Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review
Abstract
:1. Introduction
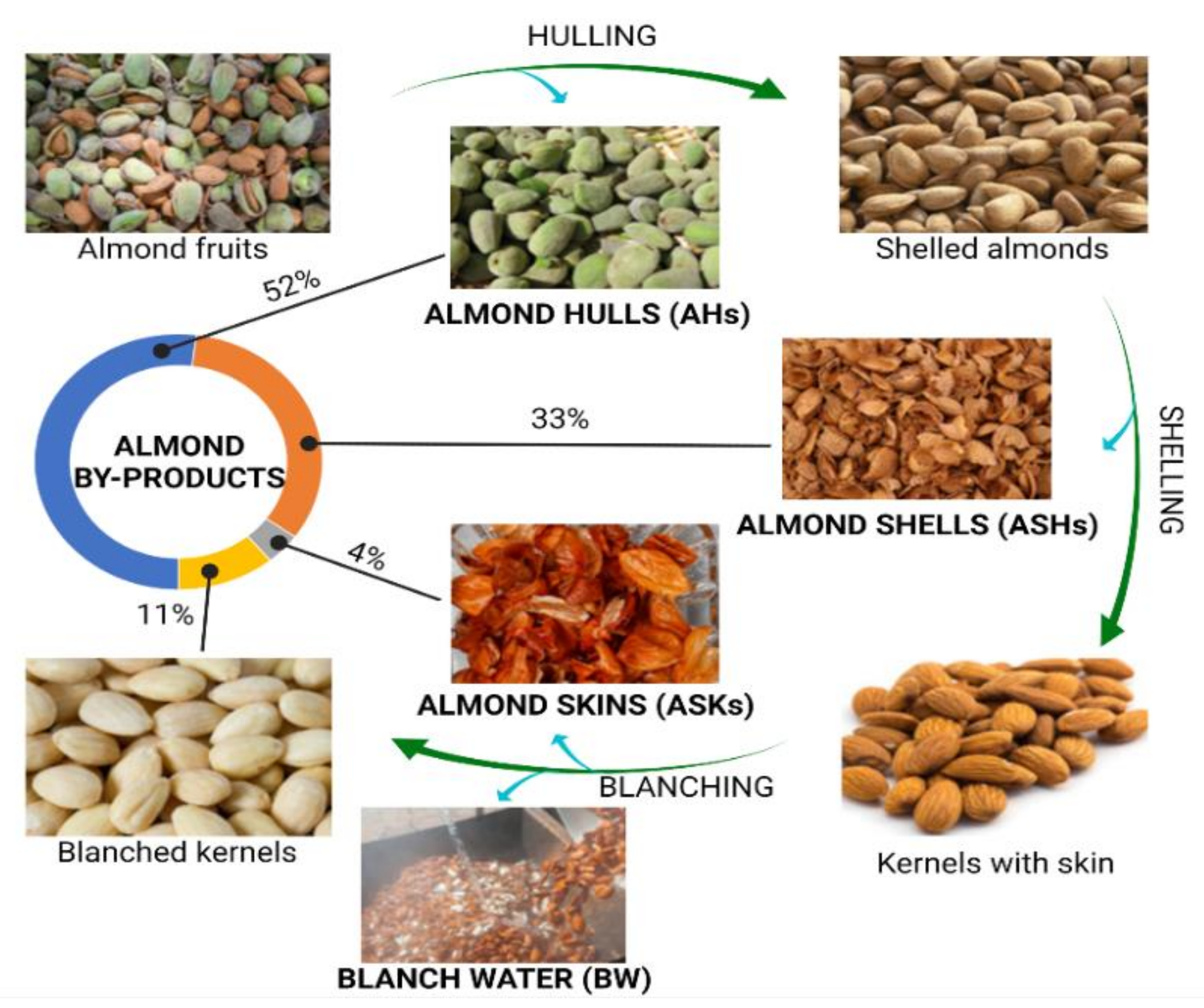
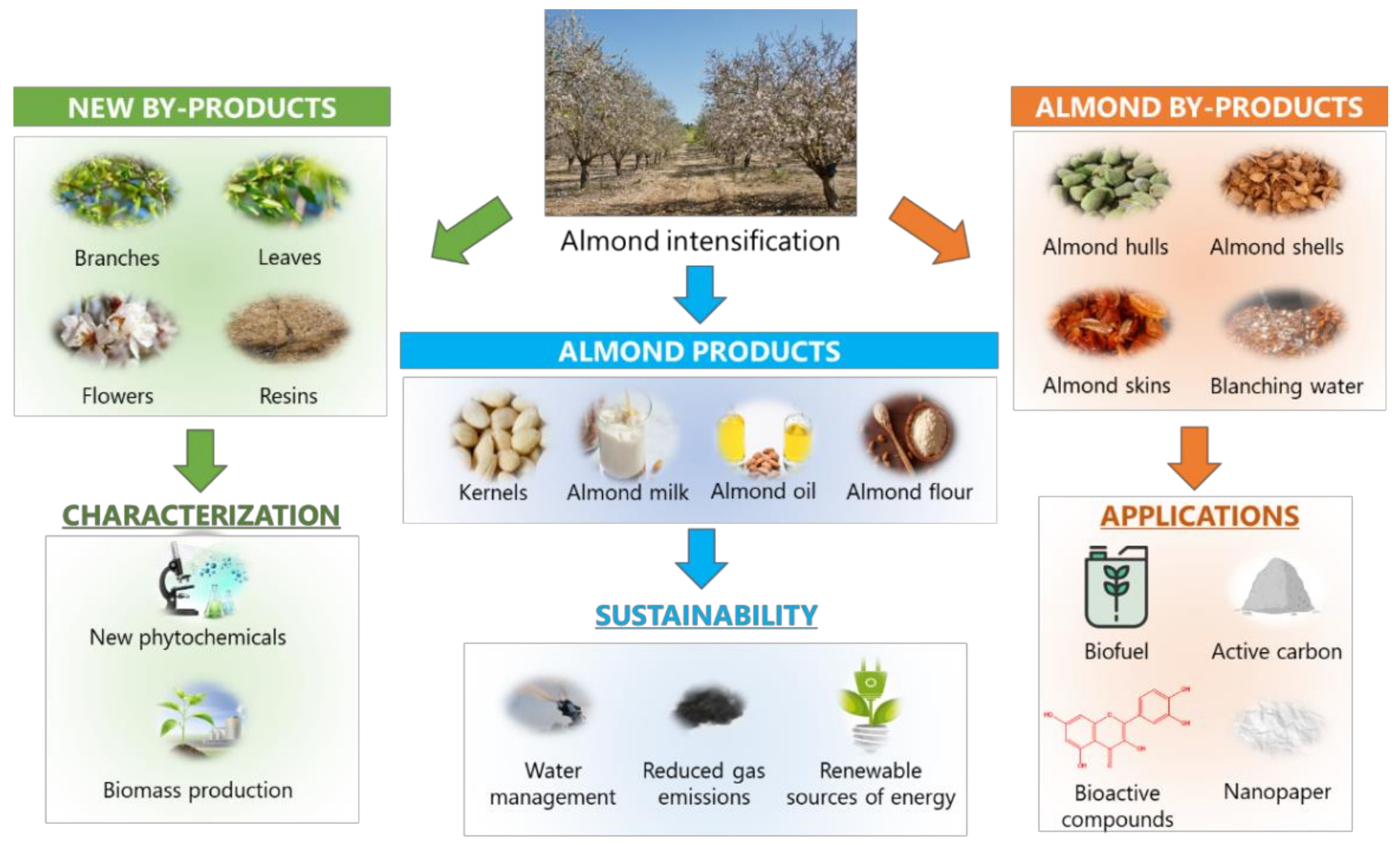
1.1. Almond By-Products
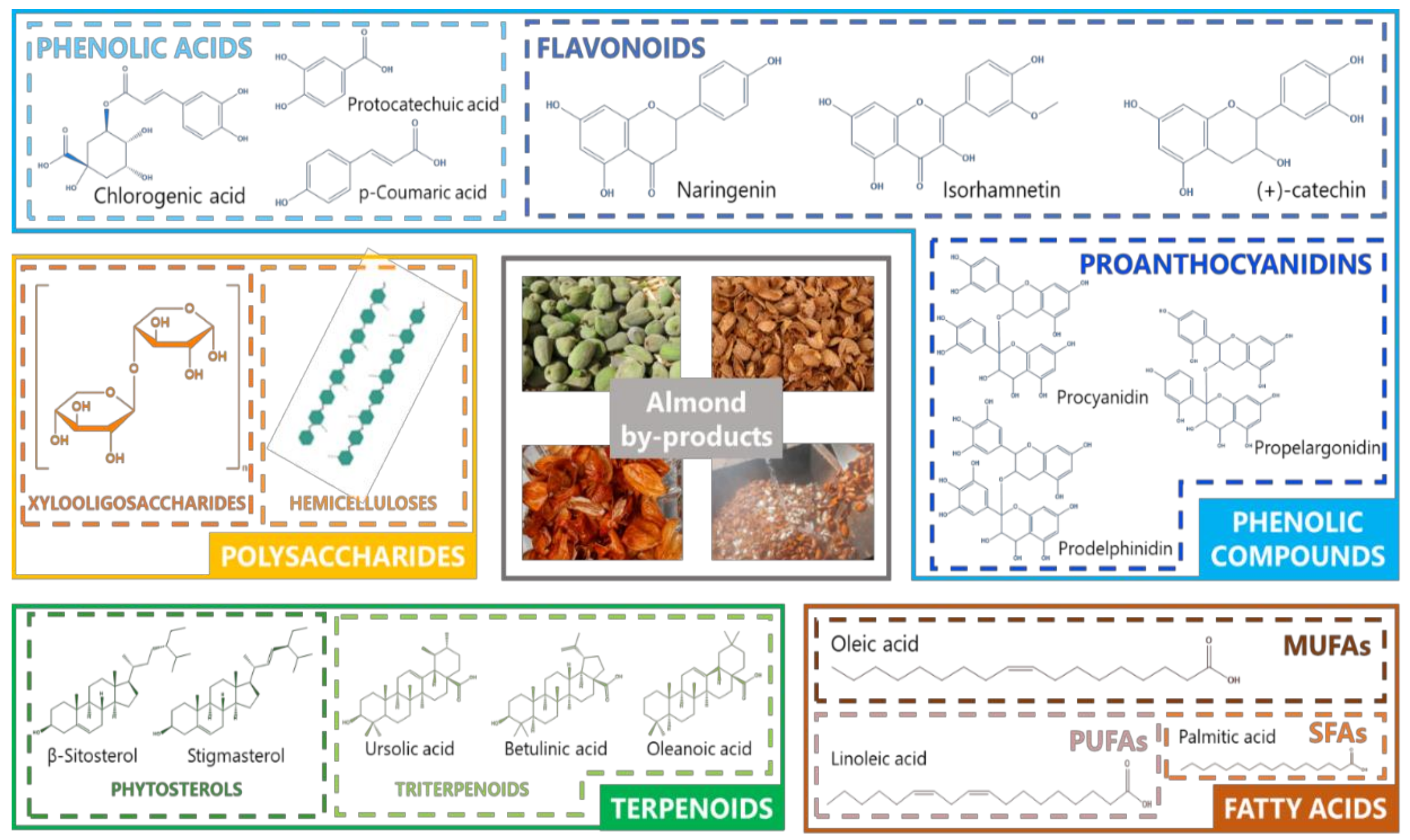
1.2. Bioactive Compounds from Almond By-Products
2. Extraction Technologies
2.1. Conventional Extraction
| Extraction | By-Product | Extraction Procedure | Bioactive Compounds | References |
|---|---|---|---|---|
| Conventional | AHs | 70% EtOH, 50 °C, 6 h | Phenolic acids, catechins | [19] |
| AHs | 70% EtOH, 24 h | Total phenolic and flavonoid contents | [42] | |
| AHs | Maceration with EtOAc, 24 h | Total phenolic and flavonoid contents | [43] | |
| AHs, ASHs | Soxhlet extraction with MeOH, 80 °C, 30 min | Total phenolic content | [22] | |
| ASHs | HAE with 80% γ-valerolactone,75 mM H2SO4, 140 °C, 30 min | Lignin and hemicelluloses | [44] | |
| MAE | ASKs | 70% EtOH, 2450 MHz, 100 W, 60 s | Flavonol rutinosides | [45] |
| ASHs | Choline chloride-oxalic acid, 800 W, 1 min | Lignin | [46] | |
| AKs | 0.5 M NaOH, 2450 MHz, 800 W, 23–67 °C, 3 min | Lignans | [47] | |
| UAE | AHs | 51.2% EtOH, 40 kHz, 300 W, 13 min | Phenolic acids, catechin | [19] |
| ASKs | Water, 20 kHz, 100 W, 20 min | Phenolic compounds, lipids, proteins | [48] | |
| ASKs | PEG, 40 kHz, 120 W, 30 min | Proanthocyanidins, chlorogenic acid | [49] | |
| BW | n.d. | Total phenolic content | [50] | |
| SFE | ASHs | Petroleum ether, 40–60 °C, 90 min, 11 kPa | Holo-cellulose, lignin | [51] |
| AKs | Butane, −0.09 MPa, recovery with N2 at −4 °C | Total phenolic, phytosterol, tocopherol, and tocotrienol contents | [52] | |
| EAE | ASHs | Endoxylanase from Thermomyces lanuginosus, pH 5.5, 50 °C | Xylooligosaccharides | [26] |
| ASHs | Cellulase and β-glucosidase, pH 4.85, 48.5 °C | Lignin and cellulose-enriched solids | [53] |
2.2. Microwave-Assisted Extraction
2.3. Ultrasound-Assisted Extraction
2.4. Supercritical Fluid-Assisted Extraction
2.5. Enzyme-Assisted Extraction
3. Food Fortification Using Almond By-Products
4. Limitations on the Applicability of Almond By-Products
4.1. Allergens
4.2. Mycotoxins
4.3. Cyanogenic Compounds
5. Sustainability and Future Perspectives for Almond Revalorization
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taş, N.G.; Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Esfahlan, A.J.; Jamei, R.; Esfahlan, R.J. The importance of almond (Prunus amygdalus L.) and its by-products. Food Chem. 2010, 120, 349–360. [Google Scholar] [CrossRef]
- Sottile, F.; Massaglia, S.; Peano, C. Ecological and economic indicators for the evaluation of almond (Prunus dulcis L.) orchard renewal in sicily. Agriculture 2020, 10, 301. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. Valorization Challenges to Almond Residues: Phytochemical Composition and Functional Application. Molecules 2017, 22, 1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Hao, J.; Wang, W. Study of almond shell characteristics. Materials 2018, 11, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thodberg, S.; Del Cueto, J.; Mazzeo, R.; Pavan, S.; Lotti, C.; Dicenta, F.; Neilson, E.H.J.; Møller, B.L.; Sánchez-Pérez, R. Elucidation of the amygdalin pathway reveals the metabolic basis of bitter and sweet almonds (Prunus dulcis). Plant. Physiol. 2018, 178, 1096–1111. [Google Scholar] [CrossRef] [Green Version]
- Garrido, I.; Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, R.; Sharma, S. Almond. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer: Berlin/Heidleberg, Germany, 2020; pp. 423–452. [Google Scholar]
- Martins, I.M.; Chen, Q.; Oliver Chen, C.Y. Emerging Functional Foods Derived from Almonds. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 445–469. [Google Scholar]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Review about Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9, 1646. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.; Lanari, S.; Santulli, C.; Pettinari, C. Use of almond shells and rice husk as fillers of poly(Methyl Methacrylate) (PMMA) composites. Materials 2017, 10, 872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite profiling and antioxidant activity of the polar fraction of Italian almonds (Toritto and Avola): Analysis of seeds, skins, and blanching water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef]
- Swanson, K.L.; Bill, H.M.; Asmus, J.; Heguy, J.M.; DePeters, E.J. Feeding high amounts of almond hulls to lactating cows. J. Dairy Sci. 2021, 104, 8846–8856. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, M.; Sachdeva, S.; Puri, S.K. An efficient multiphase bioprocess for enhancing the renewable energy production from almond shells. Energy Convers. Manag. 2020, 203, 112235. [Google Scholar] [CrossRef]
- Rahimian, R.; Zarinabadi, S. A review of Studies on the Removal of Methylene Blue Dye from Industrial Wastewater Using Activated Carbon Adsorbents Made from Almond Bark. Prog. Chem. Biochem. Res. J. 2020, 3, 251–268. [Google Scholar]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS identification of phenolic antioxidants from agricultural residues: Almond hulls and grape pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant constituents of almond [Prunus dulcis (Mill.) D.A. Webb] hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Vecchia, S.B.D.; Giovine, F.; Kbaier, H.B.H.; Bouzouita, N.; Pereira, L.B.; Zeppa, G. Characterization of polyphenolic compounds extracted from different varieties of almond hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-products. Food Anal. Methods 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- An, J.; Liu, J.; Liang, Y.; Ma, Y.; Chen, C.; Cheng, Y.; Peng, P.; Zhou, N.; Zhang, R.; Addy, M.; et al. Characterization, bioavailability and protective effects of phenolic-rich extracts from almond hulls against pro-oxidant induced toxicity in Caco-2 cells. Food Chem. 2020, 322, 126742. [Google Scholar] [CrossRef]
- Sfahlan, A.J.; Mahmoodzadeh, A.; Hasanzadeh, A.; Heidari, R.; Jamei, R. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chem. 2009, 115, 529–533. [Google Scholar] [CrossRef]
- Isfahlan, A.J.; Mahmoodzadeh, A.; Hassanzadeh, A.; Heidari, R.; Jamei, R. Antioxidant and antiradical activities of phenolic extracts from Iranian almond (Prunus amygdalus L.) hulls and shells. Turkish J. Biol. 2010, 34, 165–173. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Moure, A.; Pazos, M.; Medina, I.; Domínguez, H.; Parajó, J.C. Antioxidant activity of extracts produced by solvent extraction of almond shells acid hydrolysates. Food Chem. 2007, 101, 193–201. [Google Scholar] [CrossRef]
- Singh, R.D.; Nadar, C.G.; Muir, J.; Arora, A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019, 241, 118237. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of different processing treatments on almond (Prunus dulcis) bioactive compounds, antioxidant activities, fatty acids, and sensorial characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Bolling, B.W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16, 346–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food matrix effects of polyphenol bioaccessibility from almond skin during simulated human digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Faulks, R.M.; Bisignano, C.; Waldron, K.W.; Narbad, A.; Wickham, M.S.J. In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiol. Lett. 2010, 304, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of proanthocyanidins in almond blanch water by HPLC-ESI-QqQ-MS/MS and MALDI-TOF/TOF MS. Food Res. Int. 2012, 49, 798–806. [Google Scholar] [CrossRef]
- Takeoka, G.; Dao, L.; Teranishi, R.; Wong, R.; Flessa, S.; Harden, L.; Edwards, R. Identification of three triterpenoids in almond hulls. J. Agric. Food Chem. 2000, 48, 3437–3439. [Google Scholar] [CrossRef] [PubMed]
- Amico, V.; Barresi, V.; Condorelli, D.; Spatafora, C.; Tringali, C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): Identification and structure-activity relationships. J. Agric. Food Chem. 2006, 54, 810–814. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F. Tree Nuts: Composition, Phytochemicals, and Health Effects: An Overview. In Tree Nuts: Composition, Phytochemicals, and Health Effects; Alasalvar, C., Shahidi, F., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 1–11. [Google Scholar]
- Karimi, Z.; Firouzi, M.; Dadmehr, M.; Javad-Mousavi, S.A.; Bagheriani, N.; Sadeghpour, O. Almond as a nutraceutical and therapeutic agent in Persian medicine and modern phytotherapy: A narrative review. Phyther. Res. 2021, 35, 2997–3012. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Tiwari, B. Conventional extraction techniques: Solvent extraction. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 171–189. [Google Scholar]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Ainane, A.; Ainane, T.; Gharby, S.; Rharrabti, Y. Variability of oil content and its physicochemical traits from the main almond [Prunus dulcis Mill. DA Webb] cultivars grown under contrasting environments in north-eastern Morocco. Biocatal. Agric. Biotechnol. 2021, 32, 101952. [Google Scholar] [CrossRef]
- Ozcan, M.M.; Matthaus, B.; Aljuhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Gassem, M.A.; Alqah, H.A.S. Effect of almond genotypes on fatty acid composition, tocopherols and mineral contents and bioactive properties of sweet almond (Prunus amygdalus Batsch spp. dulce) kernel and oils. J. Food Sci. Technol. 2020, 57, 4182–4192. [Google Scholar] [CrossRef] [PubMed]
- Allaith, S.A.; Alfekaik, D.F.; Alssirag, M.A. Identification of Pistacia vera and Prunus amygdalus Batsch seed oils using GC-MS as useful methodology for chemical classification. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012061. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Numonov, S.; Aisa, H.A. Chemical and Pharmacological Evaluation of Hulls of Prunus dulcis Nuts. Int. J. Anal. Chem. 2019, 2019, 5861692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tlili, N.; Kirkan, B.; Sarikurkcu, C. LC–ESI–MS/MS characterization, antioxidant power and inhibitory effects on α-amylase and tyrosinase of bioactive compounds from hulls of Amygdalus communis: The influence of the extracting solvents. Ind. Crops Prod. 2019, 128, 147–152. [Google Scholar] [CrossRef]
- Corti, A.; Torrens, E.; Montané, D. Acid-catalyzed fractionation of almond shells in γ-valerolactone/water. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Kammoun, M.; Berchem, T.; Richel, A. Ultrafast Lignin Extraction from Unusual Mediterranean Lignocellulosic Residues. JoVE 2021, e61997. [Google Scholar] [CrossRef]
- Nemes, S.M.; Orsat, V. Evaluation of a Microwave-Assisted Extraction Method for Lignan Quantification in Flaxseed Cultivars and Selected Oil Seeds. Food Anal. Methods 2012, 5, 551–563. [Google Scholar] [CrossRef]
- Tabib, M.; Tao, Y.; Ginies, C.; Bornard, I.; Rakotomanomana, N.; Remmal, A.; Chemat, F. A one-pot ultrasound-assisted almond skin separation/polyphenols extraction and its effects on structure, polyphenols, lipids, and proteins quality. Appl. Sci. 2020, 10, 3628. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.Y.; Qiang, Q.Q.; Zhang, Z.Q. Ultrasound-assisted extraction and preliminary purification of proanthocyanidins and chlorogenic acid from almond (Prunus dulcis) skin. J. Sep. Sci. 2014, 37, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Demirbaş, A. Conversion of agricultural residues to fuel products via supercritical fluid extraction. Energy Sources 2004, 26, 1095–1103. [Google Scholar] [CrossRef]
- Qi, Z.; Xiao, J.; Ye, L.; Chuyun, W.; Chang, Z.; Shugang, L.; Fenghong, H. The effect of the subcritical fluid extraction on the quality of almond oils: Compared to conventional mechanical pressing method. Food Sci. Nutr. 2019, 7, 2231–2241. [Google Scholar] [CrossRef]
- Morales, A.; Hernández-Ramos, F.; Sillero, L.; Fernández-Marín, R.; Dávila, I.; Gullón, P.; Erdocia, X.; Labidi, J. Multiproduct biorefinery based on almond shells: Impact of the delignification stage on the manufacture of valuable products. Bioresour. Technol. 2020, 315, 123896. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Bouaoudia-Madi, N.; Boulekbache-Makhlouf, L.; Madani, K.; Silva, A.M.S.; Dairi, S.; Oukhmanou-Bensidhoum, S.; Cardoso, S.M. Optimization of ultrasound-assisted extraction of polyphenols from Myrtus communis L. Pericarp. Antioxidants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungmunnithum, D.; Elamrani, A.; Abid, M.; Drouet, S.; Kiani, R.; Garros, L.; Kabra, A.; Addi, M.; Hano, C. A quick, green and simple ultrasound-assisted extraction for the valorization of antioxidant phenolic acids from moroccan almond cold-pressed oil residues. Appl. Sci. 2020, 10, 3313. [Google Scholar] [CrossRef]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Ge, X.L.; Shi, T.; Wang, H.; Zhang, J.; Zhang, Z.Q. Development of an Aqueous Polyethylene Glycol-Based Extraction and Recovery Method for Almond (Prunus armeniaca L.) Protein. Food Anal. Methods 2016, 9, 3319–3326. [Google Scholar] [CrossRef]
- Özbek, H.N.; Koçak Yanık, D.; Fadıloğlu, S.; Keskin Çavdar, H.; Göğüş, F. Microwave-assisted extraction of non-polar compounds from pistachio hull and characterization of extracts. Grasas Aceites 2018, 69, 260. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Martinez-Ramos, T.; Benedito-Fort, J.; Watson, N.J.; Ruiz-López, I.I.; Che-Galicia, G.; Corona-Jiménez, E. Effect of solvent composition and its interaction with ultrasonic energy on the ultrasound-assisted extraction of phenolic compounds from Mango peels (Mangifera indica L.). Food Bioprod. Process. 2020, 122, 41–54. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of nonconventional green extraction technologies in process industries: Challenges, limitations and perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Abbas, K.A.; Mohamed, A.; Abdulamir, A.S.; Abas, H.A. A review on supercritical fluid extraction as new analytical method. Am. J. Biochem. Biotechnol. 2008, 4, 345–353. [Google Scholar] [CrossRef]
- Kai Bin, L.; Janakiraman, A.K.; Razak, F.S.A.; Uddin, A.B.M.H.; Sarker, M.Z.I.; Ming, L.C.; Goh, B.H. Supercritical fluid technology and its pharmaceutical applications: A revisit with two decades of progress. Indian J. Pharm. Educ. Res. 2020, 54, s1–s11. [Google Scholar] [CrossRef]
- Pinto, D.; De La Luz Cádiz-Gurrea, M.; Sut, S.; Ferreira, A.S.; Leyva-Jimenez, F.J.; Dall’acqua, S.; Segura-Carretero, A.; Delerue-Matos, C.; Rodrigues, F. Valorisation of underexploited Castanea sativa shells bioactive compounds recovered by supercritical fluid extraction with CO2: A response surface methodology approach. J. CO2 Util. 2020, 40, 101194. [Google Scholar] [CrossRef]
- Balvardi, M.; Mendiola, J.A.; Castro-Gómez, P.; Fontecha, J.; Rezaei, K.; Ibáñez, E. Development of Pressurized Extraction Processes for Oil Recovery from Wild Almond (Amygdalus scoparia). JAOCS J. Am. Oil Chem. Soc. 2015, 92, 1503–1511. [Google Scholar] [CrossRef]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Zeroual, A.; Gharby, S.; Rharrabti, Y. On the natural variability of kernel oil content in almond [Prunus dulcis Mill. DA Webb]: An Overview. J. Anal. Sci. Appl. Biotechnol. 2020, 2, 16–22. [Google Scholar] [CrossRef]
- Čolić, S.D.; Zec, G.; Nati, M.; Fotirić Akšić, M.M. Almond (Prunus dulcis) oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer Nature: Basingstoke, UK, 2019; pp. 149–180. [Google Scholar]
- Leo, L.; Rescio, L.; Ciurlia, L.; Zacheo, G. Supercritical carbon dioxide extraction of oil and α-tocopherol from almond seeds. J. Sci. Food Agric. 2005, 85, 2167–2174. [Google Scholar] [CrossRef]
- Hernández Becerra, E.; De Jesús Pérez López, E.; Zartha Sossa, J.W. Recovery of Biomolecules from Agroindustry by Solid-Liquid Enzyme-Assisted Extraction: A Review. Food Anal. Methods 2021, 14, 1744–1777. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction of bioactives. In Food Bioactives; Puri, M., Ed.; Springer: Berlin/Heidleberg, Germany, 2017; pp. 171–201. [Google Scholar]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- De Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; de Moura Bell, J.M.L.N. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Williams, S.R.O.; Chaves, A.V.; Deighton, M.H.; Jacobs, J.L.; Hannah, M.C.; Ribaux, B.E.; Morris, G.L.; Wales, W.J.; Moate, P.J. Influence of feeding supplements of almond hulls and ensiled citrus pulp on the milk production, milk composition, and methane emissions of dairy cows. J. Dairy Sci. 2018, 101, 2072–2083. [Google Scholar] [CrossRef]
- Wang, J.; Kong, F.; Kim, W.K. Effect of almond hulls on the performance, egg quality, nutrient digestibility, and body composition of laying hens. Poult. Sci. 2021, 100, 101286. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Ceballos, S.J.; Johnson, P.C.; Niemeier, D.; Pitesky, M.; VanderGheynst, J.S. Cultivation of black soldier fly larvae on almond byproducts: Impacts of aeration and moisture on larvae growth and composition. J. Sci. Food Agric. 2018, 98, 5893–5900. [Google Scholar] [CrossRef] [Green Version]
- Kacem, I.; Martinez-Saez, N.; Kallel, F.; Ben Jeddou, K.; Boisset Helbert, C.; Ellouze Chaabouni, S.; del Castillo, M.D. Use of almond shell as food ingredient. Eur. Food Res. Technol. 2017, 243, 2115–2126. [Google Scholar] [CrossRef]
- Boyce, J.; Assa’ad, A.; Burks, A.; Jones, S.; Sampson, H.; Wood, R.; Plaut, M.; Cooper, S.; Fenton, M.; Arshad, S. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Furlong, T.J.; Muñoz-Furlong, A.; Burks, A.W.; Sampson, H.A. A voluntary registry for peanut and tree nut allergy: Characteristics of the first 5149 registrants. J. Allergy Clin. Immunol. 2001, 108, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B.P.P. Almond allergens: Molecular characterization, detection, and clinical relevance. J. Agric. Food Chem. 2012, 60, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Sethi, D.K.; Salunke, D.M. Purification, identification and preliminary crystallographic studies of Pru du amandin, an allergenic protein from Prunus dulcis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 32–35. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Albillos, S.M.; Guo, F.; Howard, A.; Fu, T.-J.; Kothary, M.H.; Zhang, Y.-Z. Crystal structure of prunin-1, a major component of the almond (Prunus dulcis) allergen amandin. J. Agric. Food Chem. 2009, 57, 8643–8651. [Google Scholar] [CrossRef] [PubMed]
- Tawde, P.; Venkatesh, Y.P.; Wang, F.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning and characterization of profilin (Pru du 4), a cross-reactive almond (Prunus dulcis) allergen. J. Allergy Clin. Immunol. 2006, 118, 915–922. [Google Scholar] [CrossRef]
- Willison, L.N.; Tripathi, P.; Sharma, G.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Cloning, expression and patient IgE reactivity of recombinant Pru du 6, an 11S globulin from almond. Int. Arch. Allergy Immunol. 2011, 156, 267–281. [Google Scholar] [CrossRef]
- Bolhaar, S.; Van Ree, R.; Ma, Y.; Bruijnzeel-Koomen, C.; Vieths, S.; Hoffmann-Sommergruber, K.; Knulst, A.C.; Zuidmeer, L. Severe allergy to sharon fruit caused by birch pollen. Int. Arch. Allergy Immunol. 2005, 136, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kosma, P.; Sjölander, S.; Landgren, E.; Borres, M.P.; Hedlin, G. Severe reactions after the intake of soy drink in birch pollen-allergic children sensitized to Gly m 4. Acta Paediatr. 2011, 100, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Kabasser, S.; Hafner, C.; Chinthrajah, S.; Sindher, S.B.; Kumar, D.; Kost, L.E.; Long, A.J.; Nadeau, K.C.; Breiteneder, H.; Bublin, M. Identification of Pru du 6 as a potential marker allergen for almond allergy. Allergy 2021, 76, 1463–1472. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, T. Almond allergens: Update and perspective on identification and characterization. J. Sci. Food Agric. 2020, 100, 4657–4663. [Google Scholar] [CrossRef]
- Bayman, P.; Baker, J.L.; Mahoney, N.E. Aspergillus on tree nuts: Incidence and associations. Mycopathologia 2002, 155, 161–169. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Lima, N. Incidence and diversity of the fungal genera Aspergillus and Penicillium in Portuguese almonds and chestnuts. Eur. J. Plant Pathol. 2013, 137, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Zaied, C.; Abid, S.; Bouaziz, C.; Chouchane, S.; Jomaa, M.; Bacha, H. Ochratoxin A levels in spices and dried nuts consumed in Tunisia. Food Addit. Contam. Part B 2010, 3, 52–57. [Google Scholar] [CrossRef]
- Kluczkovski, A.M. Fungal and mycotoxin problems in the nut industry. Curr. Opin. Food Sci. 2019, 29, 56–63. [Google Scholar] [CrossRef]
- Varga, J.; Frisvad, J.; Samson, R. A reappraisal of fungi producing aflatoxins. World Mycotoxin J. 2009, 2, 263–277. [Google Scholar] [CrossRef]
- Cressey, P.; Saunders, D.; Goodman, J. Cyanogenic glycosides in plant-based foods available in New Zealand. Food Addit. Contam. Part A 2013, 30, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Drochioiu, G.; Arsene, C.; Murariu, M.; Oniscu, C. Analysis of cyanogens with resorcinol and picrate. Food Chem. Toxicol. 2008, 46, 3540–3545. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Molecular mechanism of amygdalin action in vitro: Review of the latest research. Immunopharmacol. Immunotoxicol. 2018, 40, 212–218. [Google Scholar] [CrossRef]
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of the gut microbiota on amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys. Rep. 2018, 14, 125–132. [Google Scholar] [CrossRef]
- Bromley, J.; Hughes, B.G.M.; Leong, D.C.S.; Buckley, N.A. Life-threatening interaction between complementary medicines: Cyanide toxicity following ingestion of amygdalin and vitamin C. Ann. Pharmacother. 2005, 39, 1566–1569. [Google Scholar] [CrossRef]
- Go, M.-R.; Kim, H.-J.; Yu, J.; Choi, S.-J. Toxicity and toxicokinetics of amygdalin in Maesil (Prunus mume) syrup: Protective effect of Maesil against amygdalin toxicity. J. Agric. Food Chem. 2018, 66, 11432–11440. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-M.; Kwon, H. Metabolites of amygdalin under simulated human digestive fluids. Int. J. Food Sci. Nutr. 2010, 61, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.J. Amygdalin analogs for the treatment of psoriasis. Future Med. Chem. 2013, 5, 799–808. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Wu, L.-J.; Wang, W.-X.; Xie, P.-J.; Chen, Y.-H.; Wang, F. Amygdalin-A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef]
- Wahab, M.F.; Breitbach, Z.S.; Armstrong, D.W.; Strattan, R.; Berthod, A. Problems and pitfalls in the analysis of amygdalin and its epimer. J. Agric. Food Chem. 2015, 63, 8966–8973. [Google Scholar] [CrossRef]
- Sanchez-Verlaan, P.; Geeraerts, T.; Buys, S.; Riu-Poulenc, B.; Cabot, C.; Fourcade, O.; Mégarbane, B.; Genestal, M. An unusual cause of severe lactic acidosis: Cyanide poisoning after bitter almond ingestion. Intensive Care Med. 2011, 37, 168. [Google Scholar] [CrossRef]
- Harald, S.; Wollny, C.; Oster, I.; Tutdibi, E.; Gortner, L.; Gottschling, S.; Meyer, S. Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wien. Med. Wochenschr. 2015, 165, 185–188. [Google Scholar] [CrossRef]
- Suchard, J.R.; Wallace, K.L.; Gerkin, R.D. Acute cyanide toxicity caused by apricot kernel ingestion. Ann. Emerg. Med. 1998, 32, 742–744. [Google Scholar] [CrossRef]
- Cigolini, D.; Ricci, G.; Zannoni, M.; Codogni, R.; De Luca, M.; Perfetti, P.; Rocca, G. Hydroxocobalamin treatment of acute cyanide poisoning from apricot kernels. Emerg. Med. J. 2011, 28, 804–805. [Google Scholar] [CrossRef] [Green Version]
- Vogel, S.N.; Sultan, T.R.; Ten Eyck, R.P. Cyanide poisoning. Clin. Toxicol. 1981, 18, 367–383. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Lipan, L.; Gutiérrez-Gordillo, S.; Durán Zuazo, V.H.; Jančo, I.; Hernández, F.; Rodríguez, B.C.; Carbonell-Barrachina, Á.A. Deficit irrigation and its implications for HydroSOStainable almond production. Agronomy 2020, 10, 1632. [Google Scholar] [CrossRef]
- Kendall, A.; Marvinney, E.; Brodt, S.; Zhu, W. Life Cycle-based Assessment of Energy Use and Greenhouse Gas Emissions in Almond Production, Part I: Analytical Framework and Baseline Results. J. Ind. Ecol. 2015, 19, 1008–1018. [Google Scholar] [CrossRef]
- Olatunji, O.O.; Akinlabi, S.; Madushele, N.; Adedeji, P.A.; Ndolomingo, M.J.; Meshack, T. Blended tropical almond residue for fuel production: Characteristics, energy benefits, and emission reduction potential. J. Clean. Prod. 2020, 267, 122013. [Google Scholar] [CrossRef]
- Remón, J.; Latorre-Viu, J.; Matharu, A.S.; Pinilla, J.L.; Suelves, I. Analysis and optimisation of a novel ‘almond-refinery’ concept: Simultaneous production of biofuels and value-added chemicals by hydrothermal treatment of almond hulls. Sci. Total Environ. 2021, 765, 142671. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Benzina, M.; Ammar, N. Preparation, modification and industrial application of activated carbon from almond shell. J. Ind. Eng. Chem. 2013, 19, 2092–2099. [Google Scholar] [CrossRef]
- Thitame, P.V.; Shukla, S.R. Removal of lead (II) from synthetic solution and industry wastewater using almond shell activated carbon. Environ. Prog. Sustain. Energy 2017, 36, 1628–1633. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from almond (Prunus dulcis) shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Aguado, R.; Cuevas, M.; Pérez-Villarejo, L.; Martínez-Cartas, M.L.; Sánchez, S. Upgrading almond-tree pruning as a biofuel via wet torrefaction. Renew. Energy 2020, 145, 2091–2100. [Google Scholar] [CrossRef]
| Almond By-Products | Administration | Fortified Food | Nutritional and Technological Effects | References |
|---|---|---|---|---|
| AHs | 7–20% in cow feedstock | Milk | Increased fat content, reduced protein content, no effect in lactose and total solids | [13] |
| 4.0 kg of dry matter/day in cow feedstock | Milk | No effect in fat, protein and lactose contents or fatty acids | [82] | |
| 7.5 and 15% in laying hens feedstock | Eggs | No effect in egg quality | [83] | |
| AHs and ASHs | Bed of feedstock | Edible larvae | Increased harvest weight, harvest yield and calcium content | [84] |
| ASHs | 3–15.3% alkali extract | Biscuit | Increased TDF, SDF and IDF, a* value, and hardness; reduced L* and b* values, sensory scores for color; no effect in sensory scores for flavor, crispness, mouthfeel, hardness, and overall acceptance | [85] |
| ASKs | (30–100 g/kg) | Wheat flour dough | Increased water absorption, dough stability, tenacity/extensibility ratio, L* and a* value; reduced softening index, deformation energy, b* value; no effect in dough development time | [50] |
| Allergens | Mw | Biochemical Functions | Effects on Food Processing | Clinical Effects |
|---|---|---|---|---|
| Pru du (γ-conglutin) | 45 kDa | Vicillin storage protein | n.d. | Unclear symptoms |
| Pru du 1 | 17 kDa | Protection against pathogens and environmental stresses | Wet heat protection | Reduction of immunoglobulin E (IgE)-mediated reactivity. Mild immune reaction |
| Pru du 2 | 23–27 kDa | Protection against pathogens and osmotic stresses | Heat and pH protection | Potent immunogenicity |
| Pru du 2S | 12 kDa | Nut storage protein | Heat resistance | Unclear symptoms |
| Pru du 3 | 9 kDa | Lipid transfer protein | Heat and pH protection | Systemic and life-threatening symptoms |
| Pru du 4 | 14 kDa | Actin-binding protein | Heat dissipation | Mild immune response |
| Pru du 5 | 10 kDa | Involvement in protein synthesis | Thermal stability | IgE-mediated allergic reactions |
| Pru du 6 (amandin) | 360 kDa | Storage protein | Thermal stability | Severe IgE-mediated allergic reactions |
| Research Model | Dose 1 | Toxic Events | References |
|---|---|---|---|
| 58-year-old healthy woman | 50 bitter almonds | Dizziness, vomit, encephalopathy, severe lactic acidosis | [112] |
| 4-year-old male child with malignant brain disease | 2000 mg/day | Severe metabolic and lactic acidosis, unresponsiveness | [113] |
| 41-year-old healthy woman | 15 g | Metabolic acidosis, respiratory insufficiency, hypothermia | [114] |
| 35-year-old mentally ill woman | >20 almonds | Fast apnea, hypoxia, and respiratory insufficiency | [115] |
| 57-year-old woman with breast cancer | Overdose of amygdalin | Death, HCN accumulation of 218 µg/dL | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A.; et al. Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods 2021, 10, 1823. https://doi.org/10.3390/foods10081823
Garcia-Perez P, Xiao J, Munekata PES, Lorenzo JM, Barba FJ, Rajoka MSR, Barros L, Mascoloti Sprea R, Amaral JS, Prieto MA, et al. Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods. 2021; 10(8):1823. https://doi.org/10.3390/foods10081823
Chicago/Turabian StyleGarcia-Perez, Pascual, Jianbo Xiao, Paulo E. S. Munekata, Jose M. Lorenzo, Francisco J. Barba, Muhammad Shahid Riaz Rajoka, Lillian Barros, Rafael Mascoloti Sprea, Joana S. Amaral, Miguel A. Prieto, and et al. 2021. "Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review" Foods 10, no. 8: 1823. https://doi.org/10.3390/foods10081823
APA StyleGarcia-Perez, P., Xiao, J., Munekata, P. E. S., Lorenzo, J. M., Barba, F. J., Rajoka, M. S. R., Barros, L., Mascoloti Sprea, R., Amaral, J. S., Prieto, M. A., & Simal-Gandara, J. (2021). Revalorization of Almond By-Products for the Design of Novel Functional Foods: An Updated Review. Foods, 10(8), 1823. https://doi.org/10.3390/foods10081823













