Effects of Combined Aerosolization with Ultraviolet C Light-Emitting Diode on Enterohemorrhagic Escherichia coli and Staphylococcus aureus Attached to Soft Fresh Produce
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains
2.2. Culture Preparation
2.3. Sample Preparation and Inoculation
2.4. Microbial Reduction Procedure of Soft Fresh Produce
2.4.1. Procedure of Aerosolization Treatment with Sanitizers
2.4.2. Procedure of Ultraviolet C Light-Emitting Diode (UVC LED) Irradiation Treatment
2.5. Microbial Analysis
2.6. Quality Evaluation during Storage at 10 and 15 °C after the Combined Treatment
2.6.1. Behaviors of EHEC and S. aureus on Soft Fresh Produce during Storage
2.6.2. Moisture Loss Analysis
2.6.3. Colour Measurement and Visual Appearance Evolution
2.7. Statistical Analysis
3. Results and Discussion
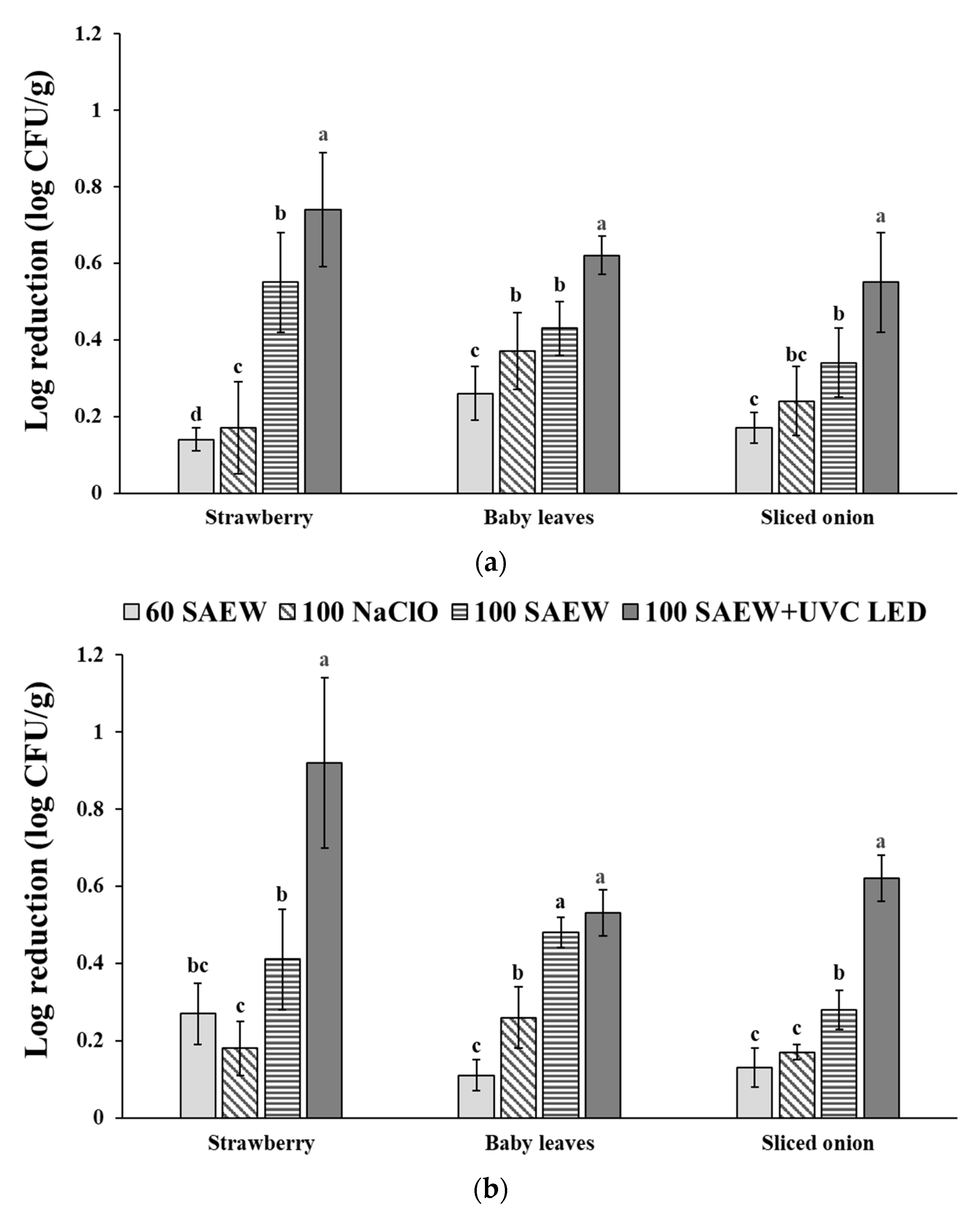
3.1. Effect of Aerosolization Alone and Combined Treatment with UVC LED on the Reduction in EHEC and S. aureus
3.2. Effect of Combined Treatment of Aerosolization with UVC LED on Behaviours of EHEC and S. aureus in Soft Fresh Produce during Storage at 10 and 15 °C
3.3. Effect of Combined Treatment of Aerosolization and UVC LED Irradiation on Moisture Loss of Soft Fresh Produce during Storage at 10 and 15 °C
3.4. Effect of Combined Treatment of Aerosolization and UVC LED on Visual Appearance Evolution and Colour Measurement of Soft Fresh Produce during Storage at 10 and 15 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food Information Statistics System (FIS). Status of Processed Food Segment Markets 2019-Convenience Food Market. 2019. Available online: https://www.Atfis.or.Kr/Article/M001050000/View.Do?ArticleId=3260&page=2&searchKey=&searchString=&searchCategory= (accessed on 11 December 2020).
- Statista (2020) Fresh-Food Meal-Kit Delivery Market Value U.S. 2016–2022. Available online: https://www.Statista.Com/Statistics/761621/Meal-Kit-Delivery-Service-Market-Value/ (accessed on 14 January 2021).
- Centers for Disease Control and Prevention (CDC). List of Selected Multistate Foodborne Outbreak Investigations. 2020. Available online: https://www.Cdc.Gov/Foodsafety/Outbreaks/Multistate-Outbreaks/Outbreaks-List.Html (accessed on 15 January 2021).
- Huang, Y.; Ye, M.; Chen, H. Inactivation of Escherichia coli O157:H7 and Salmonella spp. in Strawberry Puree by High Hydrostatic Pressure with/without Subsequent Frozen Storage. Int. J. Food Microbiol. 2013, 160, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Luna-Gierke, R.E.; Griffin, P.M.; Gould, L.H.; Herman, K.; Bopp, C.A.; Strockbine, N.; Mody, R.K. Outbreaks of Non-O157 Shiga Toxin-Producing Escherichia coli Infection: USA. Epidemiol. Infect. 2014, 142, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Eurosurveillance. National Outbreak of Shiga Toxin-Producing Escherichia coli O157: H7 Linked to Mixed Salad Leaves, United Kingdom, 2016. 2018. Available online: https://www.Eurosurveillance.Org/Content/10.2807/1560-7917.ES.2018.23.18.17-00197 (accessed on 16 January 2021).
- Kozak, G.K.; MacDonald, D.; Landry, L.; Farber, J.M. Foodborne Outbreaks in Canada Linked to Produce: 2001 through 2009. J. Food Prot. 2013, 76, 173–183. [Google Scholar] [CrossRef]
- Kim, S.R.; Chu, H.J.; Yi, S.W.; Jang, Y.J.; Shim, W.B.; Nguyen, B.H.; Kim, W.-I.; Kim, H.J.; Ryu, K. Investigation of Hazardous Microorganisms in Baby Leafy Vegetables Collected from a Korean Market and Distribution Company. J. Food Hyg. Saf. 2019, 34, 526–533. [Google Scholar] [CrossRef]
- Tango, C.N.; Wei, S.; Khan, I.; Hussain, M.S.; Kounkeu, P.F.N.; Park, J.; Kim, S.; Oh, D.H. Microbiological Quality and Safety of Fresh Fruits and Vegetables at Retail Levels in Korea. J. Food Sci. 2018, 83, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Song, B.R.; Kim, S.H.; Kim, J.K.; Han, J.A.; Kwak, H.S.; Chung, K.T.; Heo, E.J. Establishment of Microbial Criteria by Investigation of Microbial Contamination in Ready-to-Eat Foods. J. Food Hyg. Saf. 2017, 32, 348–354. [Google Scholar] [CrossRef]
- Kim, W.I.; Gwak, M.; Jo, A.R.; Ryu, S.D.; Kim, S.R.; Ryu, S.H.; Kim, H.Y.; Ryu, J.G. Investigation of Microbiological Safety of on-farm Produce in Korea. J. Food Hyg. Saf. 2017, 32, 20–26. [Google Scholar] [CrossRef]
- Quansah, J.K.; Gazula, H.; Holland, R.; Scherm, H.; Li, C.; Takeda, F.; Chen, J. Microbial Quality of Blueberries for the Fresh Market. Food Control 2019, 100, 92–96. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Food Engineering Series; Springer: Boston, MA, USA, 2017; pp. 403–432. ISBN 978-1-4939-7018-6. [Google Scholar]
- Lee, H.O.; Kim, J.Y.; Yoon, D.H.; Cha, H.S.; Kim, G.H.; Kim, B.S. Microbial Contamination in a Fresh-Cut Onion Processing Facility. Korean J. Food Preserv. 2009, 16, 567–572. [Google Scholar]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological Quality of Fresh, Minimally-Processed Fruit and Vegetables, and Sprouts from Retail Establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y. Storage Characteristics and Relationships between Microbial Growth Parameters and Shelf Life of MAP Sliced Onions. Postharvest Biol. Technol. 2006, 40, 262–268. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, X.; Li, X.; Gurtler, J.B.; Mukhopadhyay, S.; Jin, T. Inactivation of Salmonella Typhimurium and Quality Preservation of Cherry Tomatoes by In-Package Aerosolization of Antimicrobials. Food Control 2017, 73, 411–420. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, D.H. Combination Treatment of Chlorine Dioxide Gas and Aerosolized Sanitizer for Inactivating Foodborne Pathogens on Spinach Leaves and Tomatoes. Int. J. Food Microbiol. 2015, 207, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Lee, S.Y.; Park, K.H.; Chung, M.S.; Ryu, S.R.; Kang, D.H. Effect of Aerosolized Malic Acid against Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 on Spinach and Lettuce. Food Control 2012, 24, 171–176. [Google Scholar] [CrossRef]
- Oh, S.W.; Dancer, G.I.; Kang, D.H. Efficacy of Aerosolized Peroxyacetic Acid as a Sanitizer of Lettuce Leaves. J. Food Prot. 2005, 68, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Specht, I.; Cho, G.S.; Graef, V.; Stahl, M.R. UV-C-Inactivation of Microorganisms in Naturally Cloudy Apple Juice Using Novel Inactivation Equipment Based on Dean Vortex Technology. Food Control 2009, 20, 1103–1107. [Google Scholar] [CrossRef]
- Guerrero-Beltr·n, J.A.; Barbosa-C·novas, G.V. Advantages and Limitations on Processing Foods by UV Light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Green, A.; Popović, V.; Warriner, K.; Koutchma, T. The Efficacy of UVC LEDs and Low Pressure Mercury Lamps for the Reduction of Escherichia coli O157:H7 and Listeria monocytogenes on Produce. Innov. Food Sci. Emerg. Technol. 2020, 64, 102410. [Google Scholar] [CrossRef]
- Selin, H.; Keane, S.E.; Wang, S.; Selin, N.E.; Davis, K.; Bally, D. Linking Science and Policy to Support the Implementation of the Minamata Convention on Mercury. Ambio 2018, 47, 198–215. [Google Scholar] [CrossRef]
- Green, A.; Popović, V.; Pierscianowski, J.; Biancaniello, M.; Warriner, K.; Koutchma, T. Inactivation of Escherichia coli, Listeria and Salmonella by Single and Multiple Wavelength Ultraviolet-Light Emitting Diodes. Innov. Food Sci. Emerg. Technol. 2018, 47, 353–361. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, S.J.; Kim, D.K.; Kang, D.H. Fundamental Characteristics of Deep-UV Light-Emitting Diodes and Their Application to Control Foodborne Pathogens. Appl. Environ. Microbiol. 2016, 82, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Würtele, M.A.; Kolbe, T.; Lipsz, M.; Külberg, A.; Weyers, M.; Kneissl, M.; Jekel, M. Application of GaN-Based Ultraviolet-C Light Emitting Diodes—UV LEDs—For Water Disinfection. Water Res. 2011, 45, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yang, S.Y.; Yoon, K.S. Control Measures of Pathogenic Microorganisms and Shelf-Life Extension of Fresh-Cut Vegetables. Foods 2021, 10, 655. [Google Scholar] [CrossRef]
- Jiang, Y.; Ai, C.; Liao, X.; Liu, D.; Ding, T. Effect of Slightly Acidic Electrolyzed Water (SAEW) and Ultraviolet Light Illumination Pretreatment on Microflora Inactivation of Coriander. LWT 2020, 132, 109898. [Google Scholar] [CrossRef]
- Kim, D.K.; Kang, D.H. Inactivation Efficacy of a Sixteen UVC LED Module to Control Foodborne Pathogens on Selective Media and Sliced Deli Meat and Spinach Surfaces. LWT 2020, 130, 109422. [Google Scholar] [CrossRef]
- Seong, J.Y.; Kwon, K.H.; Oh, S.W. Combined Effect of Aerosolized Malic Acid and UV-C for the Inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on Fresh-Cut Lettuce. J. Food Saf. 2017, 37, e12359. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, D.K.; Kang, D.H. Using UVC Light-Emitting Diodes at Wavelengths of 266 to 279 Nanometers to Inactivate Foodborne Pathogens and Pasteurize Sliced Cheese. Appl. Environ. Microbiol. 2016, 82, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ro, E.Y.; Yoon, K.S. Comparison of Growth Kinetics of Various Pathogenic E. coli on Fresh Perilla Leaf. Foods 2013, 2, 364–373. [Google Scholar] [CrossRef]
- Cho, J.L.; Kim, C.K.; Park, J.Y.; Kim, J.M. Efficacy of Aerosolized Chlorine Dioxide in Reducing Pathogenic Bacteria on Washed Carrots. Food Sci. Biotechnol. 2017, 26, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Ha, J.W.; Park, K.H.; Chung, M.S.; Kang, D.H. Infrared Sensor–Based Aerosol Sanitization System for Controlling Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on Fresh Produce. J. Food Prot. 2014, 77, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, S.J.; Yoon, K.S. Efficacy Evaluation of Control Measures on the Reduction of Staphylococcus aureus in Salad and Bacillus cereus in Fried Rice Served at Restaurants. Foodborne Pathog. Dis. 2017, 15, 198–209. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety (MFDS). A Study on Improvement of Usage Standards for Food Sanitizers (Chlorine Dioxide, Sodium Hypochlorite). 2018. Available online: https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201900003485 (accessed on 18 July 2021).
- Han, R.; Liao, X.; Ai, C.; Ding, T.; Wang, J. Sequential Treatment with Slightly Acidic Electrolyzed Water (SAEW) and UVC Light-Emitting Diodes (UVC-LEDs) for Decontamination of Salmonella Typhimurium on Lettuce. Food Control 2021, 123, 107738. [Google Scholar] [CrossRef]
- Lee, J.H.; Chun, H.H.; Oh, D.H.; Park, J.Y.; Won, M.S.; Song, K.B. Sensory and Microbiological Qualities of Romaine Lettuce and Kale Affected by a Combined Treatment of Aqueous Chlorine Dioxide and Ultraviolet-C. Hort. Environ. Biotechnol. 2012, 53, 387–396. [Google Scholar] [CrossRef]
- Moreno, C.; Andrade-Cuvi, M.J.; Zaro, M.J.; Darre, M.; Vicente, A.R.; Concellón, A. Short UV-C Treatment Prevents Browning and Extends the Shelf-Life of Fresh-Cut Carambola. J. Food Qual. 2017, 2017, e2548791. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll breakdown in higher plants and algae. Cell. Mol. Life Sci. 1999, 56, 330–347. [Google Scholar] [CrossRef]
- Matile, P.; Hörtensteiner, S.; Thomas, H. Chlorophyll degradation. Annu. Rev. Plant Biol. 1999, 50, 67–95. [Google Scholar] [CrossRef]
- Nimitkeatkai, H.; Kim, J.G. Washing Efficiency of Acidic Electrolyzed Water on Microbial Reduction and Quality of ‘Fuji’ Apples. Kor. J. Hort. Sci. Technol. 2009, 27, 250–255. [Google Scholar]
- Massey, L.M.; Hettiarachchy, N.S.; Martin, E.M.; Ricke, S.C. Electrostatic Spray of Food-Grade Organic Acids and Plant Extract to Reduce Escherichia coli O157:H7 on Fresh-Cut Cantaloupe Cubes. J. Food Saf. 2013, 33, 71–78. [Google Scholar] [CrossRef]
- Lee, C.L.; Kim, Y.H.; Ha, S.D.; Yoon, Y.H.; Yoon, K.S. Risk assessment of Staphylococcus aureus infection in ready-to-eat Samgak-Kimbap. Korean J. Food Sci. Technol. 2020, 52, 661–669. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.B.; Sargent, S.A. Possible Influences of Water Loss and Polyphenol Oxidase Activity on Anthocyanin Content and Discoloration in Fresh Ripe Strawberry (Cv. Oso Grande) During Storage at 1 °C. J. Food Sci. 2005, 70, S79–S84. [Google Scholar] [CrossRef]
- Jalali, A.; Linke, M.; Geyer, M.; Mahajan, P.V. Shelf-Life Prediction Model for Strawberry Based on Respiration and Transpiration Processes. Food Packag. Shelf Life 2020, 25, 100525. [Google Scholar] [CrossRef]
- Nalbandi, H.; Seiiedlou, S. Sensitivity Analysis of the Precooling Process of Strawberry: Effect of Package Designing Parameters and the Moisture Loss. Food Sci. Nutr. 2020, 8, 2458–2471. [Google Scholar] [CrossRef]
- Ahmed, W.; Butt, M.S. Preserving Strawberry (Fragaria Ananasa) Using Alginate and Soy Based Edible Coatings. Am. J. Food Technol. 2014, 2, 158–161. [Google Scholar] [CrossRef][Green Version]
- de Souza, V.R.; Popović, V.; Warriner, K.; Koutchma, T. A Comparative Study on the Inactivation of Penicillium Expansum Spores on Apple Using Light Emitting Diodes at 277 nm and a Low-Pressure Mercury Lamp at 253.7 nm. Food Control 2020, 110, 107039. [Google Scholar] [CrossRef]
- Funamoto, Y.; Yamauchi, N.; Shigenaga, T.; Shigyo, M. Effects of heat treatment on chlorophyll degrading enzymes in stored broccoli (Brassica oleracea L.). Postharvest Biol. Technol. 2002, 24, 163–170. [Google Scholar] [CrossRef]
- Chairat, B.; Nutthachai, P.; Varit, S. Effect of UV-C Treatment on Chlorophyll Degradation, Antioxidant Enzyme Activities and Senescence in Chinese Kale (Brassica Oleracea Var. Alboglabra). Int. Food Res. J. 2013, 20, 623–628. [Google Scholar]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C Treatment Delays Postharvest Senescence in Broccoli Florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]






| Treatments | Storage Time | Strawberry | Baby Leaves | Sliced Onion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | ||
| Control | 0 d | 33.90 ± 0.86 a | 35.17 ± 0.88 ab | 15.68 ± 0.54 a | 35.26 ± 1.19 c | −9.96 ± 0.46 a | 11.18 ± 0.14 e | 63.00 ± 1.14 a | −1.07 ± 0.12 a | 3.26 ± 0.05 d |
| 4 d | 34.59 ± 1.57 a | 24.36 ± 1.23 c | 13.11 ± 1.39 bc | 43.07 ± 4.01 b | −10.18 ± 0.15 a | 15.28 ± 0.80 b | 62.85 ± 0.91 a | −1.12 ± 0.13 a | 6.25 ± 0.30 b | |
| 7 d | 33.84 ± 1.06 a | 9.71 ± 0.77 d | 11.20 ± 0.20 d | 62.74 ± 3.33 a | −9.96 ± 0.54 a | 28.87 ± 0.13 a | 62.70 ± 0.62 a | −1.14 ± 0.02 a | 8.74 ± 0.18 a | |
| 100 SAEW | 0 d | 34.68 ± 0.84 a | 35.57 ± 0.47 a | 16.10 ± 0.38 a | 34.28 ± 2.18 c | −10.07 ± 0.57 a | 11.79 ± 0.59 de | 62.01 ± 0.40 ab | −1.05 ± 0.10 a | 3.36 ± 0.33 d |
| 4 d | 35.06 ± 0.79 a | 23.05 ± 4.39 c | 13.63 ± 0.79 b | 35.60 ± 3.56 c | −9.97 ± 0.34 a | 14.79 ± 0.89 b | 62.31 ± 0.84 a | −1.14 ± 0.06 a | 6.34 ± 0.37 b | |
| 7 d | 35.07 ± 0.90 a | 9.54 ± 0.90 d | 11.76 ± 0.27 d | 59.07 ± 1.31 a | −10.52 ± 0.47 a | 28.13 ± 1.43 a | 61.97 ± 0.54 ab | −1.16 ± 0.16 a | 8.66 ± 0.89 a | |
| 100 SAEW + UVC LED | 0 d | 34.57 ± 0.92 a | 35.33 ± 0.63 a | 15.92 ± 0.72 a | 34.10 ± 0.23 c | −10.24 ± 0.36 a | 11.08 ± 0.68 e | 61.25 ± 1.29 ab | −1.06 ± 0.12 a | 3.50 ± 0.20 d |
| 4 d | 34.11 ± 0.39 a | 34.17 ± 0.60 ab | 13.07 ± 0.37 bc | 36.94 ± 0.84 c | −9.96 ± 0.22 a | 12.94 ± 0.35 cd | 61.72 ± 1.42 ab | −1.09 ± 0.13 a | 4.94 ± 0.53 c | |
| 7 d | 34.33 ± 0.79 a | 31.53 ± 1.10 b | 11.76 ± 0.27 cd | 35.99 ± 1.74 c | −9.53 ± 0.50 a | 13.64 ± 0.89 bc | 60.14 ± 0.51 b | −1.24 ± 0.06 a | 6.12 ± 0.35 b | |
| Treatments | Storage Time | Strawberry | Baby Leaves | Sliced Onion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | ||
| Control | 0 d | 34.57 ± 0.83 a | 34.85 ± 0.71 a | 16.13 ± 0.63 a | 35.62 ± 3.23 d | −9.98 ± 0.38 a | 11.67 ± 1.04 d | 60.83 ± 1.08 a | −1.04 ± 0.12 a | 3.35 ± 0.17 c |
| 4 d | 34.82 ± 0.60 a | 16.05 ± 1.82 c | 14.07 ± 0.50 b | 51.95 ± 0.48 b | −10.06 ± 1.02 a | 24.35 ± 1.68 b | 60.71 ± 1.14 a | −1.19 ± 0.24 a | 7.79 ± 0.29 b | |
| 7 d | 35.22 ± 0.19 a | 9.40 ± 0.85 d | 10.99 ± 0.64 c | 63.91 ± 1.26 a | −10.48 ± 0.68 a | 31.85 ± 1.33 a | 61.42 ± 1.66 a | −1.06 ± 0.08 a | 12.07 ± 1.65 a | |
| 100 SAEW | 0 d | 34.43 ± 0.63 a | 35.16 ± 0.41 a | 16.39 ± 0.86 a | 35.19 ± 1.91 d | −9.86 ± 0.29 a | 11.24 ± 0.73 d | 61.95 ± 1.46 a | −1.06 ± 0.17 a | 3.33 ± 0.11 c |
| 4 d | 34.60 ± 0.86 a | 18.72 ± 2.05 c | 14.08 ± 0.28 b | 46.15 ± 0.80 c | −10.43 ± 0.50 a | 15.84 ± 0.55 c | 61.14 ± 0.85 a | −1.14 ± 0.07 a | 7.65 ± 0.48 b | |
| 7 d | 34.90 ± 0.42 a | 10.33 ± 0.89 d | 10.60 ± 0.67 c | 61.12 ± 0.39 a | −9.63 ± 0.48 a | 26.05 ± 1.81 b | 62.48 ± 1.45 a | −1.03 ± 0.09 a | 11.41 ± 0.88 a | |
| 100 SAEW + UVC LED | 0 d | 34.71 ± 0.25 a | 34.79 ± 0.41 a | 16.25 ± 0.25 a | 34.01 ± 0.37 d | −9.56 ± 0.21 a | 11.26 ± 0.87 d | 61.31 ± 0.61 a | −1.13 ± 0.07 a | 3.45 ± 0.13 c |
| 4 d | 34.77 ± 0.83 a | 34.42 ± 0.62 a | 13.94 ± 0.31 b | 34.46 ± 1.43 d | −9.96 ± 0.54 a | 14.31 ± 0.56 c | 62.26 ± 2.71 a | −1.18 ± 0.14 a | 6.67 ± 0.18 b | |
| 7 d | 34.86 ± 0.11 a | 30.59 ± 2.68 b | 11.33 ± 0.35 c | 35.21 ± 0.63 d | −10.08 ± 0.39 a | 15.19 ± 0.43 c | 61.56 ± 0.75 a | −1.14 ± 0.08 a | 7.27 ± 0.29 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Kim, G.-H.; Yoon, K.-S. Effects of Combined Aerosolization with Ultraviolet C Light-Emitting Diode on Enterohemorrhagic Escherichia coli and Staphylococcus aureus Attached to Soft Fresh Produce. Foods 2021, 10, 1834. https://doi.org/10.3390/foods10081834
Lee C-L, Kim G-H, Yoon K-S. Effects of Combined Aerosolization with Ultraviolet C Light-Emitting Diode on Enterohemorrhagic Escherichia coli and Staphylococcus aureus Attached to Soft Fresh Produce. Foods. 2021; 10(8):1834. https://doi.org/10.3390/foods10081834
Chicago/Turabian StyleLee, Chae-Lim, Geun-Hyang Kim, and Ki-Sun Yoon. 2021. "Effects of Combined Aerosolization with Ultraviolet C Light-Emitting Diode on Enterohemorrhagic Escherichia coli and Staphylococcus aureus Attached to Soft Fresh Produce" Foods 10, no. 8: 1834. https://doi.org/10.3390/foods10081834
APA StyleLee, C.-L., Kim, G.-H., & Yoon, K.-S. (2021). Effects of Combined Aerosolization with Ultraviolet C Light-Emitting Diode on Enterohemorrhagic Escherichia coli and Staphylococcus aureus Attached to Soft Fresh Produce. Foods, 10(8), 1834. https://doi.org/10.3390/foods10081834






