Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Heat-Set Protein Gels
2.3. Confocal Raman Microscopy (CRM)
2.3.1. Specimen Preparation
2.3.2. Equipment and Software
2.3.3. Acquisition of Single Spectra
2.3.4. Confocal Raman Imaging
2.4. Analysis of Spectral Data and Image Processing
2.4.1. Method 1: Univariate Analysis
2.4.2. Method 2: Multivariate Analysis by Comparison with Reference Spectra
2.4.3. Method 3: Multivariate Analysis by Automatic Detection of Components
2.5. Confocal Laser Scanning Microscopy (CLSM)
3. Results and Discussion
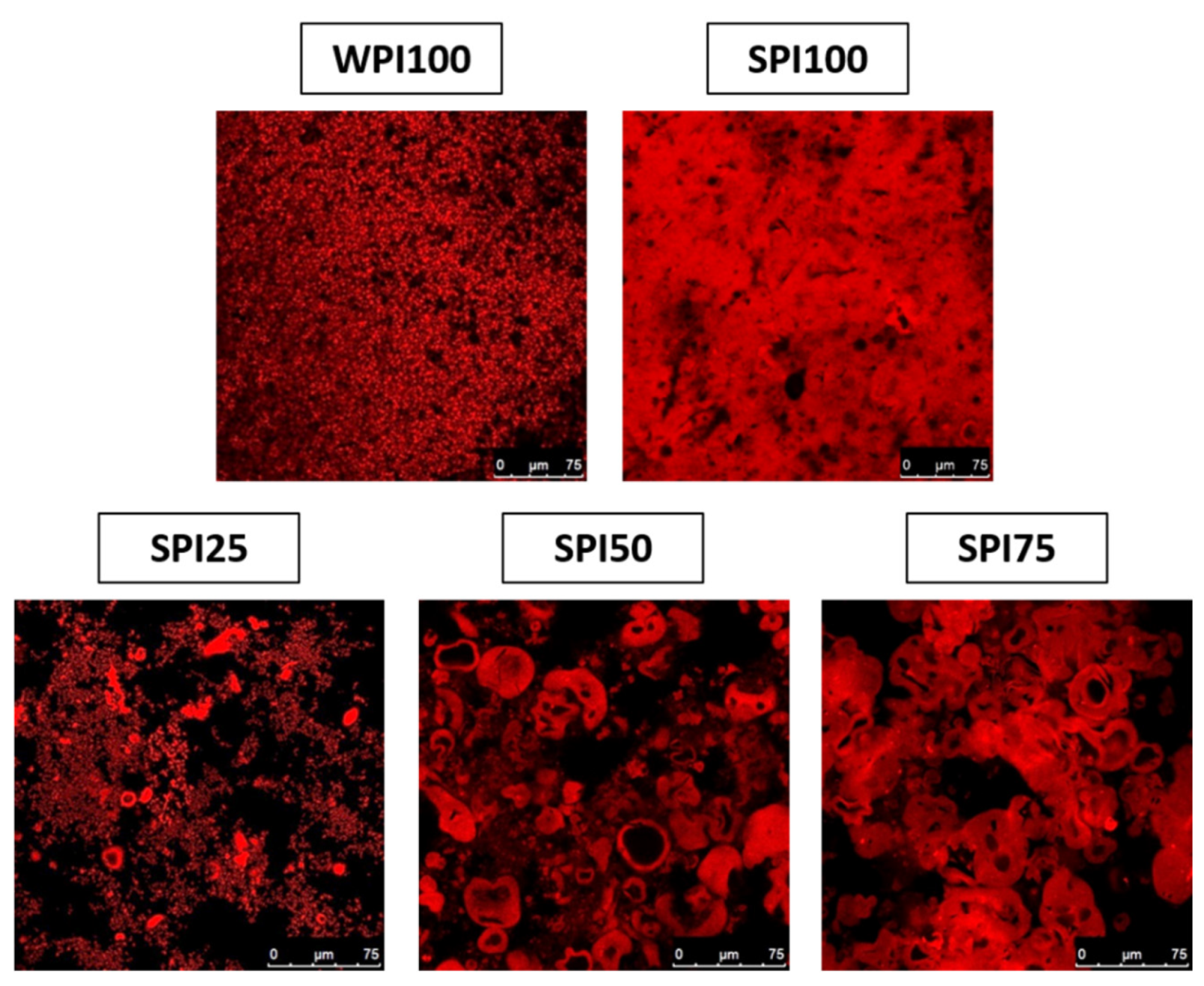
3.1. Protein Gels Preparation and CLSM Micrographs
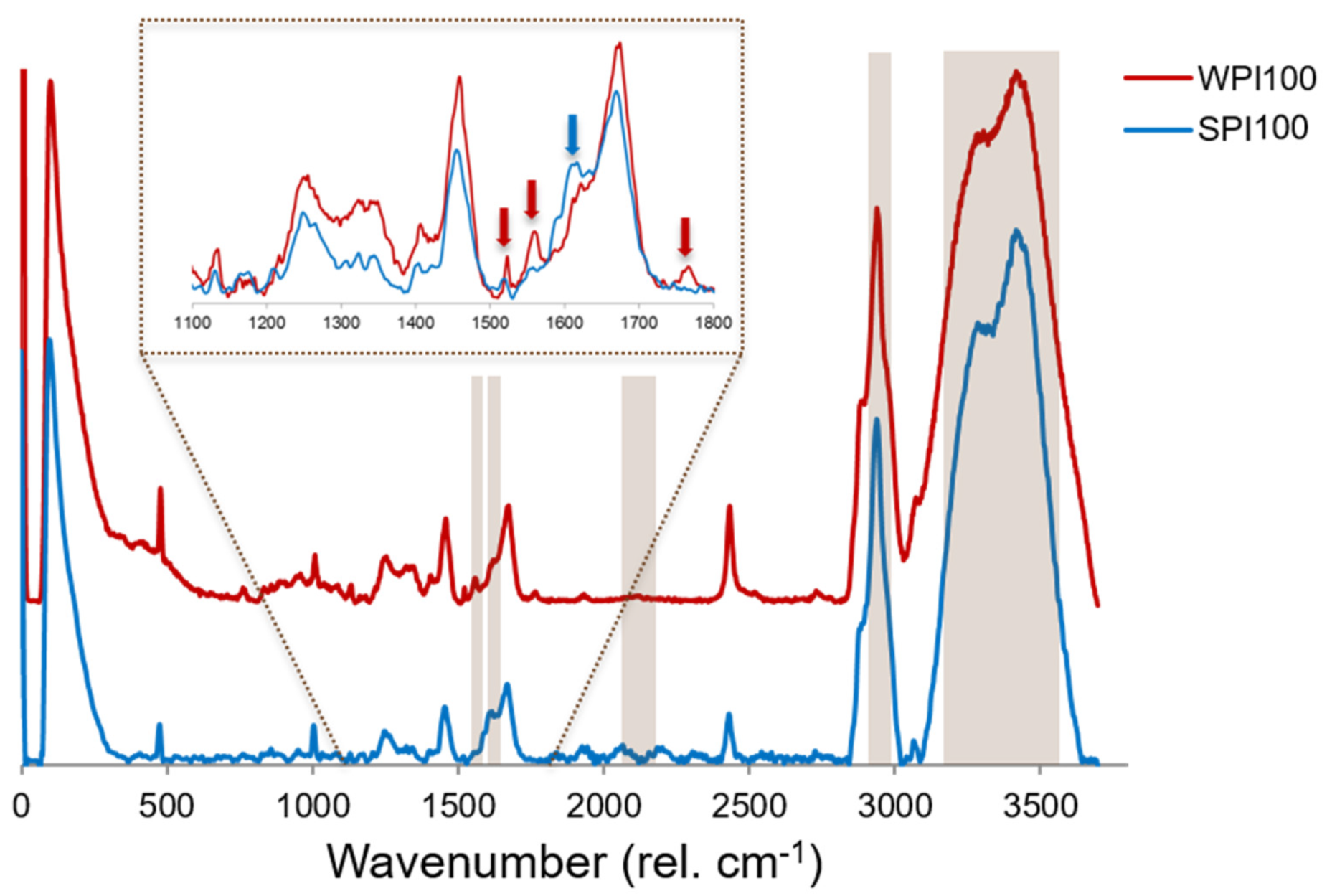
3.2. Raman Spectra of Reference Protein Gels
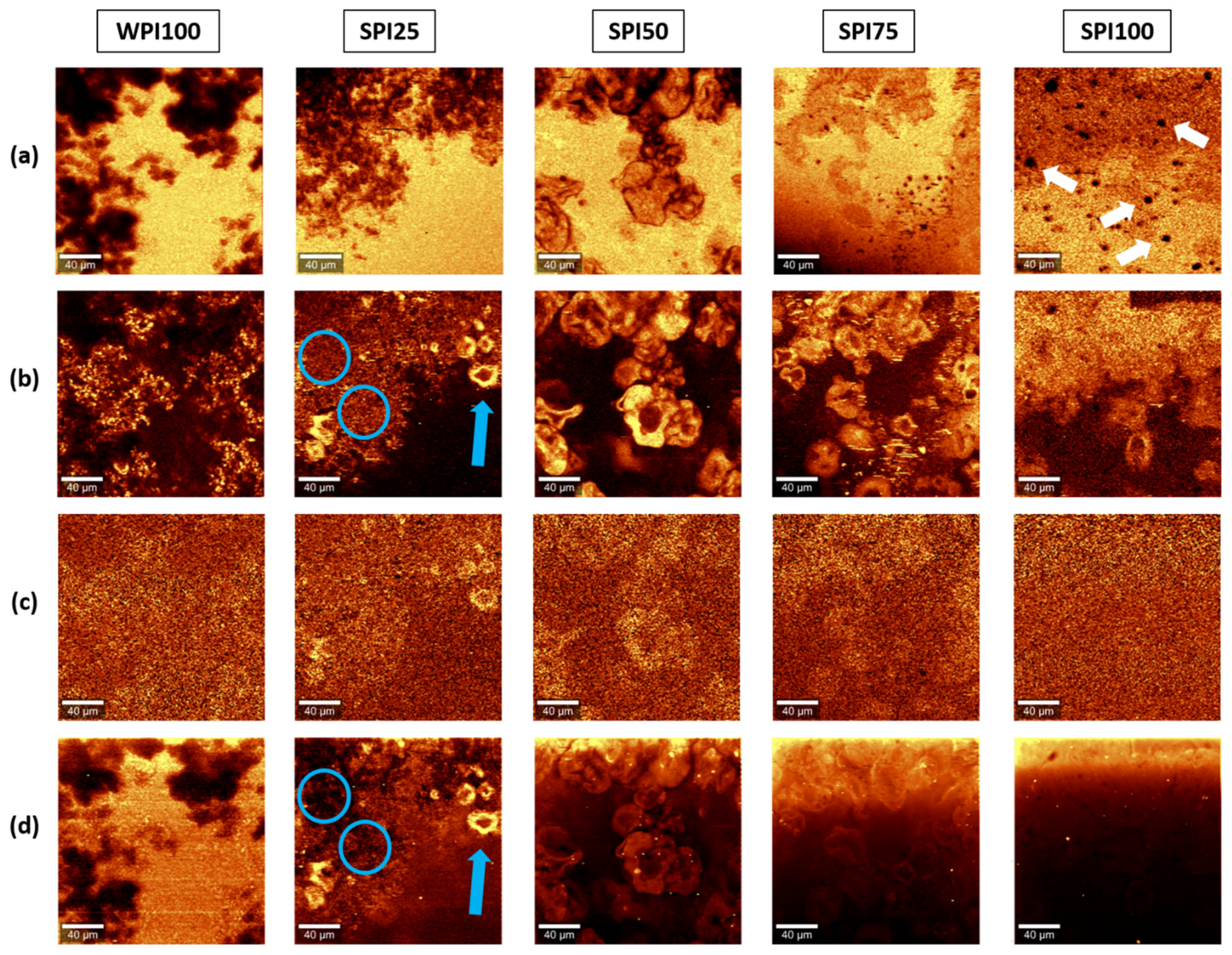
3.3. Confocal Raman Imaging: Univariate Analysis
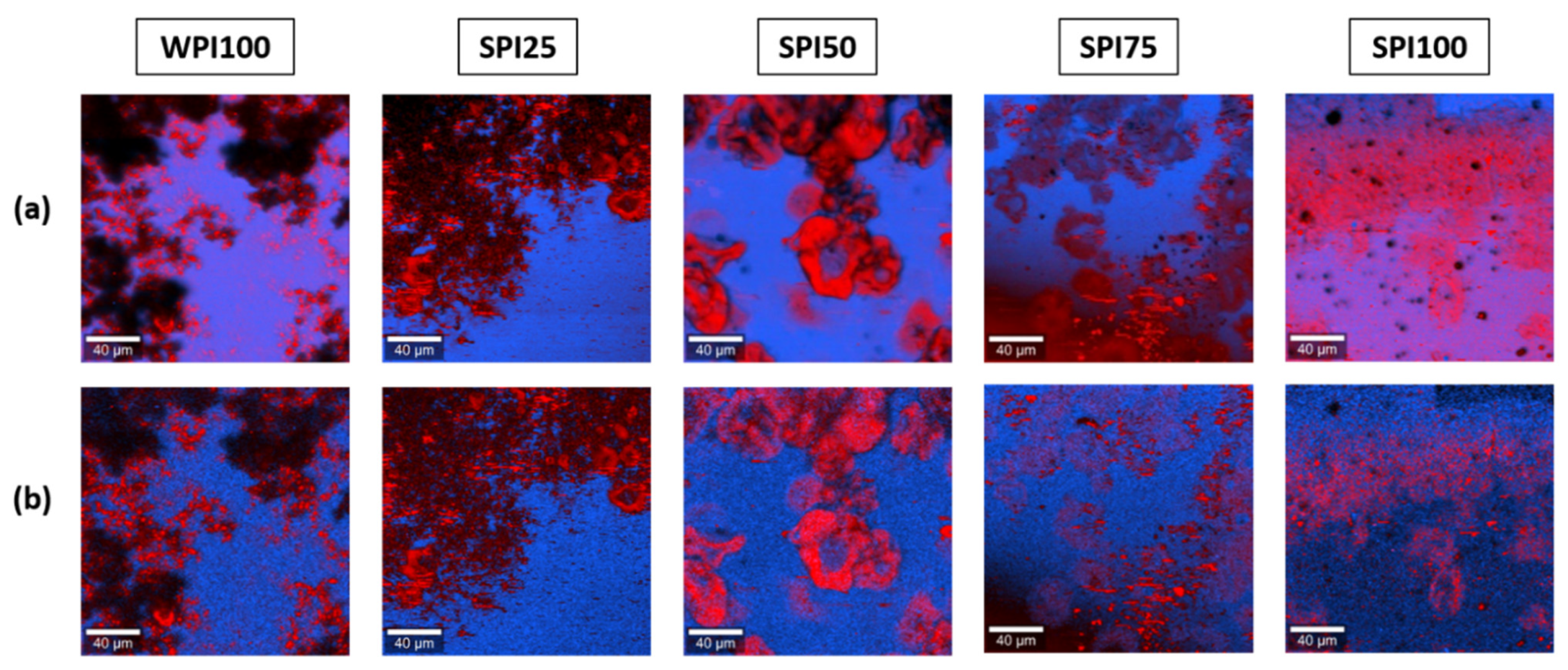
3.4. Multivariate Analysis
3.4.1. Comparison with Reference Materials
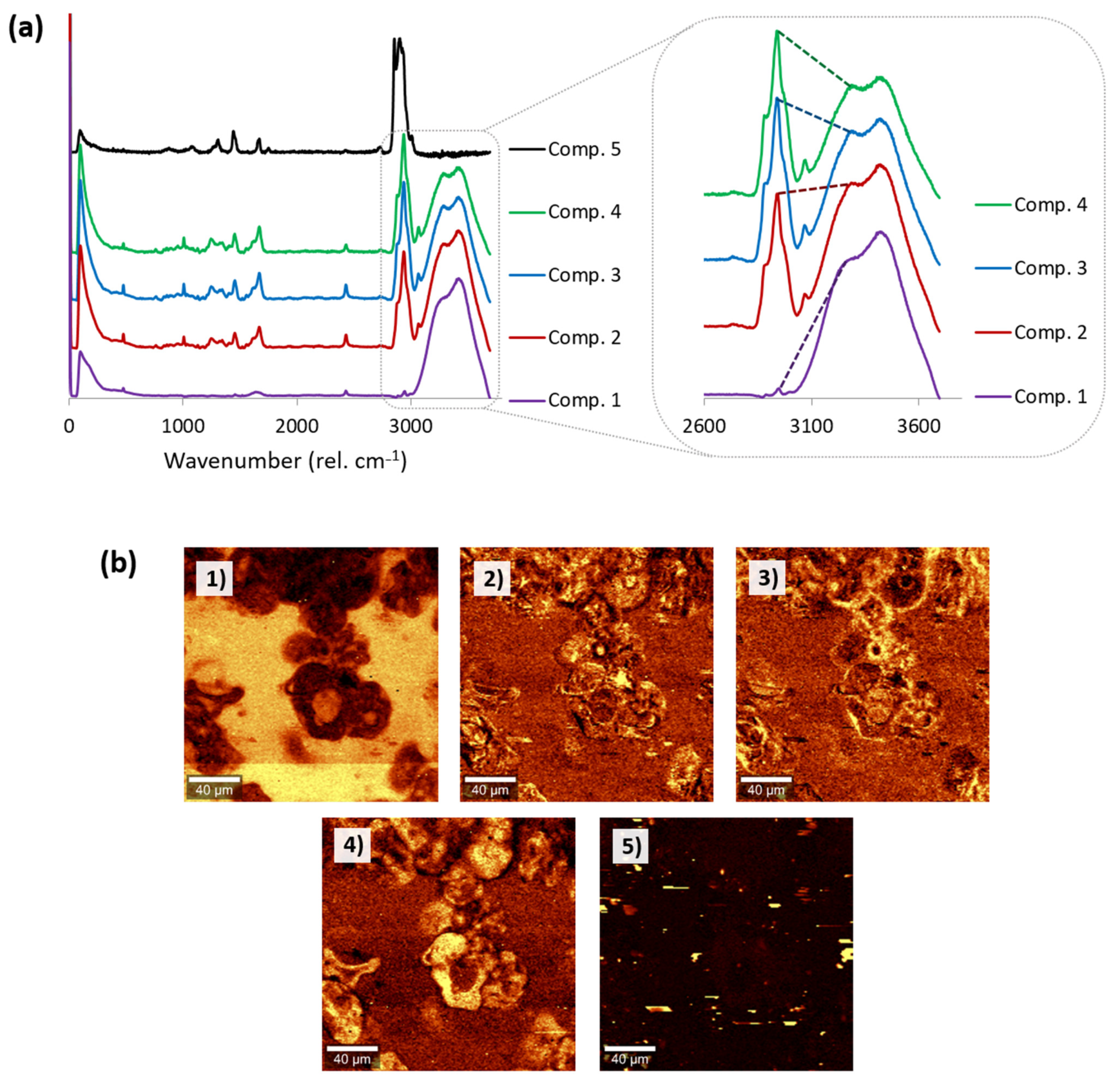
3.4.2. Detection of Main Components without the Input of Reference Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McWatters, K.H.; Cherry, J.P. Emulsification, foaming and protein solubility properties of defatted soybean, peanut, field pea and pecan flours. J. Food Sci. 1977, 42, 1444–1447. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey Proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Patel, S. Emerging trends in nutraceutical applications of whey protein and its derivatives. J. Food Sci. Technol. 2015, 52, 6847–6858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef]
- Van Vliet, T.; Lakemond, C.M.M.; Visschers, R.W. Rheology and structure of milk protein gels. Curr. Opin. Colloid Interface Sci. 2004, 9, 298–304. [Google Scholar] [CrossRef]
- Kuhn, K.R.; Cavallieri, Â.L.F.; Da Cunha, R.L. Cold-set whey protein gels induced by calcium or sodium salt addition. Int. J. Food Sci. Technol. 2010, 45, 348–357. [Google Scholar] [CrossRef]
- Nadathur, S.R.; Wanasundara, J.P.D.; Scanlin, L. Proteins in the Diet: Challenges in Feeding the Global Population. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 1–19. [Google Scholar] [CrossRef]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A Potential Source of Protein and Other Nutrients for Feed and Food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Dijkstra, D.S.; Linnemann, A.R.; van Boekel, T.A.J.S. Towards sustainable production of protein-rich foods: Appraisal of eight crops for Western Europe. PART II: Analysis of the technological aspects of the production chain. Crit. Rev. Food Sci. Nutr. 2003, 43, 481–506. [Google Scholar] [CrossRef] [PubMed]
- Aiking, H. Future protein supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Okolie, C.L.; Qian, H.; Ohanenye, I.C.; Agyei, D.; Aluko, R.E. Ribulose-1, 5-bisphosphate carboxylase as a sustainable and promising plant source of bioactive peptides for food applications. Trends Food Sci. Technol. 2017, 69, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Onwezen, M.C.; Bouwman, E.P.; Reinders, M.J.; Dagevos, H. A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat. Appetite 2021, 159, 105058. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Cachon, R.; Cayot, N. Pisum sativum vs. Glycine max, a comparative review of nutritional, physicochemical, and sensory properties for food uses. Trends Food Sci. Technol. 2020, 95, 196–204. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants–potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: A double-blind, cross-over trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Munoz, L.; Brodkorb, A.; Gómez-Mascaraque, L.; Corredig, M. Effect of heat treatment on the digestion behavior of pea and rice protein dispersions and their blends, studied using the semi-dynamic INFOGEST digestion method. Food Funct. 2021. [Google Scholar] [CrossRef]
- Loveday, S.M. Food Proteins: Technological, Nutritional, and Sustainability Attributes of Traditional and Emerging Proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef]
- Jose, J.; Pouvreau, L.; Martin, A.H. Mixing whey and soy proteins: Consequences for the gel mechanical response and water holding. Food Hydrocoll. 2016, 60, 216–224. [Google Scholar] [CrossRef]
- McCann, T.H.; Guyon, L.; Fischer, P.; Day, L. Rheological properties and microstructure of soy-whey protein. Food Hydrocoll. 2018, 82, 434–441. [Google Scholar] [CrossRef]
- Patil, G.; Patel, A.; Gupta, S.; Rajor, R. Manufacture of whey-soy beverages: A review. J. Food Sci. Technol. 1984, 21, 340–345. [Google Scholar]
- Kolar, C.; Cho, I.; Watrous, W. Vegetable protein application in yogurt, coffee creamers and whip toppings. J. Am. Oil Chem. Soc. 1979, 56 Pt 3, 389–391. [Google Scholar] [CrossRef]
- Langton, M.; Aström, A.; Hermansson, A.-M. Texture as a reflection of microstructure. Food Qual. Prefer. 1996, 7, 185–191. [Google Scholar] [CrossRef]
- Van den Berg, L.; Van Vliet, T.; Van der Linden, E.; Van Boekel, M.; Van de Velde, F. Breakdown properties and sensory perception of whey proteins/polysaccharide mixed gels as a function of microstructure. Food Hydrocoll. 2007, 21, 961–976. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Rigby, N.M.; Brodkorb, A.; Mackie, A.R. Dairy food structures influence the rates of nutrient digestion through different in vitro gastric behaviour. Food Hydrocoll. 2017, 67, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Dieing, T.; Hollricher, O.; Toporski, J. Confocal Raman Microscopy; Springer: Berlin/Heidelberg, Germany, 2011; Volume 158. [Google Scholar]
- Auty, M.A.; Twomey, M.; Guinee, T.P.; Mulvihill, D.M. Development and application of confocal scanning laser microscopy methods for studying the distribution of fat and protein in selected dairy products. J. Dairy Res. 2001, 68, 417–427. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Kilcawley, K.; Hennessy, D.; Tobin, J.T.; O’Callaghan, T.F. Raman spectroscopy: A rapid method to assess the effects of pasture feeding on the nutritional quality of butter. J. Dairy Sci. 2020, 103, 8721–8731. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Strobel, S.A.; Rai, R.; Jeoh, T.; Nitin, N. Multiscale imaging approaches for simultaneously mapping distribution of multiple components in infant formula powders. J. Food Eng. 2020, 281, 109999. [Google Scholar] [CrossRef]
- Benito-González, I.; Martínez-Sanz, M.; López-Rubio, A.; Gómez-Mascaraque, L.G. Confocal Raman imaging as a useful tool to understand the internal microstructure of multicomponent aerogels. J. Raman Spectrosc. 2020, 51. [Google Scholar] [CrossRef]
- Huen, J.; Weikusat, C.; Bayer-Giraldi, M.; Weikusat, I.; Ringer, L.; Lösche, K. Confocal Raman microscopy of frozen bread dough. J. Cereal Sci. 2014, 60, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.P.; Holroyd, S.E.; Reid, D.C.; Gordon, K.C. Raman imaging processed cheese and its components. J. Raman Spectrosc. 2017, 48, 374–383. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Trana, C.; O’Callaghan, T.; Hogan, S.A. Use of confocal Raman imaging to understand the microstructure of anhydrous milk fat-based oleogels. Food Struct. 2021. under review. [Google Scholar]
- Comfort, S.; Howell, N.K. Gelation properties of soya and whey protein isolate mixtures. Food Hydrocoll. 2002, 16, 661–672. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Nicolai, T.; Chassenieux, C. Exploiting salt induced microphase separation to form soy protein microcapsules or microgels in aqueous solution. Biomacromolecules 2017, 18, 2064–2072. [Google Scholar] [CrossRef]
- Stieger, M.; van de Velde, F. Microstructure, texture and oral processing: New ways to reduce sugar and salt in foods. Curr. Opin. Colloid Interface Sci. 2013, 18, 334–348. [Google Scholar] [CrossRef]
- Stephani, R.; Oliveira, K.d.S.; de Almeida, C.E.R.; Perrone, Í.T.; de Carvalho, A.F.; de Oliveira, L.F.C.; Almeida, M.R. Raman spectroscopy as a tool to identify modification of whey protein concentrate (WPC) during shelf life. Food Packag. Shelf Life 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, C.C.; Lin, L.L.; Chen, W. The effect of disulfide bonds on protein folding, unfolding, and misfolding investigated by FT–Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 940–947. [Google Scholar] [CrossRef]
- Herrero, A.M.; Jiménez-Colmenero, F.; Carmona, P. Elucidation of structural changes in soy protein isolate upon heating by Raman spectroscopy. Int. J. Food Sci. Technol. 2009, 44, 711–717. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Lin, M.; Vardhanabhuti, B. Raman spectroscopic characterization of structural changes in heated whey protein isolate upon soluble complex formation with pectin at near neutral pH. J. Agric. Food Chem. 2012, 60, 12029–12035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ao, Q.; Chen, F.; Xue, W.; Li, L.; Liu, J. Effect of reverse micelle on conformation of soy globulins: A Raman study. Food Chem. 2009, 116, 176–182. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; Hédoux, A.; Guinet, Y.; Paccou, L.; Petit, J.; Six, T.; Delaplace, G. Analysis by Raman spectroscopy of the conformational structure of whey proteins constituting fouling deposits during the processing in a heat exchanger. J. Food Eng. 2012, 110, 86–94. [Google Scholar] [CrossRef]
- Baeten, V. Raman spectroscopy in lipid analysis. Lipid Technol. 2010, 22, 36–38. [Google Scholar] [CrossRef]
- Baeten, V.; Hourant, P.; Morales, M.T.; Aparicio, R. Oil and Fat Classification by FT-Raman Spectroscopy. J. Agric. Food Chem. 1998, 46, 2638–2646. [Google Scholar] [CrossRef]
| Filter Code | Spectral Range (rel. cm−1) |
|---|---|
| Water | 3153.6–3553.6 |
| Protein | 2907.9–2982.7 |
| SPI | 1607.1–1637.1 |
| Fluorescence * | 2040.3–2115.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Mascaraque, L.G.; Pinho, S.C. Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy. Foods 2021, 10, 2179. https://doi.org/10.3390/foods10092179
Gómez-Mascaraque LG, Pinho SC. Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy. Foods. 2021; 10(9):2179. https://doi.org/10.3390/foods10092179
Chicago/Turabian StyleGómez-Mascaraque, Laura G., and Samantha C. Pinho. 2021. "Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy" Foods 10, no. 9: 2179. https://doi.org/10.3390/foods10092179
APA StyleGómez-Mascaraque, L. G., & Pinho, S. C. (2021). Microstructural Analysis of Whey/Soy Protein Isolate Mixed Gels Using Confocal Raman Microscopy. Foods, 10(9), 2179. https://doi.org/10.3390/foods10092179







