Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach
Abstract
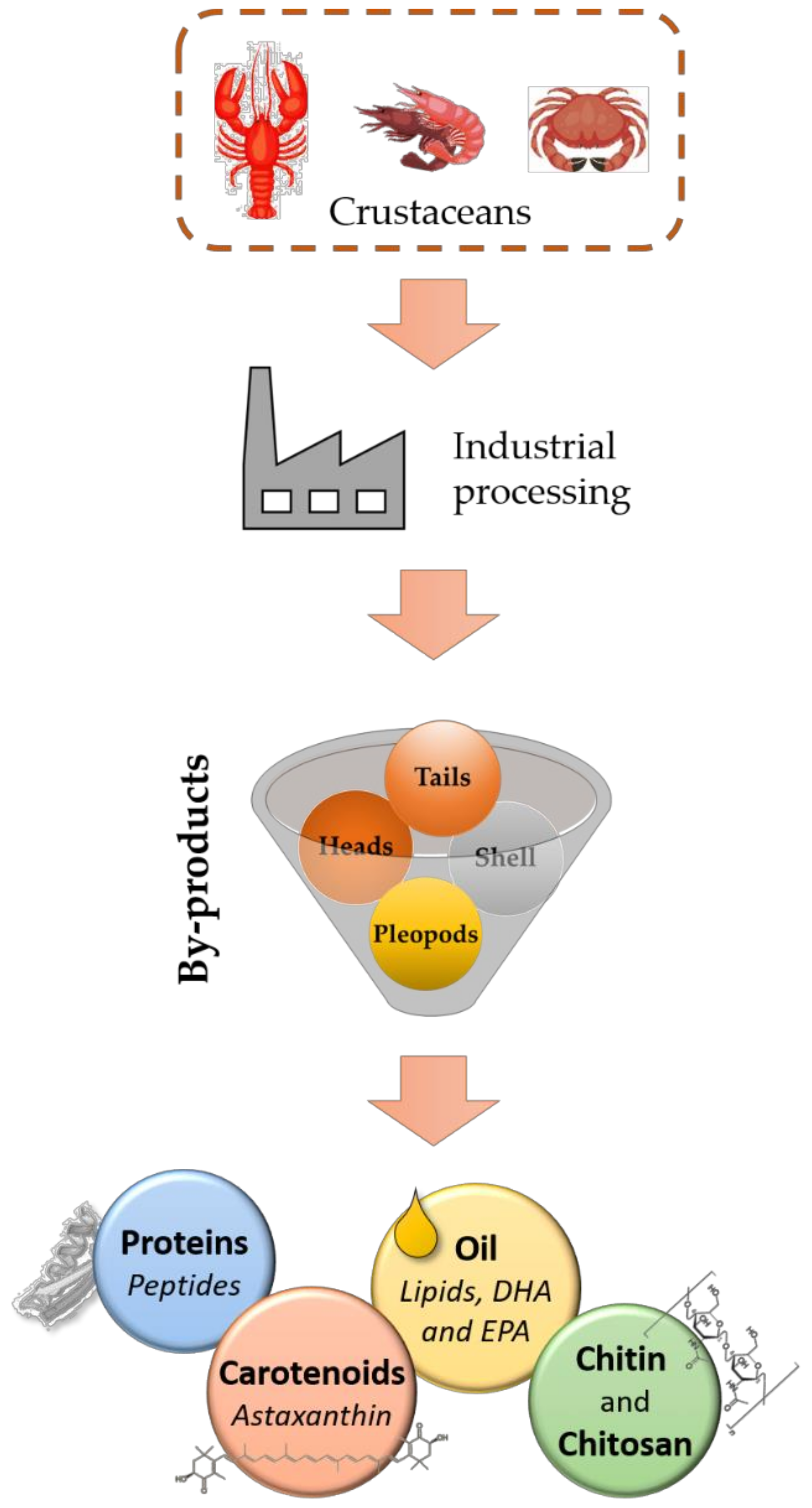
:1. Introduction
2. Crustacean By-Products as a Source of Valuable Compounds
2.1. Proteins
2.2. Lipids
2.3. Carotenoids Pigments
2.4. Chitin, Chitosan, and Derived Compounds
3. Innovative Non-Thermal Technologies for Recovery of Bioactive and Other Valuable Compounds from Crustacean By-Products
3.1. Supercritical Fluid Extraction (SFE)
3.2. High-Pressure Extraction (HPE)
3.3. Pulsed Electric Fields (PEF)
3.4. Ultrasound-Assisted Extraction (UAE)
3.5. Comparison of Technologies
4. Re-Use of Ingredients from Crustacean By-Products in Seafood and Food Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture: Challenges and Opportunities; FAO: Rome, Italy, 2014. [Google Scholar]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on super-critical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Karakoltsidis, P.A.; Zotos, A.; Constantinides, S.M. Composition of the Commercially Important Mediterranean Finfish, Crustaceans, and Molluscs. J. Food Compos. Anal. 1995, 8, 258–273. [Google Scholar] [CrossRef]
- Ruiz-Salmón, I.; Margallo, M.; Laso, J.; Villanueva-Rey, P.; Mariño, D.; Quinteiro, P.; Dias, A.C.; Nunes, M.L.; Marques, A.; Feijoo, G.; et al. Addressing challenges and opportunities of the European seafood sector under a circular economy framework. Curr. Opin. Environ. Sci. Health 2020, 13, 101–106. [Google Scholar] [CrossRef]
- Jacob, C.; Noirot, C.; Anglada, C.; Binet, T. The benefits of integrating socioeconomic dimensions of circular economy practices in the seafood sector. Curr. Opin. Environ. Sci. Health 2021, 22, 100255. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Cudennec, B.; Ravallec-Plé, R.; Courois, E.; Fouchereau-Peron, M. Peptides from fish and crustacean by-products hydroly-sates stimulate cholecystokinin release in STC-1 cells. Food Chem. 2008, 111, 970–975. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.S.; Ghaly, A.E.; Arab, F. Unconventional Approach for Demineralization of Deproteinized Crustacean Shells for Chitin Production. Am. J. Biochem. Biotechnol. 2007, 3, 1–9. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable Valorization of Seafood Processing By-Product/Discard. Energy Environ. Sustain. 2018, 111–139. [Google Scholar] [CrossRef]
- Mizani, M.; Aminlari, M.; Khodabandeh, M. An Effective Method for Producing a Nutritive Protein Extract Powder from Shrimp-head Waste. Food Sci. Technol. Int. 2005, 11, 49–54. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Barber, A.R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Zhang, W.; Barber, A.R.; Su, P.; He, S. Significant Enrichment of Polyunsaturated Fatty Acids (PUFAs) in the Lipids Extracted by Supercritical CO2 from the Livers of Australian Rock Lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Vieira, G.H.F.; Martin, A.M.; Saker-Sampaiao, S.; Omar, S.; Goncalves, R.C.F. Studies on the enzymatic hydrolysis of Brazilian lobster (Panulirus spp) processing wastes. J. Sci. Food Agric. 1995, 69, 61–65. [Google Scholar] [CrossRef]
- Ya, T.; Simpson, B.K.; Ramaswamy, H.; Yaylayan, V.; Smith, J.P.; Hudon, C. Carotenoproteins from lobster waste as a potential feed supplement for cultured salmonids. Food Biotechnol. 1991, 5, 87–93. [Google Scholar] [CrossRef]
- Sowmya, R.; Rathinaraj, K.; Sachindra, N.M. An Autolytic Process for Recovery of Antioxidant Activity Rich Carotenoprotein from Shrimp Heads. Mar. Biotechnol. 2011, 13, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, S.S.; Sawant, P.B.; Xavier, K.A.M.; Srivastava, P.P.; Dube, K.; Sawant, B.T.; Chadha, N.K. Dietary caroteno-protien extracted from shrimp shell waste augments growth, feed utilization, physio-metabolic responses and colouration in Oscar, Astronotus ocellatus (Agassiz, 1831). Aquaculture 2021, 534, 736303. [Google Scholar] [CrossRef]
- Santos, S.D.; Cahú, T.B.; Firmino, G.O.; de Castro, C.C.M.M.B.; Carvalho, L.B.; Bezerra, R.S.; Filho, J.L.L. Shrimp Waste Extract and Astaxanthin: Rat Alveolar Macrophage, Oxidative Stress and Inflammation. J. Food Sci. 2012, 77, H141–H146. [Google Scholar] [CrossRef]
- Venugopal, V. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean, 1st ed.; CRC Press: Boca Ratón, FL, USA, 2008. [Google Scholar]
- Yan, N.; Chen, X. Sustainability: Don’t waste seafood waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kijroongrojana, K.; Prodpran, T. Chemical, physical, rheological and sensory properties of biscuit fortified with protein hydrolysate from cephalothorax of Pacific white shrimp. J. Food Sci. Technol. 2019, 56, 1145–1154. [Google Scholar] [CrossRef]
- Karimi, N.; Nikoo, M.; Gavlighi, H.A.; Gheshlaghi, S.P.; Regenstein, J.M.; Xu, X. Effect of pacific white shrimp (Litopenaeus vannamei) protein hydrolysates (SPH) and (−)-epigallocatechin gallate (EGCG) on sourdough and bread quality. LWT—Food Sci. Technol. 2020, 131, 109800. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Development, properties, and stability of antioxidant shrimp muscle protein films incorporating carotenoid-containing extracts from food by-products. LWT—Food Sci. Technol. 2015, 64, 189–196. [Google Scholar] [CrossRef]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, S. State of the Art Manufacturing of Protein Hydrolysates. In Protein Hydrolysates in Biotechnology, 1st ed.; Pasupuleti, V., Demain, A., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 11–32. [Google Scholar]
- Montero, P.; Gómez-Guillén, M.C. Recovery and Functionality of Wash Water Protein from Krill Processing. J. Agric. Food Chem. 1998, 46, 3300–3304. [Google Scholar] [CrossRef]
- Santín, E.P.; Calvo, M.; López-Caballero, M.; Montero, P.; Gómez-Guillén, M.C. Compositional properties and bioactive potential of waste material from shrimp cooking juice. LWT—Food Sci. Technol. 2013, 54, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Ramyadevi, D.; Subathira, A.; Saravanan, S. Potential recovery of protein from shrimp waste in aqueous two phase system. 2231. Res. J. Chem. Sci. 2012, 2, 47–52. [Google Scholar]
- Gómez-Estaca, J.; Montero, P.; Gómez-Guillén, M.C. Chemical characterization of wash water biomass from shrimp surimi processing and its application to develop functional edible films. J. Food Sci. Technol. 2018, 55, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- Sahena, F.; Zaidul, I.; Jinap, S.; Karim, A.; Abbas, K.; Norulaini, N.; Omar, A. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Albalat, A.; Nadler, L.E.; Foo, N.; Dick, J.R.; Watts, A.J.R.; Philp, H.; Neil, D.M.; Monroig, O. Lipid composition of oil extracted fromwasted norway lobster (Nephrops norvegicus) heads and comparison with oil extracted from antarctic krill (Euphasia superba). Mar. Drugs 2016, 14, 219. [Google Scholar] [CrossRef] [Green Version]
- Takeungwongtrakul, S.; Benjakul, S.; H-Kittikun, A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): Compositions and deterioration as affected by iced storage. Food Chem. 2012, 134, 2066–2074. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and stability of carote-noid-containing lipids from hepatopancreas of pacific white shrimp (Litopenaeus vannamei). J. Food Process. Preserv. 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Montero, P.; López-Caballero, M.; Baccan, G.; Gómez-Estaca, J. Bioactive and technological functionality of a lipid extract from shrimp (L. vannamei) cephalothorax. LWT—Food Sci. Technol. 2018, 89, 704–711. [Google Scholar] [CrossRef] [Green Version]
- López-Saiz, C.-M.; Suárez-Jiménez, G.-M.; Plascencia-Jatomea, M.; Burgos-Hernández, A. Shrimp Lipids: A Source of Cancer Chemopreventive Compounds. Mar. Drugs 2013, 11, 3926–3950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Saiz, C.M.; Hernández, J.; Cinco-Moroyoqui, F.J.; Velázquez, C.; Ocaño-Higuera, V.M.; Plascencia-Jatomea, M.; Robles-Sánchez, M.; Machi-Lara, L.; Burgos-Hernández, A. Antimutagenic compounds of white shrimp (Litopenaeus vannamei): Isolation and structural elucidation. Evidence-based Complement. Altern. Med. 2016, 2016, 8148215. [Google Scholar]
- Gómez-Estaca, J.; Calvo, M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oehlenschläger, J. Cholesterol content in seafood, data from the last decade: A review. In Seafood Research from Fish to Dish, 1st ed.; Luten, J.B., Jacobsen, C., Bekaert, K., Saebø, A., Oehlenschläger, J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 41–57. [Google Scholar]
- Aryee, A.N.; Simpson, B.K. Comparative studies on the yield and quality of solvent-extracted oil from salmon skin. J. Food Eng. 2009, 92, 353–358. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Encapsulation of an astaxan-thin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin-cashew gum complex. Food Hydrocoll. 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-Kittikun, A. Wall materials and the presence of antioxidants influence encapsulation efficiency and oxidative stability of micro-encapsulated shrimp oil. Eur. J. Lipid Sci. Technol. 2014, 117, 450–459. [Google Scholar] [CrossRef]
- Marín-Peñalver, D.; Alemán, A.; Montero, M.P.; Gómez-Guillén, M.C. Entrapment of natural compounds in spray-dried and heat-dried iota-carrageenan matrices as functional ingredients in surimi gels. Food Funct. 2021, 12, 2137–2147. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultra-sonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Benjakul, S. Fortification of Skim Milk with Nanoliposomes Loaded with Shrimp Oil: Properties and Storage Stability. J. Am. Oil Chem. Soc. 2020, 97, 929–940. [Google Scholar] [CrossRef]
- Babu, C.M.; Chakrabarti, R.; Sambasivarao, K.R.S. Enzymatic isolation of carotenoid-protein complex from shrimp head waste and its use as a source of carotenoids. LWT—Food Sci. Technol. 2008, 41, 227–235. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Ramaswamy, H.S.; Yu, Y.; Zhu, S.; Wang, J. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus Vannamei Boone): Effect on Yield and Antioxidant Activity. J. Food Process. Eng. 2016, 40, 12353. [Google Scholar] [CrossRef]
- Dmytrów, I.; Szymczak, M.; Szkolnicka, K.; Kamiński, P. Development of functional acid curd cheese (Tvarog) with antioxidant activity containing astaxanthin from shrimp shells preliminary experiment. Foods 2021, 10, 895. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Favaro-Trindade, C.S. Physico-Chemical Properties, Stability, and Potential Food Applications of Shrimp Lipid Extract Encapsulated by Complex Coacervation. Food Bioprocess Technol. 2018, 11, 1596–1604. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT—Food Sci. Technol. 2016, 70, 229–236. [Google Scholar] [CrossRef]
- López, M.; Arce, L.; Garrido, J.; Ríos, A.; Valcárcel, M. Selective extraction of astaxanthin from crustaceans by use of supercritical carbon dioxide. Talanta 2004, 64, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, K.V.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Zou, Y.; Robbens, J.; Heyndrickx, M.; Debode, J.; Raes, K. Bioprocessing of marine crustacean side-streams into bioactives: A review. J. Chem. Technol. Biotechnol. 2021, 96, 1465–1474. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Alishahi, A.; Aïder, M. Applications of Chitosan in the Seafood Industry and Aquaculture: A Review. Food Bioprocess Technol. 2012, 5, 817–830. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans in Food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef] [PubMed]
- No, H.; Meyers, S.; Prinyawiwatkul, W.; Xu, Z. Applications of Chitosan for Improvement of Quality and Shelf Life of Foods: A Review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef] [PubMed]
- Birolli, W.; Delezuk, J.A.D.M.; Campana-Filho, S.P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Zhao, Y.; Oh, K.-T.; Nguyen, V.-N.; Park, R.-D. Enzymatic deacetylation of chitin by extracellular chitin deacetylase from a newly screened Mortierella sp. DY-52. J. Microbiol. Biotechnol. 2008, 18, 759–766. [Google Scholar]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Lin, S.-B.; Lin, Y.-C.; Chen, H.-H. Low molecular weight chitosan prepared with the aid of cellulase, lysozyme and chitinase: Characterisation and antibacterial activity. Food Chem. 2009, 116, 47–53. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT—Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Hrnčič, M.K.; Škerget, M. Industrial applications of supercritical fluids: A review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [Green Version]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Krishna, C.R.; Mohan, C.M. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Camargo, A.D.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibanez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- Charest, D.J.; Balaban, M.Ö.; Marshall, M.R.; Cornell, J.A. Astaxanthin Extraction from Crawfish Shells by Supercritical CO2 with Ethanol as Cosolvent. J. Aquat. Food Prod. Technol. 2001, 10, 81–96. [Google Scholar] [CrossRef]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent Selection for Supercritical Fluid Extraction of Astaxanthin and Other Carotenoids from Penaeus monodon Waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Camargo, A.P.; Meireles, M.Â.A.; Ferreira, A.L.; Saito, E.; Cabral, F.A. Extraction of ω-3 fatty acids and astaxanthin from Brazilian redspotted shrimp waste using supercritical CO2 + ethanol mixtures. J. Supercrit. Fluids 2012, 61, 71–77. [Google Scholar] [CrossRef]
- Mezzomo, N.; Martínez, J.; Maraschin, M.; Ferreira, S.R.S. Pink shrimp (P. brasiliensis and P. paulensis) residue: Supercritical fluid extraction of carotenoid fraction. J. Supercrit. Fluids 2013, 74, 22–33. [Google Scholar] [CrossRef]
- Amiguet, V.T.; Kramp, K.L.; Mao, J.; McRae, C.; Goulah, A.; Kimpe, L.E.; Blais, J.M.; Arnason, J.T. Supercritical carbon dioxide extraction of polyunsaturated fatty acids from Northern shrimp (Pandalus borealis Kreyer) processing by-products. Food Chem. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Ibarra-Garza, I.P.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.D.J.; Alemán-Nava, G.S.; García-Pérez, J.S.; Parra-Saldívar, R. Green extraction technologies for high-value metabolites from algae: A review. Biofuels Bioprod. Biorefining 2017, 11, 215–231. [Google Scholar] [CrossRef]
- Sevenich, R.; Mathys, A. Continuous Versus Discontinuous Ultra-High-Pressure Systems for Food Sterilization with Focus on Ultra-High-Pressure Homogenization and High-Pressure Thermal Sterilization: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 646–662. [Google Scholar] [CrossRef]
- Shouqin, Z.; Junjie, Z.; Changzhen, W. Novel high pressure extraction technology. Int. J. Pharm. 2004, 278, 471–474. [Google Scholar] [CrossRef]
- Du, J.; He, J.; Yu, Y.; Zhu, S.; Li, J. Astaxanthin extracts from shrimp (Litopenaeus vannamei) Discards Assisted by High Pressure Processing. In Proceedings of the 2013 ASABE Annual International Meeting, Kansas City, MI, USA, 21–24 July 2013. [Google Scholar] [CrossRef]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Comparison Between High-Pressure Processing and Chemical Extraction: Astaxanthin Yield from Six Species of Shrimp Carapace. J. Diet. Suppl. 2018, 15, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Irna, C.; Jaswir, I.; Othman, R.; Jimat, D.N. Antioxidant and antimicrobial activities of astaxanthin from Penaeus monodon in comparison between chemical extraction and High Pressure Processing (HPP). Int. Food Res. J. 2017, 24, 508–513. [Google Scholar]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Yin, Y.; Yan, X.; Wang, Y. Application of Pulsed Electric Field for Treatment of Fish and Seafood. In Handbook of Electroporation, 1st ed.; Miklavčič, D., Ed.; Springer: Chem, Switzerland, 2017; Volume 4, pp. 2637–2655. [Google Scholar]
- Li, M.; Lin, J.; Chen, J.; Fang, T. Pulsed Electric Field-Assisted Enzymatic Extraction of Protein from Abalone (Haliotis Discus HannaiIno) Viscera. J. Food Process. Eng. 2016, 39, 702–710. [Google Scholar] [CrossRef]
- Luo, W.-B.; Han, Z.; Zeng, X.-A.; Yu, S.-J.; Kennedy, J.F. Study on the degradation of chitosan by pulsed electric fields treatment. Innov. Food Sci. Emerg. Technol. 2010, 11, 587–591. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Effect of pre-treatments on yield and properties of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasonic assisted process. LWT—Food Sci. Technol. 2019, 100, 106–113. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-E-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted from Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). Eur. J. Lipid Sci. Technol. 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kijroongrojana, K.; Prodpran, T.; Agustini, T.W. Yield and chemical composition of lipids extracted from solid residues of protein hydrolysis of Pacific white shrimp cephalothorax using ultrasound-assisted extraction. Food Biosci. 2018, 26, 169–176. [Google Scholar] [CrossRef]
- Ngo, T.H.D.; Ngo, D.N. Effects of low–frequency ultrasound on heterogenous deacetylation of chitin. Int. J. Biol. Macromol. 2017, 104, 1604–1610. [Google Scholar] [CrossRef]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, A.K.; Weiss, J. Sonication-Assisted Extraction of Chitin from North Atlantic Shrimps (Pandalus borealis). J. Agric. Food Chem. 2006, 54, 5894–5902. [Google Scholar] [CrossRef]
- Baxter, S.; Zivanovic, S.; Weiss, J. Molecular weight and degree of acetylation of high-intensity ultrasonicated chitosan. Food Hydrocoll. 2005, 19, 821–830. [Google Scholar] [CrossRef]
- Liu, H.; Bao, J.; Du, Y.; Zhou, X.; Kennedy, J.F. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohydr. Polym. 2006, 64, 553–559. [Google Scholar] [CrossRef]
- Savitri, E.; Juliastuti, S.R.; Handaratri, A.; Sumarno, S.; Roesyadi, A. Degradation of chitosan by sonication in very-low-concentration acetic acid. Polym. Degrad. Stab. 2014, 110, 344–352. [Google Scholar] [CrossRef]
- Aoude, C.; Zhang, R.; Barba, F.J.; Grimi, N.; Galanakis, C.M.; Prasad, K.N. Cost and safety issues of emerging technologies against conventional techniques. In Food Waste Recovery, 1st ed.; Galanakis, C.M., Ed.; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 279–294. [Google Scholar]
- Zougagh, M.; Valcárcel, M.; Ríos, A. Supercritical fluid extraction: A critical review of its analytical usefulness. TrAC Trends Anal. Chem. 2004, 23, 399–405. [Google Scholar] [CrossRef]
- Tsiaka, T.; Zoumpoulakis, P.; Sinanoglou, V.J.; Makris, C.; Heropoulos, G.A.; Calokerinos, A.C. Response surface methodology toward the optimization of high-energy carotenoid extraction from Aristeus antennatus shrimp. Anal. Chim. Acta 2015, 877, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.; Corcuff, R.; Goulet, C.; Godefroy, S.B.; Doyen, A.; Beaulieu, L. Valorization of snow crab (Chionoecetes opilio) cooking effluents for food applications. J. Sci. Food Agric. 2020, 100, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Gómez-Guillén, M.C. Shrimp (Litopenaeus vannamei) muscle proteins as source to develop edible films. Food Hydrocoll. 2014, 41, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.S.; Dora, K.C. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. 2011, 51, 449–457. [Google Scholar] [CrossRef] [Green Version]
- De Holanda, H.D.; Netto, F.M. Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S. Potential application of seafood-derived peptides as bifunctional ingredients, antioxidant–cryoprotectant: A review. J. Funct. Foods 2015, 19, 753–764. [Google Scholar] [CrossRef]
- Ruttanapornvareesakul, Y.; Somjit, K.; Otsuka, A.; Hara, K.; Osatomi, K.; Osako, K.; Kongpun, O.; Nozaki, Y. Cryoprotective effects of shrimp head protein hydrolysate on gel forming ability and protein denaturation of lizardfish surimi during frozen storage. Fish. Sci. 2006, 72, 421–428. [Google Scholar] [CrossRef]
- Taksima, T.; Limpawattana, M.; Klaypradit, W. Astaxanthin encapsulated in beads using ultrasonic atomizer andapplication in yogurt as evaluated by consumer sensory profile. LWT—Food Sci. Technol. 2015, 62, 431–437. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Özogul, F. Chitosan role for shelf-life extension of seafood. Environ. Chem. Lett. 2019, 18, 61–74. [Google Scholar] [CrossRef]
- Kamil, J. Antioxidative activity of chitosans of different viscosity in cooked comminuted flesh of herring (Clupea harengus). Food Chem. 2002, 79, 69–77. [Google Scholar] [CrossRef]
- Kim, K.W.; Thomas, R.L. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007, 101, 308–313. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tanaka, M.; Ishizaki, S.; Suthidham, R.; Sungpech, O. Effect of chitin and chitosan on gelling properties of surimi from barred garfish (Hemiramphus far). J. Sci. Food Agric. 2001, 81, 102–108. [Google Scholar] [CrossRef]
- Li, X.; Xia, W. Effects of chitosan on the gel properties of salt-soluble meat proteins from silver carp. Carbohydr. Polym. 2010, 82, 958–964. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Phatchrat, S.; Tanaka, M. Chitosan Affects Transglutaminase-Induced Surimi Gelation. J. Food Biochem. 2003, 27, 53–66. [Google Scholar] [CrossRef]
- Kataoka, J.; Ishizaki, S.; Tanaka, M. Effects of Chitosan on Gelling Properties of Low Quality Surimi. J. Muscle Foods 1998, 9, 209–220. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural Preservatives for Extending the Shelf-Life of Seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arancibia, M.Y.; Alemán, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Development of active films of chitosan isolated by mild extraction with added protein concentrate from shrimp waste. Food Hydrocoll. 2015, 43, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Alemán, A.; González, F.; Arancibia, M.Y.; López-Caballero, M.E.; Montero, P.; Gómez-Guillén, M.C. Comparative study between film and coating packaging based on shrimp concentrate obtained from marine industrial waste for fish sausage preservation. Food Control. 2016, 70, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Xavier, K.M.; Kannuchamy, N.; Balange, A.K.; Chouksey, M.K.; Gudipati, V. Functionality of chitosan in batter formulations for coating of fish sticks: Effect on physicochemical quality. Carbohydr. Polym. 2017, 169, 433–440. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Ninan, G.; Joshy, C.G.; Parvathy, U.; Zynudheen, A.A.; Lalitha, K.V. Effect of chitosan on shelf life of re-structured fish products from pangasius (Pangasianodon hypophthalmus) surimi during chilled storage. J. Food Sci. Technol. 2016, 53, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somjit, K.; Ruttanapornwareesakul, Y.; Hara, K.; Nozaki, Y. The cryoprotectant effect of shrimp chitin and shrimp chitin hydrolysate on denaturation and unfrozen water of lizardfish surimi during frozen storage. Food Res. Int. 2005, 38, 345–355. [Google Scholar] [CrossRef]
- Kumar, K.S.; Chrisolite, B.; Sugumar, G.; Bindu, J.; Venkateshwarlu, G. Shelf life extension of tuna fillets by gelatin and chitosan based edible coating incorporated with clove oil. Fish. Technol. 2018, 55, 104–113. [Google Scholar]
- El-Obeid, T.; Yehia, H.M.; Sakkas, H.; Lambrianidi, L.; Tsiraki, M.I.; Savvaidis, I.N. Shelf-life of smoked eel fillets treated with chitosan or thyme oil. Int. J. Biol. Macromol. 2018, 114, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Arancibia, M.Y.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Chitosan coatings enriched with active shrimp waste for shrimp preservation. Food Control. 2015, 54, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Tsiligianni, M.; Papavergou, E.; Soultos, N.; Magra, T.; Savvaidis, I.N. Effect of chitosan treatments on quality parameters of fresh refrigerated swordfish (Xiphias gladius) steaks stored in air and under vacuum conditions. Int. J. Food Microbiol. 2012, 159, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.-J.; Su, W.-H.; Chen, H.-C.; Pan, C.-L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Wijesekara, I.; Vo, T.-S.; Van Ta, Q.; Kim, S.-K. Marine food-derived functional ingredients as potential anti-oxidants in the food industry: An overview. Food Res. Int. 2011, 44, 523–529. [Google Scholar] [CrossRef]
- Ramezani, Z.; Zarei, M.; Raminnejad, N. Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control. 2015, 51, 43–48. [Google Scholar] [CrossRef]
- Osheba, A.; Sorour, M.; Abdou, S. Effect of chitosan nanoparticles as active coating on chemical quality and oil uptake of fish fingers. J. Agric. Environ. Sci. 2013, 2, 1–14. [Google Scholar]



| Species | By-Products | Compounds | Extraction Conditions | Optimum Condition | Yield and Characteristics of Products’ | References |
|---|---|---|---|---|---|---|
| Australian Rock Lobsters (Jasus edwardsii) | Livers | Lipids | P: 25, 30, 35 MPa T: 50 °C CO2 flow rate: 0.434 kg/h Time: 240 min | 35 MPa and 50 °C for 4 h: | 94% of lipid yield, 4 and 7 times higher content of DHA and EPA, respectively, compared to those obtained by Soxhlet extraction | [15] |
| Tiger shrimp (Penaeus monodon) | Head and shells | Astaxanthin and other carotenoids | T: 60 °C, P: 20 MPa Co-solvents: ethanol, water, methanol, 50% (v/v) ethanol in water, 50% (v/v) methanol in water, 70% (v/v) ethanol in water, and 70% (v/v) methanol in water. | 50% (v/v) ethanol in water | Carotenoid yield: 84.02 ± 0.8 μg/g dry weight (DW), Extracted astaxanthin complex: 58.03 ± 0.1 μg/g DW free astaxanthin content: 12.25 ± 0.9 μg/g DW | [75] |
| Pink shrimp (Penaeus brasiliensis and Penaeus paulensis) | Head, shell, and tail | Astaxanthin and other carotenoids | Moisture content (11.21–46.30%), solvent flow rate (8.3–13.3 g/min), T: 40–60 °C P: 100–300 bar co-solvent (hexane: isopropanol solution, 50:50, and sunflower oil) | Solvent: CO2 + 2% hexane: isopropanol solution, 50:50 Flow rate: 13.3 g CO2/min 11.21%: moisture content P: 300 bar T: 333.15 K | Global yield (amount of extract removed by the solvent and related to the solvent power, i.e., to the process temperature and pressure): 4.2 ± 0.2 | [77] |
| Brazilian redspotted shrimp (Farfantepenaeus paulensis) | Head, shell and tail | Astaxanthin and ω3 fatty acid (EPA + DHA) | CO2/Ethanol Etahnol 5, 10 and 15% wt, P: 300 bar, T: 50 °C. | 15% wt of ethanol. | 93.8% and 65.2% for lipids and astaxanthin Total lipid extraction yield increased to 136% increasing ethanol from 5 to 15% wt. | [76] |
| Brazilian redspotted shrimp (Farfantepenaeus paulensis) | Head, shell and tail | Lipids, astaxanthin | P: 200–400 bar T: 40–60 °C | 43 °C and 370 bar | Astaxanthin: 39% recovery Lipids yield similar under different conditions (1.74% to 2.21%) Possibility to fractionate oil | [70] |
| Northern shrimp (Pandalus borealis) | Head, shell and tail | Lipids (EPA+DHA) | Low P: 15 MPa, 50 °C Moderate P: 35 MPa; 40 °C | 35 MPa; 40 °C | Total Fatty Acids: 795 mg/g Oil rich in ω-3 PUFAs (EPA:78 mg/g, DHA:79.7 mg/g) | [78] |
| Louisiana crawfish (Procambarus clarkii) | Shell and tail | Astaxanthin | T: 50–60–70 °C P: 13.8–22.4–31.0 MPa, Moisture content: freeze-dried 0–25–50%. | 75 °C, 24.1 MPa, and 13% moisture. | Predicted maximum extractable astaxanthin: 207.6 mg/kg | [74] |
| Species | By-Products | Compound | Extraction Conditions | Optimum Conditions | Yield and Characteristics of Products’ | References |
|---|---|---|---|---|---|---|
| Pacific white shrimp (Litopenaeus vannamei) | Head and shell | Astaxanthin | P: 0.1–600 MPa, liquid-to-solid ratio (10 to 50 mL/g), and pressure holding time (0–20 min) | P: 210 MPa P holding time:9.2 min, liquid-solid-ratio: 32 mL/g | 89.12 μg/g | [82] |
| Pacific white shrimp (Litopenaeus vannamei) | Shells | Astaxanthin | P: 0.1–600 MPa, holding times (0–20 min), different solvents (acetone, dichloromethane, and ethanol), and solvent to solid ratios (10–50 mL/g) | Ethanol, liquid to solid ratio of 20 mL/g and 200 MPa for 5min. | 71.1 μg/g, better antioxidant activity in the extract than conventional solvent extraction | [49] |
| Rainbow Shrimp (Parapenaeopsis sculptili) Bird shrimp (Metapenaeus lysianassa) Giant river prawn (Macrobrachium rosenbergii) Shrimp (Metapenaeopsis hardwickii) Banana shrimp (Penaeus merguiensis) Giant tiger prawn (Penaeus monodon) | Head, shell, and tail | Astaxanthin | P: 210 MPa, time 10 min, solvent mixture of acetone and methanol (7:3, v/v). | Higher total carotenoid and astaxanthin yield obtained for P. monodon | Total carotenoid: 68.26 µg/mL astaxanthin yield: 59.9744 µg/gdw | [83] |
| Species | By-Products | Compounds | Extraction Conditions | Optimum Conditions | Yield and Characteristics of Products | References |
|---|---|---|---|---|---|---|
| Pacific white shrimp (Litopenaeus vannamei) | Head | Lipids and carotenoids | Pulse and continuous Mode, sonication time (15, 20, 25, and 30 min), amplitudes of 50– 90%, 4 °C | 80% amplitude with continuous mode, for 25 min. | 50% yield, Extract richer in free fatty acids and higher oxidation level | [93] |
| Pacific white shrimp (Litopenaeus vannamei) | Head | Lipids and carotenoids | Frequency: 20 kHz, Power: 750 W, amplitudes: 60–100% | 80% amplitude for 10 min at 4 °C | Lipid yield: 10–11 g/100 g, carotenoids yields: 8.6–8.8 mg/g lipid, higher lipid oxidation and hydrolysis | [94] |
| Giant river prawn (Macrobrachium rosenbergii) | Shell | Chitin | Demineralization in 0.25 M HCl (1:40 solid-to-solvent, w/v) at 40 °C, sonicated for 0, 1, and 4 h. Deproteinization in 0.25 M NaOH (1:15 solid-to-solvent, w/v) at 40 °C, sonicated for 0, 1, and 4 h | 4 h | Lower content of proteins (7.45%) and deacetylation degree (61.4%) | [96] |
| Pacific white shrimp (Litopenaeus vannamei) | Shell | Chitosan | Deacetylation: NaOH (35%–65%, w/w), ratio of chitin (1:15, w/v), 80 °C, 360 min., frequency of 37 kHz and power of 300 W | Deacetylation rate improved with concentration of NaOH below 45% (w/w) | Higher solubility of chitosan | [95] |
| Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| SFE |
|
| [71,101] |
| HPE |
|
| [49,81] |
| PEF |
|
| [85,87] |
| UAE |
|
| [91,94] |
| By-Product | Compond | Function | Seafood Product | Application | Findings | References |
|---|---|---|---|---|---|---|
| Shrimp Shell | Chitosan | Antioxidant | Rohu (Labeo rohita) fish sticks | Addition of 0.5%, 1%, 1.5%, and 2% of chitosan gel in batter for fish stick coating | Increase in chitosan gel concentration reduced oil absorption from 65–78%. Reduced total volatile basic nitrogen (TVBN), PV, and TBARS. Lipid oxidation decreased as the chitosan inclusion in batter increased. | [121] |
| Shrimp shell (Metapenaeus dobsoni) | Chitosan | Gelling | Surimi from Pangasius (Pangasianodonhypophthalmus) | Three different formulations by incorporating corn starch (10%) and chitosan (0.75%). A formulation containing only cornstarch (10%) was used as a control. | Reduction of total volatile basic nitrogen (TVBN), free fatty acids (FFA), peroxide value (PV), 2-thiobarbituric acid reactive substances (TBARS), and microbial count of the product during chilled storage. Extended the shelf-life of 17 days in comparison with the control of 10 days. | [122] |
| Shrimp cephalothorax, shell, and tail (Penaeus monodon and Penaeus sindicus) | Shrimp protein hydrolysate (SPH) | Antioxidant | Whole Croaker fish (Johnius gangeticus) | Dipping in various concentrations of SPH Solution (0.1%, 0.2%, and 0.5% (w/v) of 5 mg/mL concentration SPH solution) | Lowered TBA values of fillet and maintained yellowishness of skin color during 10 days of refrigerated storage at 4 °C and limited the increase of PV and FFA values. | [106] |
| Shrimp head (Pandalus eous, Metapenaeus endeavouri, Penaeus monodon) | Shrimp protein hydrolysate (SPH) | Cryoprotectant | Lizardfish (Saurida spp.) surimi. | Lizardfish surimi with 5% (dried matter) of any of the three SPH | Stabilized freeze-induced denaturation of myofibrillar protein and enhance gel-forming ability of surimi during frozen storage. Decreased the whiteness of all kamaboko. | [109] |
| Shrimp Shell (Penaeus monodon), (Metapenaeus endeavouri) (Macrobrachium rosenbergii) | Shrimp Chitin Hydrolysate (SCH) | Cryoprotectant | Lizardfish (Saurida spp.) surimi. | Lizardfish surimi with 5% (dried matter) of shrimp chitin hydrolysates | Delayed freeze-induced protein denaturation and increased the amount of unfrozen water in surimi stored at 25 °C for 6 months. | [123] |
| Crab shells | Chitosan | Antioxidant | Cooked comminuted flesh of herring (Clupea harengus) | Solutions with 50, 100, and 200 ppm of chitosan with a viscosity of 14, 57, and 360 cP, added directly on the minced fish | PV and TBARS were both reduced following treatment of the fish before cooking with 50, 100, and 200 ppm of chitosan 14, 57, and 360 cP. Inhibition of oxidation was concentrated-dependent and highest for chitosan 14 cP. | [112] |
| Prawn shell | Chitin and Chitosan | Gelling | Surimi from barred garfish (Hemiramphus far) | Chitin or chitosans with different degrees of deacetylation 65%, 83%, 88%, 99% DD) and concentrations were added to the surimi | Chitosan with 65.6% DD at 15 mg/g resulted in the maximum increases in both breaking force and deformation of suwari and kamaboko gels. | [114] |
| Species | By-Products | Microorganism Reduced and/or Inhibited | Application | References |
|---|---|---|---|---|
| Tuna fillets (Euthynnus affinis) | Shrimp shell | Aerobic plate count (APC) Pseudomonas spp. Aeromonas hydrophila Salmonella enteritidis Klebsiella sp. Bacillus firmus Bacillus cereus Micrococcus sp. Escherichia coli Salmonella paratyphi Vibrio cholera Salmonella typhi Staphylococcus aureus | Fillets were dipped in edible chitosan coatings (chitosan conc. 1%) | [124] |
| Smoked European eel (Anguilla Anguilla) stored under vacuum packaging (VP) at 4 °C | Crab shell | Pseudomonas spp. Shewaella spp. and yeasts/molds | Fillets were dipped in chitosan solution (2.0% w/v). | [125] |
| Pacific white shrimp (Litopenaus vannamei) | Shrimp processing by-products | Total bacterial counts (TBC) H2S-producer organisms Luminescent bacteria Total aerobic mesophiles Pseudomonas spp. Enterobacteriaceae Lactic acid bacteria | Chitosan coatings (chitosan conc. 2% w/w) | [126] |
| Fresh swordfish steaks (Xiphia gladius) | Crab shells | Total Viable Counts (TVC) Pseudomonas spp. H2S-producing bacteria Lactic acid bacteria Enterobacteriaceae | Chitosan with a concentration of 0.045% w/w, added by spraying it directly onto the product. | [127] |
| Salmon fillets (Oncorhynchus nereka) | Shrimp shells | Mesophiles, Psychrotrophs, coliforms, Aeromonas spp., and Vibrio spp. | Soaked in various concentrations of chitosan solutions (0.2%, 0.5%, or 1.0% in 0.1 N HCl, adjusted to pH 6.0 with 1 N NaOH) | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Aguiar Saldanha Pinheiro, A.C.; Martí-Quijal, F.J.; Barba, F.J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods 2021, 10, 2030. https://doi.org/10.3390/foods10092030
De Aguiar Saldanha Pinheiro AC, Martí-Quijal FJ, Barba FJ, Tappi S, Rocculi P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods. 2021; 10(9):2030. https://doi.org/10.3390/foods10092030
Chicago/Turabian StyleDe Aguiar Saldanha Pinheiro, Ana Cristina, Francisco J. Martí-Quijal, Francisco J. Barba, Silvia Tappi, and Pietro Rocculi. 2021. "Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach" Foods 10, no. 9: 2030. https://doi.org/10.3390/foods10092030










