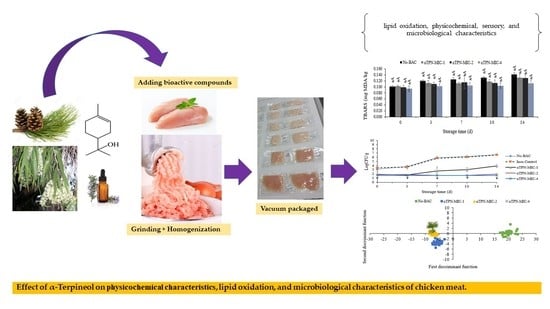
Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Meat Samples and Experimental Design
2.2. Physicochemical Properties
2.2.1. Measurement of pH
2.2.2. Color Measurement
- -
- Chroma: C* = [(a*)2 + (b*)2]1/2.
- -
- Hue angle: h* = tan−1 (arctangent) (b*/a*).
2.2.3. Measurement of Water Holding Capacity (WHC)
2.2.4. Determination of Metmyoglobin, Deoxymyoglobin, and Oxymyoglobin Pigments
- % MetMb = (−0.159𝑅1 − 0.085𝑅2 + 1.262𝑅3 − 0.520) ∗ 100
- % DeoMb = (−0.543𝑅1 + 1.594𝑅2 + 0.552𝑅3 − 1.329) ∗ 100
- % OxyMb = (0.722𝑅1 − 1.432𝑅2 − 1.659𝑅3 + 2.599) ∗ 100
- *𝑅1 = 𝐴582/𝐴557, 𝑅2 = 𝐴557/𝐴525, and 𝑅3 = 𝐴503/𝐴525
2.3. Determinations of Thiobarbituric Acid-Reactive Substances (TBARS)
2.4. Microbiological Properties
2.4.1. In Vitro Anti-Microbial Activity of BACs
2.4.2. Disc Diffusion Assay
2.4.3. Minimal Inhibition Concentration (MIC)—Micro-Dilution Method
2.5. Determination of Aerobic Mesophilic Counts (AMCs), Pseudomonas lundensis, Listeria monocytogenes, and Salmonella Typhimurium in Meat
2.5.1. Preparation of Bacterial Strains and Inocula
2.5.2. Bacterial Inoculation on Chicken Meat
2.5.3. Microbial Enumeration
2.6. Electronic Nose Analysis
2.7. Statistical Analysis
3. Results
3.1. Evaluation of the In Vitro Antimicrobial Activity of BACs
3.1.1. Using Disc Diffusion Assay
3.1.2. Using the MIC Method
3.2. Physicochemical Properties
3.2.1. pH of Meat
3.2.2. Color Values
3.2.3. Water Holding Capacity
3.2.4. Meat Pigments (Metmyoglobin, Deoxymyoglobin, and Oxymyoglobin)
3.3. Thiobarbituric Acid-Reactive Substances (TBARS)
3.4. Microbiological Characteristics of Chicken Meat
3.5. Electronic Nose
4. Discussion
4.1. Evaluation of the In Vitro Antimicrobial Activity of BACs
4.2. Effect of αTPN on the Physicochemical Properties of Chicken Meat
4.3. Effect of αTPN on the TBARS Values of Chicken Meat
4.4. Effect of αTPN on the Microbiological Properties of Chicken Meat
4.5. Effect of αTPN on the Smell Detection by Electronic-Nose in Chicken Meat
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Total weight | 100 | g | ||||||
| MIC1: | 2.5 | g αTPN in | 1000 | g final mixture | let 1 mL αTPN is 1 g αTPN | |||
| if | 2.5 | g αTPN in | 1000 | g final mixture | ||||
| then | 0.25 | g αTPN in | 100 | g final mixture | ||||
| αTPN | 0.25 | g | ||||||
| + | ethanol | 1.25 | g | =5× weight of αTPN | ||||
| αTPN + ethanol | 1.5 | g | ||||||
| + | DW | 3.45 | g | |||||
| αTPN + DW + ethanol | 5 | g | 5 | % of meat | ||||
| + | meat | 95 | g | 95 | % of total weight | |||
| 100 | g | total weight | ||||||
| The concentration of αTPN in MIC-2 were ×2, and in MIC-4 were ×4 | ||||||||
References
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [Green Version]
- Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- FAO. World Livestock 2011—Livestock in Food Security. Available online: http://www.fao.org/3/i2373e/i2373e00.htm (accessed on 8 May 2020).
- Iulietto, M.F.; Sechi, P.; Borgogni, E.; Cenci-Goga, B.T. Meat Spoilage: A Critical Review of a Neglected Alteration Due to Ropy Slime Producing Bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Hayat, M.N.; Kaka, U.; Sazili, A.Q. Assessment of Physicochemical Characteristics and Microbiological Quality in Broiler Chicken Breast Muscle (Pectoralis major) Subjected to Different Temperatures and Lengths of Cold Transportation. Foods 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Sant’Ana, A.S.; Igarashi, M.C.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Prevalence, populations and pheno- and genotypic characteristics of Listeria monocytogenes isolated from ready-to-eat vegetables marketed in São Paulo, Brazil. Int. J. Food Microbiol. 2012, 155, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Coronado, S.A.; Trout, G.R.; Dunshea, F.R.; Shah, N.P. Antioxidant effects of rosemary extract and whey powder on the oxidative stability of wiener sausages during 10 months frozen storage. Meat Sci. 2002, 62, 217–224. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Morrissey, P.A.; Sheehy, P.J.; Galvin, K.; Kerry, J.P.; Buckley, D.J. Lipid stability in meat and meat products. Meat Sci. 1998, 49S1, S73–S86. [Google Scholar] [CrossRef]
- Sampels, S. Oxidation and Antioxidants in Fish and Meat from Farm to Fork. Food Ind. 2013, 114–144. [Google Scholar]
- WHO (Ed.) WHO Estimates of the Global Burden of Foodborne Diseases; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-156516-5. [Google Scholar]
- Mohammed, M. Phage typing or CRISPR typing for epidemiological surveillance of Salmonella Typhimurium? BMC Res. Notes 2017, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Eng, S.-K.; Pusparajah, P.; Mutalib, N.-S.A.; Ser, H.-L.; Chan, K.-G.; Lee, L.-H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef] [Green Version]
- EFSA. EU summary report on zoonoses, zoonotic agents and food-borne outbreaks 2012. EFSA J. 2014, 12, 3547. [Google Scholar]
- Molina, L.; Bernal, P.; Udaondo, Z.; Segura, A.; Ramos, J.-L. Complete Genome Sequence of a Pseudomonas putida Clinical Isolate, Strain H8234. Genome Announc. 2013, 1, e00496-13. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, D.; Russo, F.; Nasi, A.; Ferranti, P.; Villani, F. Mesophilic and Psychrotrophic Bacteria from Meat and Their Spoilage Potential In Vitro and in Beef. Appl. Environ. Microbiol. 2009, 75, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Mellor, G.E.; Bentley, J.A.; Dykes, G.A. Evidence for a role of biosurfactants produced by Pseudomonas fluorescens in the spoilage of fresh aerobically stored chicken meat. Food Microbiol. 2011, 28, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.C. Food Safety Control in the Poultry Industry, 1st ed.; CRC Press LLC: Boca Raton, FL, USA, 2005; ISBN 978-1-84569-023-6. [Google Scholar]
- Loncarevic, S.; Tham, W.; Danielsson-Tham, M.L. Occurrence of Listeria species in broilers pre- and post-chilling in chlorinated water at two slaughterhouses. Acta Vet. Scand. 1994, 35, 149–154. [Google Scholar] [CrossRef]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- EFSA. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J. 2017, 15, 5077. [Google Scholar]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus aureus and analysis of associated bacterial communities on food industry surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Moaddabdoost Baboli, Z.; Schimmel, K.; Jafari, S.M.; Williams, L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci. Technol. 2020, 96, 61–78. [Google Scholar] [CrossRef]
- Shin, J.; Harte, B.; Ryser, E.; Selke, S. Active packaging of fresh chicken breast, with allyl isothiocyanate (AITC) in combination with modified atmosphere packaging (MAP) to control the growth of pathogens. J. Food Sci. 2010, 75, M65–M71. [Google Scholar] [CrossRef]
- Ward, S.M.; Delaquis, P.J.; Holley, R.A.; Mazza, G. Inhibition of spoilage and pathogenic bacteria on agar and pre-cooked roast beef by volatile horseradish distillates. Food Res. Int. 1998, 31, 19–26. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Bertani, G.; Maoloni, A.; Bernini, V.; Levante, A.; Neviani, E.; Lazzi, C. Antimicrobial Activity of Fermented Vegetable Byproduct Extracts for Food Applications. Foods 2021, 10, 1092. [Google Scholar] [CrossRef]
- FDA. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101 (accessed on 9 May 2020).
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Preedy, V.R. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-416644-8. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Friedrich, L.; Kisko, G.; Ayari, E.; Nemeth, C.; Dalmadi, I. Use of allyl-isothiocyanate and carvacrol to preserve fresh chicken meat during chilling storage. Czech. J. Food Sci. 2019, 37, 417–424. [Google Scholar] [CrossRef]
- Hussein, K.N.; Friedrich, L.; Pinter, R.; Németh, C.; Kiskó, G.; Dalmadi, I. Effect of linalool and piperine on chicken meat quality during refrigerated conditions. Acta Aliment. 2019, 48, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Elhatmi, M.; Taktak, M.A.; Nasri, M.; Zouari, N. Effect of rosemary leaves and essential oil on turkey sausage quality. Acta Aliment. 2015, 44, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Piñon, M.I.; Alarcon-Rojo, A.D.; Renteria, A.L.; Mendez, G.; Janacua-Vidales, H. Reduction of microorganisms in marinated poultry breast using oregano essential oil and power ultrasound. Acta Aliment. 2015, 44, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, Isolation and Characterization of Bioactive Compounds from Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.G.B.; Brito, R.G.; Santos, P.L.; Araújo-Filho, H.G.; Quintans, J.S.S.; Menezes, P.P.; Serafini, M.R.; Carvalho, Y.M.B.G.; Silva, J.C.; Almeida, J.R.G.S.; et al. α-Terpineol, a monoterpene alcohol, complexed with β-cyclodextrin exerts antihyperalgesic effect in animal model for fibromyalgia aided with docking study. Chem. Biol. Interact. 2016, 254, 54–62. [Google Scholar] [CrossRef]
- Bicas, J.L.; Neri-Numa, I.A.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Pastore, G.M. Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem. Toxicol. 2011, 49, 1610–1615. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Quintans, L., Jr.; de Almeida, R.N. Evolution of the Anticonvulsant Activity of α-Terpineol. Pharm. Biol. 2007, 45, 69–70. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar] [PubMed]
- Pandey, S.K.; Tandon, S.; Ahmad, A.; Singh, A.K.; Tripathi, A.K. Structure-activity relationships of monoterpenes and acetyl derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2013, 69, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.G.; Marques, R.B.; de Santana, M.F.; Santos, A.B.; Brito, F.A.; Barreto, E.O.; De Sousa, D.P.; Almeida, F.R.; Badauê-Passos, D., Jr.; Antoniolli, A.R.; et al. α-Terpineol Reduces Mechanical Hypernociception and Inflammatory Response. Basic Clin. Pharmacol. Toxicol. 2012, 111, 120–125. [Google Scholar] [CrossRef]
- Commission Internationale de L’Eclairage (CIE). Colorimetry, 2nd ed.; CIE: Viena, Austria, 1986; Publication CIE 15.2-1986; ISBN 3-900-734-00-3. [Google Scholar]
- Dias, M.; Soares, N.; Borges, S.; Sousa, M.; Nunes, C.; Oliveira, I.; Medeiros, E. Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef]
- Grau, R.; Hamm, R. A simple method for the determination of water binding in muscles. Naturswissenschaften 1953, 40, 29–30. [Google Scholar] [CrossRef]
- Utama, D.T.; Lee, S.G.; Baek, K.H.; Chung, W.S.; Chung, I.A.; Jeon, J.T.; Lee, S.K. High pressure processing for dark-firm-dry beef: Effect on physical properties and oxidative deterioration during refrigerated storage. Asian-Australas J. Anim. Sci. 2017, 30, 424–431. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki Revisited: Equations for Spectrophotometric Determination of Myoglobin Redox Forms in Aqueous Meat Extracts. J. Food Sci. 2004, 69, C717–C720. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Morcuende, D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- de Oliveira, T.L.; Junior, B.R. de C.L.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Phenolic carvacrol as a natural additive to improve the preservative effects of high pressure processing of low-sodium sliced vacuum-packed turkey breast ham. LWT-Food Sci. Technol. 2015, 64, 1297–1308. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, L.; Siró, I.; Dalmadi, I.; Horváth, K.; Ágoston, R.; Balla, C. Influence of various preservatives on the quality of minced beef under modified atmosphere at chilled storage. Meat Sci. 2008, 79, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Kim, J.; Marshall, M.R.; Wei, C. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Zengin, H.; Baysal, A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules 2014, 19, 17773–17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, P.; Sanchez, C.; Batlle, R.; Nerín, C. Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, C.; Yin, Z.; Jia, R.; Peng, L.; Kang, S.; Li, Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2015, 45, 1409–1413. [Google Scholar] [CrossRef] [Green Version]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Shirzadegan, K.; Falahpour, P. The physicochemical properties and antioxidative potential of raw thigh meat from broilers fed a dietary medicinal herb extract mixture. Open Vet. J. 2014, 4, 69–77. [Google Scholar]
- Bekhit, A.E.D.; Faustman, C. Metmyoglobin reducing activity. Meat Sci. 2005, 71, 407–439. [Google Scholar] [CrossRef]
- Velasco, V.; Williams, P. Improving meat quality through natural antioxidants. Chil. J. Agric. Res. 2011, 71, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Bekhit, A.E.D.; Cassidy, L.; Hurst, R.D.; Farouk, M.M. Post-mortem metmyoglobin reduction in fresh venison. Meat Sci. 2007, 75, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Astorga, M.; Capita, R.; Alonso-Calleja, C.; Moreno, B.; Del, M.; García-Fernández, C. Microbiological quality of retail chicken by-products in Spain. Meat Sci. 2002, 62, 45–50. [Google Scholar] [CrossRef]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef]
- Park, S.-N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef]
- Park, M.J.; Gwak, K.S.; Yang, I.; Kim, K.W.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Poultry meat freshness evaluation using electronic nose technology and ultra-fast gas chromatography. Mon. Chem. Chem. Mon. 2017, 148, 1631–1637. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhao, J.; Wu, M.; Chen, Q. Nondestructive detection of total volatile basic nitrogen (TVB-N) content in pork meat by integrating hyperspectral imaging and colorimetric sensor combined with a nonlinear data fusion. LWT Food Sci. Technol. 2015, 63, 268–274. [Google Scholar] [CrossRef]






| Bacterial Strains | Storage Time (h) | Bioactive Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Cymene | Linalool | Camphor | Piperine | γ-Terpinene | α-Terpineol | α-Pinene | 1,8-Cineole | Carvacrol | ||
| Pseudomonaslundensis | 24 | NI | 1.10 ± 0.06 | NI | NI | NI | 1.83 ± 0.01 | 0.93 ± 0.06 | NI | 5.19 ± 0.02 |
| 48 | NI | 1.10 ± 0.15 | NI | NI | NI | 1.41 ± 0.38 | 0.84 ± 0.18 | NI | 5.16 ± 0.17 | |
| 72 | NI | 1.03 ± 0.00 | NI | NI | NI | 0.94 ± 0.01 | 0.89 ± 0.00 | NI | 5.17 ± 0.06 | |
| Escherichia coli | 24 | NI | 7.12 ± 0.07 | 1.02 ± 0.00 | NI | NI | 3.02 ± 0.15 | 0.77 ± 0.78 | 2.50 ± 0.60 | 20.14 ± 0.73 |
| 48 | NI | 7.42 ± 0.66 | 1.20 ± 0.00 | NI | NI | 2.84 ± 0.03 | 0.53 ± 0.53 | 1.75 ± 0.22 | 16.94 ± 0.66 | |
| 72 | NI | 6.66 ± 0.30 | 1.04 ± 0.00 | NI | NI | 2.24 ± 0.57 | 0.63 ± 0.63 | 2.02 ± 0.18 | 16.76 ± 0.92 | |
| Staphylococcus aureus | 24 | 0.88 ± 0.89 | 4.01 ± 0.23 | NI | NI | 1.02 ± 1.03 | 2.83 ± 0.01 | 2.62 ± 0.77 | NI | 16.70 ± 0.29 |
| 48 | 0.78 ± 0.78 | 3.65 ± 0.23 | NI | NI | 0.73 ± 0.74 | 2.42 ± 0.72 | 1.61 ± 0.26 | NI | 16.20 ± 0.60 | |
| 72 | 0.65 ± 0.65 | 3.38 ± 0.25 | NI | NI | 0.64 ± 0.64 | 1.76 ± 0.25 | 1.44 ± 0.20 | NI | 15.86 ± 0.09 | |
| Listeria monocytogenes | 24 | NI | 3.43 ± 0.15 | NI | NI | NI | 1.86 ± 0.76 | NI | NI | 17.27 ± 1.00 |
| 48 | NI | 3.10 ± 0.02 | NI | NI | NI | 1.13 ± 0.03 | NI | NI | 17.01 ± 1.57 | |
| 72 | NI | 2.83 ± 0.36 | NI | NI | NI | 1.31 ± 0.40 | NI | NI | 17.07 ± 0.99 | |
| Salmonella Typhimurium | 24 | NI | 5.19 ± 0.37 | NI | NI | 1.06 ± 0.06 | 2.33 ± 0.04 | 1.22 ± 0.02 | 1.25 ± 0.25 | 15.15 ± 0.27 |
| 48 | NI | 4.86 ± 0.91 | NI | NI | 1.32 ± 0.02 | 2.09 ± 0.46 | 0.87 ± 0.04 | 1.10 ± 0.40 | 15.45 ± 0.34 | |
| 72 | NI | 5.22 ± 0.40 | NI | NI | 0.80 ± 0.30 | 1.99 ± 0.38 | 0.83 ± 0.15 | 1.12 ± 0.45 | 15.80 ± 0.24 | |
| Bacillus cereus | 24 | NI | 4.30 ± 0.52 | NI | NI | NI | 2.52 ± 0.60 | NI | NI | 17.60 ± 0.39 |
| 48 | NI | 3.84 ± 0.61 | NI | NI | NI | 2.07 ± 1.10 | NI | NI | 14.14 ± 0.12 | |
| 72 | NI | 3.55 ± 0.82 | NI | NI | NI | 1.50 ± 1.02 | NI | NI | 15.35 ± 0.09 | |
| Bacterial Strains | Ethanol | Bioactive Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Cymene | Linalool | Camphor | Piperine | γ-Terpinene | αTPN | α-Pinene | 1,8-Cineole | Carvacrol | ||
| Pseudomonaslundensis | NI | 0.125 | 0.125 | 0.5 | 0.5 | 0.5 | 0.125 | 1 | 0.5 | 0.25 |
| Escherichia coli | NI | 0.5 | 0.125 | 0.5 | 0.5 | 1 | 0.125 | 1 | 0.5 | 0.063 |
| Staphylococcus aureus | NI | 0.5 | 0.125 | 0.5 | 0.5 | 1 | 0.25 | 1 | 0.125 | 0.063 |
| Listeria monocytogenes | NI | 0.5 | 0.125 | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.25 | 0.125 |
| Salmonella Typhimurium | NI | 0.5 | 0.125 | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 0.25 |
| Bacillus cereus | NI | 0.5 | 0.25 | 0.5 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | 0.125 |
| Parameters | Storage Time (d) | Treatments | |||
|---|---|---|---|---|---|
| No-BAC | αTPN-MIC-1 | αTPN-MIC-2 | αTPN-MIC-4 | ||
| pH | 0 | 6.02 ± 0.02 aA | 6.02 ± 0.02 abA | 6.04 ± 0.01 aAB | 6.09 ± 0.03 aB |
| 3 | 6.00 ± 0.02 aA | 6.01 ± 0.02 aAB | 6.04 ± 0.00 aB | 6.11 ± 0.01 aC | |
| 7 | 6.00 ± 0.01 aA | 6.02 ± 0.01 abA | 6.04 ± 0.02 aA | 6.12 ± 0.00 aB | |
| 10 | 6.01 ± 0.01 aA | 6.03 ± 0.01 abAB | 6.05 ± 0.01 abB | 6.11 ± 0.02 aB | |
| 14 | 6.01 ± 0.01 aA | 6.04 ± 0.01 bB | 6.06 ± 0.01 bB | 6.12 ± 0.01 aC | |
| L* | 0 | 46.75 ± 1.01 aA | 49.82 ± 0.59 aB | 50.44 ± 0.67 aB | 55.33 ± 1.24 aC |
| 3 | 47.69 ± 0.69 aA | 49.73 ± 1.75 aA | 52.25 ± 1.24 abB | 58.56 ± 0.91 bC | |
| 7 | 47.23 ± 1.39 aA | 49.02 ± 0.96 aA | 51.95 ± 0.57 abB | 58.66 ± 1.64 bC | |
| 10 | 47.46 ± 0.66 aA | 49.32 ± 0.40 aB | 52.58 ± 1.32 bC | 59.40 ± 1.00 bD | |
| 14 | 48.30 ± 1.32 aA | 50.02 ± 0.66 aA | 52.47 ± 1.28 bB | 59.00 ± 0.79 bC | |
| a* | 0 | 1.41 ± 0.46 aA | 1.36 ± 0.38 aA | 1.66 ± 0.22 aA | 1.75 ± 0.46 aA |
| 3 | 1.66 ± 0.74 aA | 1.27 ± 9.78 aA | 1.67 ± 0.39 aA | 1.87 ± 0.74 aA | |
| 7 | 1.69 ± 0.74 aA | 1.26 ± 0.039 aA | 1.61 ± 0.44 aA | 1.79 ± 0.57 aA | |
| 10 | 1.80 ± 0.18 aA | 1.36 ± 0.18 aA | 1.52 ± 0.25 aA | 1.72 ± 0.21 aA | |
| 14 | 1.87 ± 0.44 aA | 1.37 ± 0.64 aA | 1.52 ± 0.30 aA | 1.72 ± 0.35 aA | |
| b* | 0 | 12.78 ± 0.72 aA | 12.59 ± 1.16 aA | 13.08 ± 0.68 aA | 15.29 ± 1.56 bB |
| 3 | 12.05 ± 2.11 aA | 12.58 ± 1.14 aAB | 14.79 ± 1.16 abBC | 16.90 ± 0.86 bC | |
| 7 | 11.34 ± 1.12 aA | 12.42 ± 0.30 aA | 14.71 ± 0.94 abB | 16.98 ± 1.52 bC | |
| 10 | 11.19 ± 0.81 aA | 12.26 ± 0.51 aA | 14.51 ± 0.46 abB | 16.82 ± 1.35 bC | |
| 14 | 11.66 ± 0.28 aA | 12.55 ± 0.53 aA | 15.00 ± 1.18 bB | 16.62 ± 1.08 bC | |
| C* | 0 | 12.87 ± 0.68 aA | 12.67 ± 1.18 aA | 13.18 ± 0.68 aA | 15.40 ± 1.57 aB |
| 3 | 12.18 ± 2.13 aA | 12.66 ± 1.19 aAB | 14.89 ± 1.14 abBC | 17.02 ± 0.82 aC | |
| 7 | 11.49 ± 1.06 aA | 12.48 ± 0.31 aA | 14.80 ± 0.93 abB | 17.09 ± 1.47 aC | |
| 10 | 11.33 ± 0.85 aA | 12.34 ± 0.50 aA | 14.59 ± 0.45 abB | 16.91 ± 1.34 aC | |
| 14 | 11.81 ± 0.33 aA | 12.63 ± 0.57 aA | 15.08 ± 1.16 bB | 16.71 ± 1.06 aC | |
| h* | 0 | 1.46 ± 0.04 aA | 1.46 ± 0.02 aA | 1.44 ± 0.02 aA | 1.46 ± 0.03 aA |
| 3 | 1.43 ± 0.05 aA | 1.47 ± 0.05 aA | 1.46 ± 0.03 aA | 1.46 ± 0.05 aA | |
| 7 | 1.42 ± 0.07 aA | 1.47 ± 0.03 aA | 1.46 ± 0.03 aA | 1.46 ± 0.04 aA | |
| 10 | 1.41 ± 0.03 aA | 1.46 ± 0.02 aB | 1.47 ± 0.02 aB | 1.47 ± 0.02 aB | |
| 14 | 1.41 ± 0.03 aA | 1.46 ± 0.05 aB | 1.47 ± 0.02 aB | 1.47 ± 0.02 aB | |
| WHC (%) | 0 | 2.02 ± 0.74 aA | 1.96 ± 0.48 aA | 1.81 ± 0.12 aA | 2.45 ± 0.39 aA |
| 3 | 1.74 ± 0.26 aA | 2.01 ± 0.20 aA | 1.88 ± 0.15 aA | 2.70 ± 0.22 abB | |
| 7 | 1.80 ± 0.13 aA | 2.44 ± 0.43 aAB | 3.07 ± 0.55 bB | 2.89 ± 0.17 abB | |
| 10 | 1.90 ± 0.19 aA | 2.10 ± 0.17 aAB | 2.92 ± 0.37 bBC | 3.56 ± 0.51 bC | |
| 14 | 1.96 ± 0.50 aA | 2.58 ± 0.40 aAB | 2.77 ± 0.06 bAB | 3.46 ± 0.29 bB | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, K.N.; Csehi, B.; József, S.; Ferenc, H.; Kiskó, G.; Dalmadi, I.; Friedrich, L. Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods 2021, 10, 1855. https://doi.org/10.3390/foods10081855
Hussein KN, Csehi B, József S, Ferenc H, Kiskó G, Dalmadi I, Friedrich L. Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods. 2021; 10(8):1855. https://doi.org/10.3390/foods10081855
Chicago/Turabian StyleHussein, Khabat Noori, Barbara Csehi, Surányi József, Horváth Ferenc, Gabriella Kiskó, István Dalmadi, and László Friedrich. 2021. "Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions" Foods 10, no. 8: 1855. https://doi.org/10.3390/foods10081855
APA StyleHussein, K. N., Csehi, B., József, S., Ferenc, H., Kiskó, G., Dalmadi, I., & Friedrich, L. (2021). Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods, 10(8), 1855. https://doi.org/10.3390/foods10081855