Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings)
Abstract
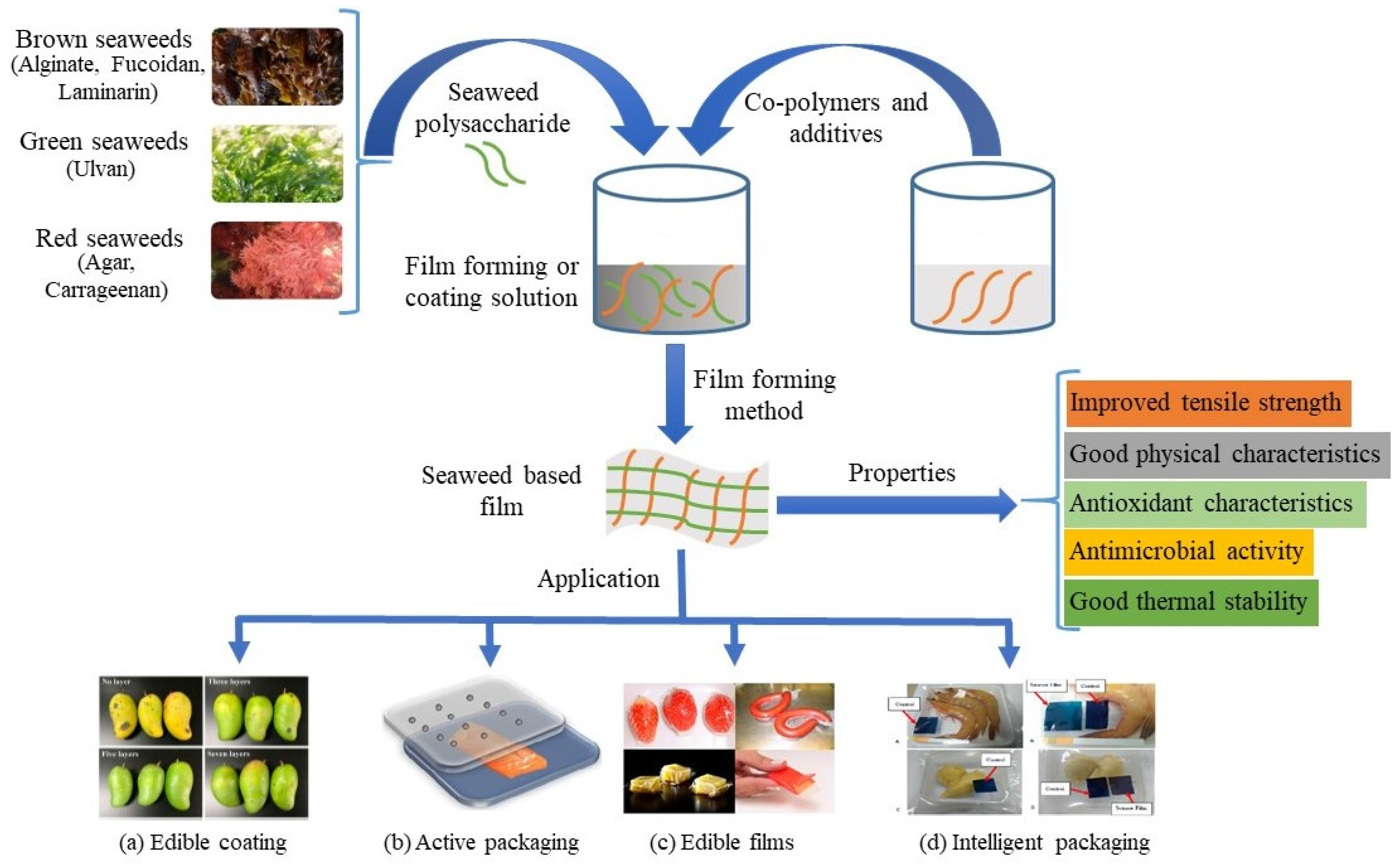
:1. Introduction
2. Seaweeds
3. Seaweeds Application as Food Contact Materials
3.1. Active Packaging
3.2. Smart and Intelligent Packaging
3.2.1. Data Carriers
3.2.2. Indicators
3.2.3. Sensors
3.3. Edible Films
3.4. Coatings
4. Effects of Seaweeds on Food Contact Materials
4.1. Mechanical Properties
4.2. Physical Properties
4.3. Chemical Properties
4.4. Thermal Properties
4.5. Antimicrobial Properties
4.6. Antioxidant Properties
5. Legal Aspects of Using Seaweed as Food Contact Material
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. Food Contact Materials. Available online: https://www.efsa.europa.eu/en/topics/topic/food-contact-materials#:~:text=Food%20contact%20materials%20are%20all,%2C%20rubber%2C%20paper%20and%20metal (accessed on 31 May 2021).
- PlasticsEurope. Plastics—the Facts 2020—an Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2020. [Google Scholar]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds polysaccharides in active food packaging: A review of recent progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Albertos, I.; Martin-Diana, A.B.; Burón, M.; Rico, D. Development of functional bio-based seaweed (Himanthalia elongata and Palmaria palmata) edible films for extending the shelflife of fresh fish burgers. Food Packag. Shelf Life 2019, 22, 100382. [Google Scholar] [CrossRef]
- Ristivojevic, P.; Jovanovic, V.; Opsenica, D.M.; Park, J.; Rollinger, J.M.; Velickovic, T.C. Rapid analytical approach for bioprofiling compounds with radical scavenging and antimicrobial activities from seaweeds. Food Chem. 2021, 334, 127562. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Cheikh, D.; Martin-Sampedro, R.; Majdoub, H.; Darder, M. Alginate bionanocomposite films containing sepiolite modified with polyphenols from myrtle berries extract. Int. J. Biol. Macromol. 2020, 165, 2079–2088. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.F.; Kong, R.Q.; Lu, W.Q.; Wang, X.Y. Characterization and application of the microencapsulated carvacrol/Check for sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G.; Liu, H.; Li, K. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 2019, 125, 1289–1298. [Google Scholar] [CrossRef]
- Jancikova, S.; Jamroz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/gelatin hydrolysate/rosemary extract composite films as active and intelligent packaging materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Bai, R.; Zhang, X.; Yuan, L.; Liu, J. Preparation of pH-sensitive and antioxidant packaging films based on κ-carrageenan and mulberry polyphenolic extract. Int. J. Biol. Macromol. 2019, 134, 993–1001. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Shi, J.; Huang, X.; Zou, X.; Zhang, D.; Zhai, X.; Yang, Z.; Li, Z.; Li, Y. Preparation and comparison of two functional nanoparticle-based bilayers reinforced with a kappa-carrageenan-anthocyanin complex. Int. J. Biol. Macromol. 2020, 165, 758–766. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.; Wang, J.; Liu, Y.; Lu, P.; Huang, L. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizzini, P.; Beltrame, E.; Zanet, V.; Vidic, J.; Manzano, M. Development and Evaluation of qPCR Detection Method and Zn-MgO/Alginate Active Packaging for Controlling Listeria monocytogenes Contamination in Cold-Smoked Salmon. Foods 2020, 9, 1353. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.A.; Yook Heng, L.; Salam, F.; Mat Zaid, M.H.; Abu Hanifah, S. A Colorimetric pH Sensor Based on Clitoria sp. and Brassica sp. for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef] [Green Version]
- Seaweed Packaging. Available online: https://seaweedpackaging.com/ (accessed on 24 May 2021).
- Nakhate, P.; van der Meer, Y. A Systematic Review on Seaweed Functionality: A Sustainable Bio-Based Material. Sustainability 2021, 13, 6174. [Google Scholar] [CrossRef]
- Kocira, A.; Kozlowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortynska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- European Commission. European Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food; European Commission: Luxembourg, 2009. [Google Scholar]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.X.; Qazi, I.M.; Pavase, T.R.; Lv, L.T. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Yildirim, S.; Rocker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Gopal, T.K.S. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Rao, D.N.; Sachindra, N.M. Modified atmosphere and vacuum packaging of meat and poultry products. Food Rev. Int. 2002, 18, 263–293. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Mills, A.; Doyle, G.; Peiro, A.M.; Durrant, J. Demonstration of a novel, flexible, photocatalytic oxygen-scavenging polymer film. J. Photochem. Photobiol. A Chem. 2006, 177, 328–331. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide)/poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhao, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Han, Y.Y.; Wang, L.J. Sodium alginate/carboxymethyl cellulose films containing pyrogallic acid: Physical and antibacterial properties. J. Sci. Food Agric. 2017, 97, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.O.; Alberti, F.V.; Matiacevich, S.B. Edible antimicrobial films based on microencapsulated lemongrass oil. J. Food Sci. Technol. 2016, 53, 832–839. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, J.P.; Bruni, G.P.; Fabra, M.J.; Zavareze, E.D.; Lopez-Rubio, A.; Martinez-Sanz, M. Development of food packaging bioactive aerogels through the valorization of Gelidium sesquipedale seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Gelatin/Carrageenan-Based Color-Indicator Film Integrated with Shikonin and Propolis for Smart Food Packaging Applications. ACS Appl. Bio Mater. 2021, 4, 770–779. [Google Scholar] [CrossRef]
- EFSA. Guidelines on submission of a dossier for safety evaluation by the EFSA of active or intelligent substances present in active and intelligent materials and articles intended to come into contact with food. EFSA J. 2009, 7, 1208. [Google Scholar]
- Barska, A.; Wyrwa, J. Innovations in the Food Packaging Market—Intelligent Packaging—A Review. Czech J. Food Sci. 2017, 35, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
- Ghaani, M.; Cozzolino, C.A.; Castelli, G.; Farris, S. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 2016, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.X.; Brody, A.L.; Qazi, I.M.; Lv, L.T.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef]
- Mlalila, N.; Kadam, D.M.; Swai, H.; Hilonga, A. Transformation of food packaging from passive to innovative via nanotechnology: Concepts and critiques. J. Food Sci. Technol. 2016, 53, 3395–3407. [Google Scholar] [CrossRef] [Green Version]
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.X.; Li, B.F.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Yerramathi, B.B.; Kola, M.; Muniraj, B.A.; Aluru, R.; Thirumanyam, M.; Zyryanov, G.V. Structural studies and bioactivity of sodium alginate edible films fabricated through ferulic acid crosslinking mechanism. J. Food Eng. 2021, 301, 110566. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, F.; Han, X.J. An edible film of sodium alginate/pullulan incorporated with capsaicin. New J. Chem. 2018, 42, 17756–17761. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Paula, G.A.; Benevides, N.M.B.; Cunha, A.P.; de Oliveira, A.V.; Pinto, A.M.B.; Morais, J.P.S.; Azeredo, H.M.C. Development and characterization of edible films from mixtures of kappa-carrageenan, i-carrageenan, and alginate. Food Hydrocoll. 2015, 47, 140–145. [Google Scholar] [CrossRef]
- Ma, D.H.; Jiang, Y.; Ahmed, S.; Qin, W.; Liu, Y.W. Physical and antimicrobial properties of edible films containing Lactococcus lactis. Int. J. Biol. Macromol. 2019, 141, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Munisamy, S.; Bhat, R. Producing novel edible films from semi refined carrageenan (SRC) and ulvan polysaccharides for potential food applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef]
- Wang, X.J.; Guo, C.F.; Hao, W.H.; Ullah, N.; Chen, L.; Li, Z.X.; Feng, X.C. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int. J. Biol. Macromol. 2018, 118, 722–730. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.T.; Zhang, H.; Dai, Y.Y.; Dong, H.Z.; Hou, H.X. Effects of hydrophobic agents on the physicochemical properties of edible agar/maltodextrin films. Food Hydrocoll. 2019, 88, 283–290. [Google Scholar] [CrossRef]
- Gomaa, M.; Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Use of seaweed and filamentous fungus derived polysaccharides in the development of alginate-chitosan edible films containing fucoidan: Study of moisture sorption, polyphenol release and antioxidant properties. Food Hydrocoll. 2018, 82, 239–247. [Google Scholar] [CrossRef]
- Garg, S.; Hemrom, A.; Syed, I.; Sivapratha, S.; Panigrahi, S.S.; Dhumal, C.V.; Sarkar, P. Nanoedible coatings for dairy food matrices. In Nanotechnology Applications in Dairy Science; Apple Academic Press: Waretown, NJ, USA, 2019; pp. 27–42. [Google Scholar]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D. Novel Techniques for Extrusion, Agglomeration, Encapsulation, Gelation, and Coating of Foods. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 379–392. [Google Scholar]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Zhou, X.; Zong, X.; Zhang, M.; Ge, Q.; Qi, J.; Liang, J.; Xu, X.; Xiong, G. Effect of konjac glucomannan/carrageenan-based edible emulsion coatings with camellia oil on quality and shelf-life of chicken meat. Int. J. Biol. Macromol. 2021, 183, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Ben Mansour, M.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Massé, A. Active exopolysaccharides based edible coatings enriched with red seaweed (Gracilaria gracilis) extract to improve shrimp preservation during refrigerated storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Banu, A.T.; Ramani, P.S.; Murugan, A. Effect of seaweed coating on quality characteristics and shelf life of tomato (Lycopersicon esculentum Mill.). Food Sci. Hum. Wellness 2020, 9, 176–183. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Boonyaritthongchai, P.; Buanong, M.; Supapvanich, S.; Wongs-Aree, C. Chitosan- and κ-carrageenan-based composite coating on dragon fruit (Hylocereus undatus) pretreated with plant growth regulators maintains bract chlorophyll and fruit edibility. Sci. Hortic. 2021, 281, 109916. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Acevedo-Fani, A.; Martin-Belloso, O. Improving the shelf life of low-fat cut cheese using nanoemulsionbased edible coatings containing oregano essential oil and mandarin fiber. Food Control 2017, 76, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. Effect of chitosan and carrageenan-based edible coatings on post-harvested longan (Dimocarpus longan) fruits. CyTA—J. Food 2018, 16, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Parreidt, T.S.; Lindner, M.; Rothkopf, I.; Schmid, M.; Muller, K. The Development of a Uniform Alginate-Based Coating for Cantaloupe and Strawberries and the Characterization of Water Barrier Properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Maizura, M.; Fazilah, A.; Norziah, M.H.; Karim, A.A. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch-alginate edible film containing lemongrass oil. J. Food Sci. 2007, 72, C324–C330. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.Y.; Chang, P.R.; Yu, J.G.; Ma, X.F. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Rhim, J.W. Effect of PLA lamination on performance characteristics of agar/kappa-carrageenan/clay bio-nanocomposite film. Food Res. Int. 2013, 51, 714–722. [Google Scholar] [CrossRef]
- Huq, T.; Salmieri, S.; Khan, A.; Khan, R.A.; Le Tien, C.; Riedl, B.; Fraschini, C.; Bouchard, J.; Uribe-Calderon, J.; Kamal, M.R.; et al. Nanocrystalline cellulose (NCC) reinforced alginate based biodegradable nanocomposite film. Carbohydr. Polym. 2012, 90, 1757–1763. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Passanha, P.; Esteves, S.; Schnherr, H.; Jhurry, D. Biomineralization potential and cellular response of PHB and PHBV blends with natural anionic polysaccharides. Mater. Sci. Eng. C 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Characteristics of thermoplastic sugar palm Starch/Agar blend: Thermal, tensile, and physical properties. Int. J. Biol. Macromol. 2016, 89, 575–581. [Google Scholar] [CrossRef]
- Lopes, J.R.; dos Reis, R.A.; Almeida, L.E. Production and characterization of films containing poly(hydroxybutyrate) (PHB) blended with esterified alginate (ALG-e) and poly(ethylene glycol) (PEG). J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef] [Green Version]
- Eghbalifam, N.; Frounchi, M.; Dadbin, S. Antibacterial silver nanoparticles in polyvinyl alcohol/sodium alginate blend produced by gamma irradiation. Int. J. Biol. Macromol. 2015, 80, 170–176. [Google Scholar] [CrossRef]
- Kadam, S.U.; Pankaj, S.K.; Tiwari, B.K.; Cullen, P.J.; O’Donnell, C.P. Development of biopolymer-based gelatin and casein films incorporating brown seaweed Ascophyllum nodosum extract. Food Packag. Shelf Life 2015, 6, 68–74. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Development and characterization of carrageenan/grapefruit seed extract composite films for active packaging. Int. J. Biol. Macromol. 2014, 68, 258–266. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef]
- Augusto, A.; Dias, J.R.; Campos, M.J.; Alves, N.M.; Pedrosa, R.; Silva, S.F.J. Influence of Codium tomentosum Extract in the Properties of Alginate and Chitosan Edible Films. Foods 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Mandal, A.; Ayton, E.; Hunt, R.; Zeller, M.A.; Sharma, S. Modification of Protein Rich Algal-Biomass to Form Bioplastics and Odor Removal. In Protein Byproducts; Singh Dhillon, G., Ed.; Academic Press: New York, NY, USA, 2016; pp. 107–117. [Google Scholar]
- Assadpour, E.; Rostamabadi, H.; Jafari, S.M. Introduction to characterization of nanoencapsulated food ingredients. In Characterization of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: New York, NY, USA, 2020; Volume 4, pp. 1–50. [Google Scholar]
- Jafarzadeh, S.; Jafari, S.M. Impact of metal nanoparticles on the mechanical, barrier, optical and thermal properties of biodegradable food packaging materials. Crit. Rev. Food Sci. Nutr. 2020, 61, 1–19. [Google Scholar]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Hasan, M.; Chong, E.W.N.; Jafarzadeh, S.; Paridah, M.T.; Gopakumar, D.A.; Tajarudin, H.A.; Thomas, S.; Khalil, H. Enhancement in the Physico-Mechanical Functions of Seaweed Biopolymer Film via Embedding Fillers for Plasticulture Application—A Comparison with Conventional Biodegradable Mulch Film. Polymers 2019, 11, 210. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Lee, S.W.; Park, J.G.; Baek, K.H. Antioxidant and antibacterial properties of essential oil extracted from an edible seaweed undaria pinnatifida. J. Food Biochem. 2017, 41, e12278. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Ritta, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.X.; Cao, M.J.; Liu, G.M.; Chen, Q.C.; Sun, L.C.; Chen, H.X. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. In vitro antioxidant and antibacterial activity of sulfated polysaccharides isolated from Spatoglossum asperum. Carbohydr. Polym. 2017, 170, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-heterocyclic derivatives with antibacterial properties from marine bacterium Bacillus subtilis associated with seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Lahare, P.; Chawla, S.P.; Sharma, A. Kappaphycus alcarezii: Its antioxidant potential and use in bioactive packaging films. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Velasco, E.M.Z.; Fundador, N.G.V. Development and Use of Antimicrobial Durian Starch-Carrageenan/Carvacrol Films. Mindanao J. Sci. Technol. 2020, 18, 118–128. [Google Scholar]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Bhutiya, P.L.; Mahajan, M.S.; Rasheed, M.A.; Pandey, M.; Hasan, S.Z.; Misra, N. Zinc oxide nanorod clusters deposited seaweed cellulose sheet for antimicrobial activity. Int. J. Biol. Macromol. 2018, 112, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Development of Biodegradable Films with Improved Antioxidant Properties Based on the Addition of Carrageenan Containing Olive Leaf Extract for Food Packaging Applications. J. Polym. Environ. 2020, 28, 123–130. [Google Scholar] [CrossRef]
- Muncke, J.; Andersson, A.-M.; Backhaus, T.; Boucher, J.M.; Almroth, B.C.; Castillo, A.C.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.-B. Impacts of food contact chemicals on human health: A consensus statement. Environ. Health 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 629/2008 of 2 July 2008 amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2008, 173, 6–9. [Google Scholar]





| Seaweed | Polysaccharide | Structure | Reference |
|---|---|---|---|
| Brown seaweeds | Alginate |  | [3] |
| Red seaweeds | Agar |  | [3] |
| Carrageenan |  | [3] | |
| Green seaweeds | Ulvan |  | [3] |
| Packaging Matrix | Properties of the Packaging Material | Reference | |
|---|---|---|---|
| Active packaging | |||
| Sodium alginate | Lemongrass oil |
| [31] |
| Furcellaran | Gelatin hydrolysate/Rosemary extract |
| [10] |
| Sodium alginate | Carboxymethyl cellulose, CaCl2, Pyrogallic acid |
| [30] |
| Gelidium sesquipedale | Polyvinyl alcohol, Nanocellulose |
| [32] |
| Fucus vesiculosus | Whey protein |
| [29] |
| Smart and intelligent packaging | |||
| κ-Carrageenan | Mulberry polyphenol extract |
| [11] |
| Carrageenan | Gelatin, Shikonin, and Propolis |
| [33] |
| κ-Carrageenan, agar | Nano-TiO2, nano-ZnO, and Clitoria ternatea Linn anthocyanin |
| [12] |
| Packaging Matrix | Properties of the Packaging Material | Reference | |
|---|---|---|---|
| k-carrageenan, i-carrageenan, and Alginate | Glycerol |
| [50] |
| Sodium alginate | Gelatin- tea polyphenols |
| [43] |
| Sodium alginate | Sodium carboxymethylcellulose, collagen, and Lactococcus lactis |
| [51] |
| Sodium alginate | Essential oils of R. officinalis L., A. herba alba Asso, O. basilicum L. and M. pulegium L. |
| [46] |
| Alginate | Pullulan and Capsaicin |
| [48] |
| Sodium alginate | Ferulic acid |
| [47] |
| Semi refined carrageenan and ulvan | Glycerol |
| [52] |
| Agar | Starch, and Maltodextrin |
| [49] |
| Agar | Nano-bacterial cellulose |
| [53] |
| Agar | Maltodextrin, beeswax, shortening, and liquid paraffin |
| [54] |
| Packaging Matrix | Properties of the Packaging Material | Reference | |
|---|---|---|---|
| Alginate | Pomegranate peel extract |
| [58] |
| Kappa-carrageenan | Konjac glucomannan and camellia oil |
| [59] |
| Gracilaria gracilis extract | Microalgal exopolysaccharides |
| [60] |
| Sargassum tenerrimum and Kappaphycus alvarezii extract | - |
| [61] |
| κ-carrageenan | Chitosan |
| [62] |
| Sodium alginate | Oregano essential oil (1.5–2.5% w/w), mandarin fibre (0.5% w/w) |
| [63] |
| Sodium alginate | Pomegranate peel extract, Chitosan |
| [45] |
| Carrageenan | Chitosan |
| [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. https://doi.org/10.3390/foods10092088
Perera KY, Sharma S, Pradhan D, Jaiswal AK, Jaiswal S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods. 2021; 10(9):2088. https://doi.org/10.3390/foods10092088
Chicago/Turabian StylePerera, Kalpani Y., Shubham Sharma, Dileswar Pradhan, Amit K. Jaiswal, and Swarna Jaiswal. 2021. "Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings)" Foods 10, no. 9: 2088. https://doi.org/10.3390/foods10092088






