | 0.143 | 1.919 | 51.176 | 15.154 | 0.915 | 0.625 | 1.921 | 3.21 | 0.405 |
| UAE-EtOH | 0.982 | 0.41 | 1.027 | 1.508 | 1.74 | 0.942 | 0.582 | 1.021 | 2.332 | 0.209 | 0.772 | 0.756 | 1.024 | 34.799 | 14.796 | 0.913 | 0.625 | 1.028 | 2.281 | 0.351 |
| TFC | R2 | SEE | RMS | A0 | A1 | R2 | SEE | RMS | B | n | R2 | SEE | RMS | C1 | C2 | R2 | SEE | RMS | E0 | E1 |
| CSLE-H2O | 0.686 | 0.266 | 4.711 | 2.08 | 0.107 | 0.969 | 0.002 | 1.355 | 2.097 | 0.06 | 0.915 | 0.005 | 1.356 | 8.28 | 3.335 | 0.97 | 0.002 | 1.355 | 2.086 | 0.14 |
| CSLE-EtOH | 0.951 | 0.049 | 1.581 | 1.871 | 0.174 | 0.98 | 0.02 | 1.58 | 1.618 | 0.237 | 0.916 | 0.085 | 1.575 | 1.982 | 0.605 | 0.969 | 0.03 | 1.58 | 1.475 | 0.575 |
| CSLE-MeOH | 0.919 | 0.053 | 1.005 | 1.441 | 0.139 | 0.809 | 0.125 | 1.001 | 1.274 | 0.23 | 0.622 | 0.251 | 0.997 | 1.742 | 0.704 | 0.759 | 0.157 | 1.004 | 1.214 | 0.412 |
| UAE-H2O | 0.746 | 0.012 | 0.788 | 1.923 | 0.365 | 0.857 | 0.008 | 0.799 | 2.116 | 0.057 | 0.832 | 0.007 | 0.807 | 80.589 | 37.211 | 0.86 | 0.008 | 0.8 | 2.116 | 0.118 |
| UAE-EtOH | 0.979 | 0.077 | 0.37 | 0.847 | 1.649 | 0.989 | 0.112 | 0.394 | 1.679 | 0.354 | 0.939 | 0.174 | 0.411 | 7.492 | 3.481 | 0.966 | 0.144 | 0.397 | 1.649 | 0.431 |
| CTC | R2 | SEE | RMS | A0 | A1 | R2 | SEE | RMS | B | n | R2 | SEE | RMS | C1 | C2 | R2 | SEE | RMS | E0 | E1 |
| CSLE-H2O | 0.961 | 0.013 | 2.316 | 2.903 | 0.101 | 0.939 | 0.02 | 2.316 | 2.721 | 0.102 | 0.787 | 0.071 | 2.316 | 7.111 | 1.968 | 0.927 | 0.024 | 2.316 | 2.69 | 0.326 |
| CSLE-EtOH | 0.705 | 0.623 | 3.67 | 3.78 | 0.218 | 0.879 | 0.257 | 3.671 | 3.322 | 0.174 | 0.991 | 0.716 | 3.677 | 55.643 | 11.72 | 0.909 | 0.192 | 3.67 | 3.112 | 0.811 |
| CSLE-MeOH | 0.923 | 0.141 | 4.851 | 4.905 | 0.232 | 0.953 | 0.086 | 4.851 | 4.484 | 0.137 | 0.847 | 0.282 | 4.849 | 8.756 | 1.324 | 0.944 | 0.102 | 4.851 | 4.374 | 0.769 |
| UAE-H2O | 0.989 | 0.003 | 2.174 | 2.783 | 1.301 | 0.917 | 0.02 | 2.173 | 3.435 | 0.115 | 0.746 | 0.061 | 2.173 | 68.663 | 19.245 | 0.899 | 0.024 | 2.174 | 3.429 | 0.357 |
| UAE-EtOH | 0.979 | 0.027 | 0.906 | 1.008 | 2.999 | 0.925 | 0.098 | 0.889 | 2.529 | 0.461 | 0.845 | 0.218 | 0.852 | 7.559 | 1.969 | 0.844 | 0.2 | 0.899 | 2.481 | 0.802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgano, F.; Tolve, R.; Scarpa, T.; Caruso, M.C.; Lucini, L.; Senizza, B.; Condelli, N. Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods 2021, 10, 1810. https://doi.org/10.3390/foods10081810
Galgano F, Tolve R, Scarpa T, Caruso MC, Lucini L, Senizza B, Condelli N. Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods. 2021; 10(8):1810. https://doi.org/10.3390/foods10081810
Chicago/Turabian StyleGalgano, Fernanda, Roberta Tolve, Teresa Scarpa, Marisa Carmela Caruso, Luigi Lucini, Biancamaria Senizza, and Nicola Condelli. 2021. "Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods" Foods 10, no. 8: 1810. https://doi.org/10.3390/foods10081810
APA StyleGalgano, F., Tolve, R., Scarpa, T., Caruso, M. C., Lucini, L., Senizza, B., & Condelli, N. (2021). Extraction Kinetics of Total Polyphenols, Flavonoids, and Condensed Tannins of Lentil Seed Coat: Comparison of Solvent and Extraction Methods. Foods, 10(8), 1810. https://doi.org/10.3390/foods10081810










