Antimicrobial Effect of Acetic Acid, Sodium Hypochlorite, and Thermal Treatments against Psychrotolerant Bacillus cereus Group Isolated from Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assay
2.3. Bacterial Growth Analysis with Acetic Acid and Sodium Hypochlorite
2.4. Heat Treatment
2.5. Calculation of D- and z-Values
2.6. Statistical Analysis
3. Results
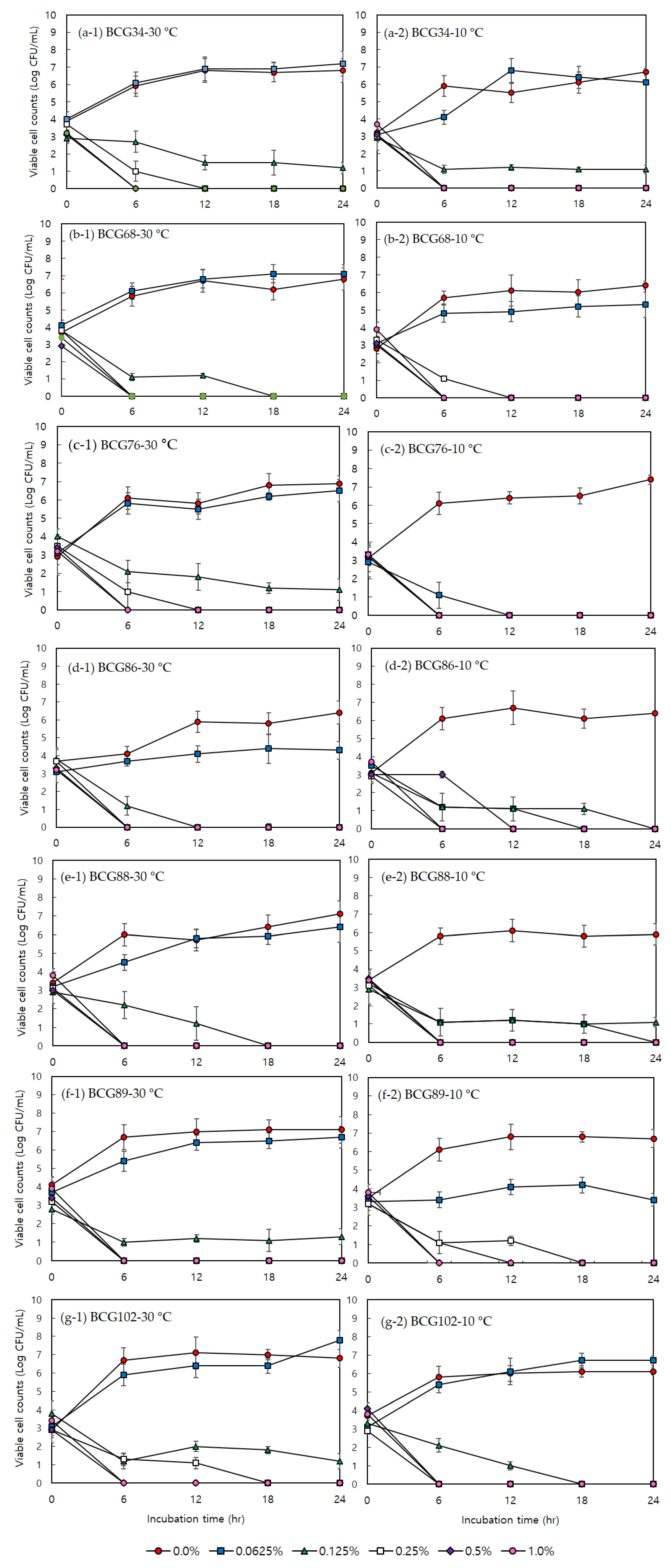
3.1. Minimum Inhibitory Concentration, Minimum Bactericidal Concentration, and Effects of Acetic Acid on Bacterial Growth
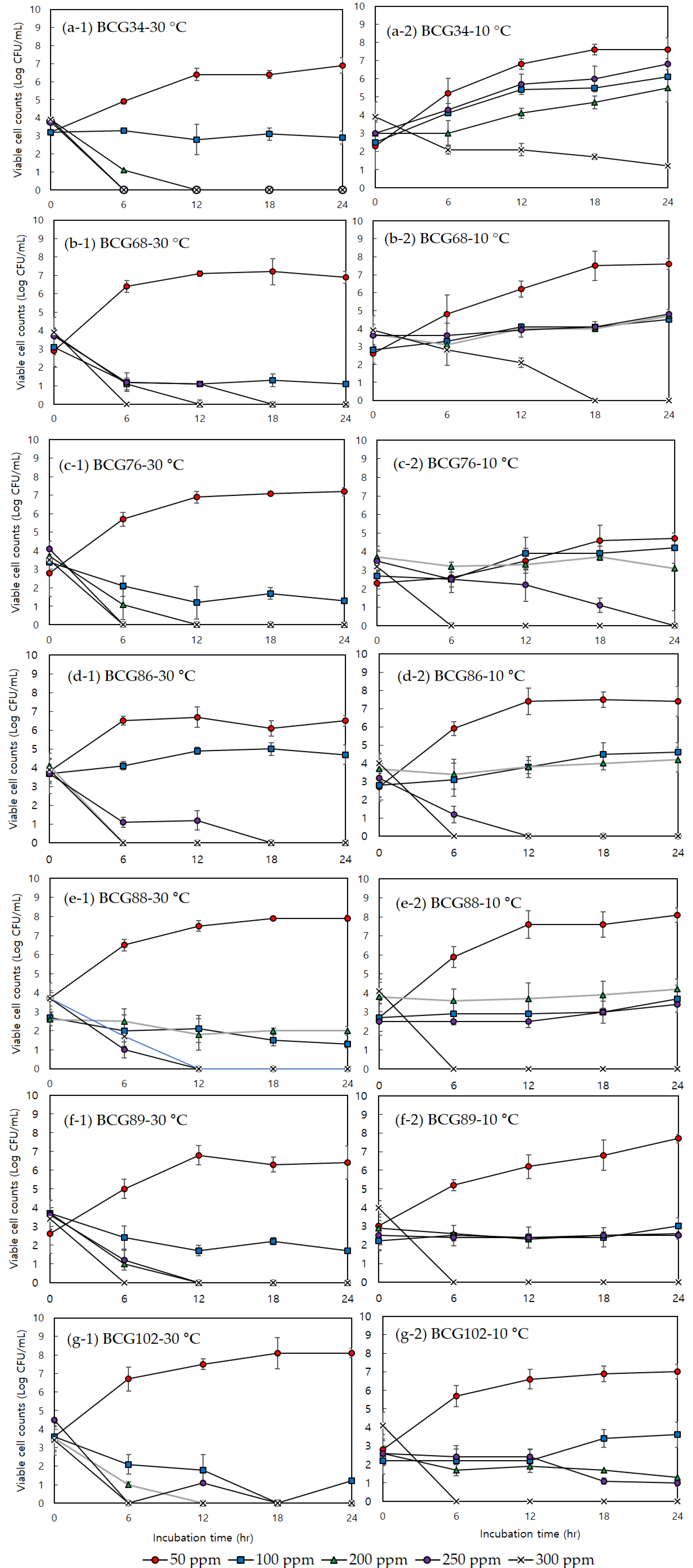
3.2. Minimum Inhibitory Concentration, Minimum Bactericidal Concentration, and Effects of Sodium Hypochlorite on Bacterial Growth
3.3. Effect of Thermal Stress on the Growth of Psychrotolerant B. cereus Group Strains at Low Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e04524. [Google Scholar] [CrossRef]
- Nicolás Lazarte, J.; Lopez, R.P.; Ghiringhelli, D.; Berón, C.M. Bacillus wiedmannii biovar thuringiensis: A specialized mosquitocidal pathogen with plasmids from diverse origins. Genome Biol. Evol. 2018, 10, 2823–2833. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.M.; Kabuki, D.Y.; Kuaye, A.Y. Growth of enterotoxin producing Bacillus cereus in meat substrate at 10 °C and 30 °C. Braz. J. Microbiol. 2012, 43, 1401–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meer, R.R.; Baker, J.; Bodyfelt, F.W.; Griffiths, M.W. Psychrotrophic Bacillus spp. in fluid milk products: A review. J. Food Prot. 1991, 54, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Beno, S.M.; Kent, D.J.; Carroll, L.M.; Martin, N.H.; Boor, K.J.; Kovac, J. Bacillus wiedmannii sp. nov., a psychrotolerant and cytotoxic Bacillus cereus group species isolated from dairy foods and dairy environments. Int. J. Syst. Evol. Microbiol. 2016, 66, 4744–4753. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.; Granum, P.E.; Buisson, C.; Bohlin, J.; Nielsen-LeRoux, C. Using an insect model to assess correlation between temperature and virulence in Bacillus weihenstephanensis and Bacillus cereus. FEMS Microbiol. Lett. 2011, 317, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Kim, H.J.; Jeong, M.; Koo, M. Enterotoxin genes, antibiotic susceptibility, and biofilm formation of low-temperature-tolerant Bacillus cereus isolated from lettuce in the cold chain. Foods 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Pittman, J.R.; Buntyn, J.O.; Posadas, G.; Nanduri, B.; Pendarvis, K.; Donaldson, J.R. Proteomic analysis of cross protection provided between cold and osmotic stress in Listeria monocytogenes. J. Proteome Res. 2014, 13, 1896–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhaes, R.; Ferreira, V.; Brandão, T.R.S.; Casquete, R.; Almeida, G.; Teixeira, P. Persistent and non-persistent strains of Listeria monocytogenes: A focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol. 2016, 57, 103–108. [Google Scholar] [CrossRef] [PubMed]
- López-Gálvez, F.; Allende, A.; Truchado, P.; Martínez-Sánchez, A.; Tudela, J.A.; Selma, M.V.; Gil, M.I. Suitability of aqueous chlorine dioxide versus sodium hypochlorite as an effective sanitizer for preserving quality of fresh-cut lettuce while avoiding by-product formation. Postharvest Biol. Technol. 2010, 55, 53–60. [Google Scholar] [CrossRef]
- Expert Group for Technical Advice on Organic Production (EGTOP). Final Report on Plant protection (III). Directorate-General for Agriculture and Rural Development. 2016. Available online: http://ec.europa.eu/info/sites/default/files/food-farming-fisheries/farming/documents/finalreportegtoponcleaningdisinfectanten.pdf (accessed on 10 August 2021).
- Rosario, D.K.; Duarte, A.L.A.; Madalao, M.C.M.; Libardi, M.C.; Teixeira, L.J.Q.; Conte, C.A., Jr.; Bernardes, P.C. Ultrasound improves antimicrobial effect of sodium hypochlorite and instrumental texture on fresh-cut yellow melon. J. Food Qual. 2018, 1–6. [Google Scholar] [CrossRef]
- Lingham, T.; Besong, S.; Ozbay, G. Antimicrobial activity of vinegar on bacterial species isolated from retail and local channel catfish (Ictalurus punctatus). J. Food Process. Technol. 2012, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Surekha, M.; Robinson, R.K.; Batt, C.A. Preservatives. Classification and properties. In Encyclopedia of Food Microbiology; Academic Press: New York, NY, USA, 2000; pp. 1710–1717. [Google Scholar]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Lv, R.; Zou, M.; Chantapakul, T.; Chen, W.; Muhammad, A.I.; Zhou, J.; Ding, T.; Ye, X.; Liu, D. Effect of ultrasonication and thermal and pressure treatments, individually and combined, on inactivation of Bacillus cereus spores. Appl. Microbiol. Biotechnol. 2019, 103, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Luo, Y.; Wu, D.; Liu, X. Effects of mild heat treatment on microbial growth and product quality of packaged fresh-cut table grapes. J. Food Sci. 2007, 72, 567–573 . [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Nur, I.T.; Munna, M.S.; Noor, R. Study of exogenous oxidative stress response in Escherichia coli, Pseudomonas spp., Bacillus spp. and Salmonella spp. Turk. J. Biol. 2014, 38, 502–509. [Google Scholar] [CrossRef]
- Redondo-Solano, M.; Burson, D.E.; Thippareddi, H. Thermal resistance of Clostridium difficile spores in peptone water and pork meat. J. Food Prot. 2016, 79, 1468–1474. [Google Scholar] [CrossRef]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food, 3rd ed.; Taylor & Francis/CRC Press: Boca Raton, FL, USA, 2005; pp. 1–10. [Google Scholar]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of acetic and citric acids to inhibit Escherichia coli O157:H7, Salmonella Typhimurium and Staphylococcus aureus in tabbouleh salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef]
- Olaimat, N.A.; Al-Nabulsi, A.A.; Osaili, M.T.; Al-Holy, M.; Ayyash, M.M.; Mehyar, F.G.; Jaradat, W.Z.; Ghoush, A.M. Survival and inhibition of Staphylococcus aureus in commercial and hydrated tahini using acetic and citric acids. Food Control. 2017, 77, 179–186. [Google Scholar] [CrossRef]
- Ivy, R.; Wiedmann, M.; Boor, K. Listeria monocytogenes grown at 7 °C shows reduced acid survival and an altered transcriptional response to acid shock compared to L. monocytogenes grown at 37 °C. Appl. Environ Microbiol. 2012, 78, 3824–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Yu, R.C.; Chou, C.C. Susceptibility of Vibrio parahaemolyticus to various environmental stresses after cold shock treatment. Int. J. Food Microbiol. 2004, 92, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Doyle, M.P.; Zhao, T.; Zhao, S. Enterohemorrhagic Escherichia coli. In Food Microbiology, Fundamentals and Frontiers; Doyle, M.P., Beuchat, L.R., Eds.; ASM Press: Washington, DC, USA, 2007; pp. 249–269. [Google Scholar]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 4, 1–20. [Google Scholar] [CrossRef]
- Chikthimmah, N.; Laborde, L.F.; Beelman, R.B. Critical factors affecting the destruction of Escherichia coli O157:H7 in apple cider treated with fumaric acid and sodium benzoate. J. Food Sci. 2003, 68, 1438–1442. [Google Scholar] [CrossRef]
- Shikongo-Nambabi, M.N.N.N.; Shoolongela, A.; Schneider, M.B. Control of bacterial contamination during marine fish processing. J. Biol. Life Sci. 2012, 3, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kwon, K.Y.; Kang, K.A.; Yoon, K.S. Effects of sodium hypochlorite and acidified sodium chlorite on the morphological, microbiological, and sensory qualities of selected vegetables. Food Sci. Biotechnol. 2011, 20, 759–766. [Google Scholar] [CrossRef]
- Guerin, A.; Dargaignaratz, C.; Broussolle, V.; Clavel, T.; Nguyen-the, C. Combined effect of anaerobiosis, low pH and cold temperatures on the growth capacities of psychrotrophic Bacillus cereus. Food Microbiol. 2016, 59, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Choma, C.; Guinebretiere, M.H.; Carlin, F.; Schmitt, P.; Velge, P.; Granum, P.E.; Nguyen-The, C. Prevalence, characterization and growth of Bacillus cereus in commercial cooked chilled foods containing vegetables. J. Appl. Microbiol. 2000, 88, 617–625. [Google Scholar] [CrossRef]
- Bergholz, T.M.; Bowen, B.; Wiedmann, M.; Boor, K.J. Listeria monocytogenes shows temperature-dependent and independent responses to salt stress, including responses that induce cross-protection against other stresses. Appl. Environ. Microbiol. 2012, 78, 2602–2612. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Luu-Thi, H.; Khadka, D.B.; Michiels, C.W. Thermal inactivation parameters of spores from different phylogenetic groups of Bacillus cereus. Int. J. Food Microbiol. 2014, 189, 183–188. [Google Scholar] [CrossRef]
- Carlin, F.; Brillard, J.; Broussolle, V.; Clavel, T.; Duport, C.; Jobin, M.; Guinebretiere, M.-H.; Auger, S.; Sorokine, A.; Nguyen-The, C. Adaptation of Bacillus cereus, an ubiquitous worldwide distributed foodborne pathogen, to a changing environment. Food Res. Int. 2010, 43, 1885–1894. [Google Scholar] [CrossRef]
- Jackson, T.C.; Hardin, M.D.; Acuff, G.R. Heat resistance of Escherichia coli 0157:H7 in a nutrient medium and in ground beef patties as influenced by storage and holding temperatures. J. Food Prot. 1996, 59, 230–237. [Google Scholar] [CrossRef]
- Kaur, J.; Ledward, D.A.; Park, R.W.A.; Robson, R.L. Factors affecting the heat resistance of Escherichia coli O157:H7. J. Appl. Microbiol. 1998, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Semanchek, J.; Golden, D.A. Influence of growth temperature on inactivation and injury of Escherichia coli O157:H7 by heat, acid, and freezing. J. Food Prot. 1998, 61, 395–401. [Google Scholar] [CrossRef]
- Kim, C.; Rana, A.; Mariam, B.; Eunice, N.; Paul, K.; Crystal, W. Influence of growth temperature on thermal tolerance of leading foodborne pathogens. Food Sci Nutr. 2019, 7, 4027–4036. [Google Scholar] [CrossRef]
- Den Besten, H.M.W.; van der Mark, E.-J.; Hensen, L.; Abee, T.; Zwietering, M.H. Quantification of the effect of culturing temperature on salt-induced heat resistance of Bacillus species. J. Appl. Environ. Microbiol. 2010, 76, 4286–4292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Food Isolates | Concentration of NaOCl (ppm) | |||
|---|---|---|---|---|
| 30°C | 10 °C | |||
| MICs | MLCs | MICs | MLCs | |
| BCG34 | 25 | 100 | 50 | 300 |
| BCG68 | 25 | 50 | 50 | 200 |
| BCG76 | 25 | 50 | 100 | 200 |
| BCG86 | 25 | 100 | 100 | 200 |
| BCG88 | 25 | 50 | 100 | 200 |
| BCG89 | 25 | 100 | 100 | 200 |
| BCG102 | 25 | 100 | 100 | 300 |
| Isolates | Temperature (°C) | D-Value * (min) | R2 for D-Value | z-Value * (°C) | R2 for z-Value |
|---|---|---|---|---|---|
| BCG34 | 55 | 42.2 ± 0.37 | 0.907 | 12.9 ± 0.24 | 0.957 |
| 60 | 20.9 ± 0.17 | 0.909 | |||
| 65 | 6.1 ± 0.38 | 0.927 | |||
| BCG68 | 55 | 47.6 ± 0.01 | 0.919 | 9.9 ± 0.41 | 0.943 |
| 60 | 17.9 ± 0.33 | 0.967 | |||
| 65 | 4.7 ± 0.75 | 0.972 | |||
| BCG76 | 55 | 66.6 ± 0.01 | 0.924 | 7.7 ± 0.39 | 0.949 |
| 60 | 8.9 ± 0.97 | 0.934 | |||
| 65 | 3.3 ± 0.44 | 0.919 | |||
| BCG86 | 55 | 47.6 ± 0.01 | 0.912 | 9.4 ± 0.28 | 0.914 |
| 60 | 9.8 ± 0.76 | 0.909 | |||
| 65 | 4.2 ± 0.99 | 0.917 | |||
| BCG88 | 55 | 30.3 ± 0.01 | 0.907 | 11.2 ± 0.24 | 0.929 |
| 60 | 17.9 ± 0.37 | 0.924 | |||
| 65 | 3.9 ± 1.46 | 0.977 | |||
| BCG89 | 55 | 18.2 ± 0.01 | 0.934 | 10.3 ± 0.39 | 0.992 |
| 60 | 9.8 ± 0.88 | 0.988 | |||
| 65 | 2.8 ± 4.67 | 0.946 | |||
| BCG102 | 55 | 27.0 ± 0.01 | 0.923 | 11.5 ± 0.17 | 0.948 |
| 60 | 5.2 ± 1.47 | 0.917 | |||
| 65 | 3.6 ± 0.31 | 0.903 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-M.; Kim, H.-J.; Choi, J.-Y.; Koo, M. Antimicrobial Effect of Acetic Acid, Sodium Hypochlorite, and Thermal Treatments against Psychrotolerant Bacillus cereus Group Isolated from Lettuce (Lactuca sativa L.). Foods 2021, 10, 2165. https://doi.org/10.3390/foods10092165
Park K-M, Kim H-J, Choi J-Y, Koo M. Antimicrobial Effect of Acetic Acid, Sodium Hypochlorite, and Thermal Treatments against Psychrotolerant Bacillus cereus Group Isolated from Lettuce (Lactuca sativa L.). Foods. 2021; 10(9):2165. https://doi.org/10.3390/foods10092165
Chicago/Turabian StylePark, Kyung-Min, Hyun-Jung Kim, Ji-Yoen Choi, and Minseon Koo. 2021. "Antimicrobial Effect of Acetic Acid, Sodium Hypochlorite, and Thermal Treatments against Psychrotolerant Bacillus cereus Group Isolated from Lettuce (Lactuca sativa L.)" Foods 10, no. 9: 2165. https://doi.org/10.3390/foods10092165






