Formation and Characterization of Irreversible Sediment of Ginseng Extract
Abstract
:1. Introduction
2. Material and Methods
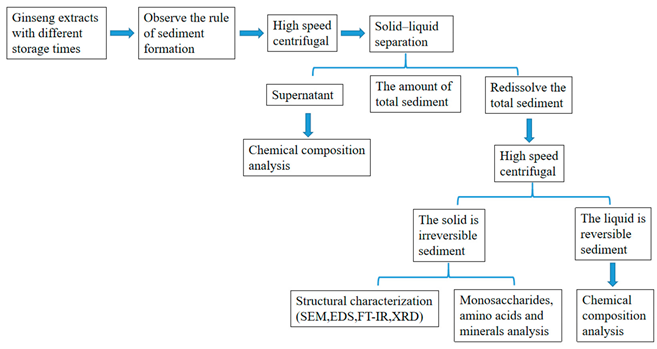
2.1. Graphical Scheme of the Approach of the Study
2.2. Preparation of a Ginseng Extract
2.3. Observation of Precipitate Formation
2.4. Preparation of RS and IRS
2.5. Determination of the Amount of Sediments
2.6. Analysis of Chemical Composition
2.6.1. Analysis of Total Sugar, Protein and Total Free Amino Acids
2.6.2. Analysis of Total Saponins
2.6.3. Analysis of Ginsenosides
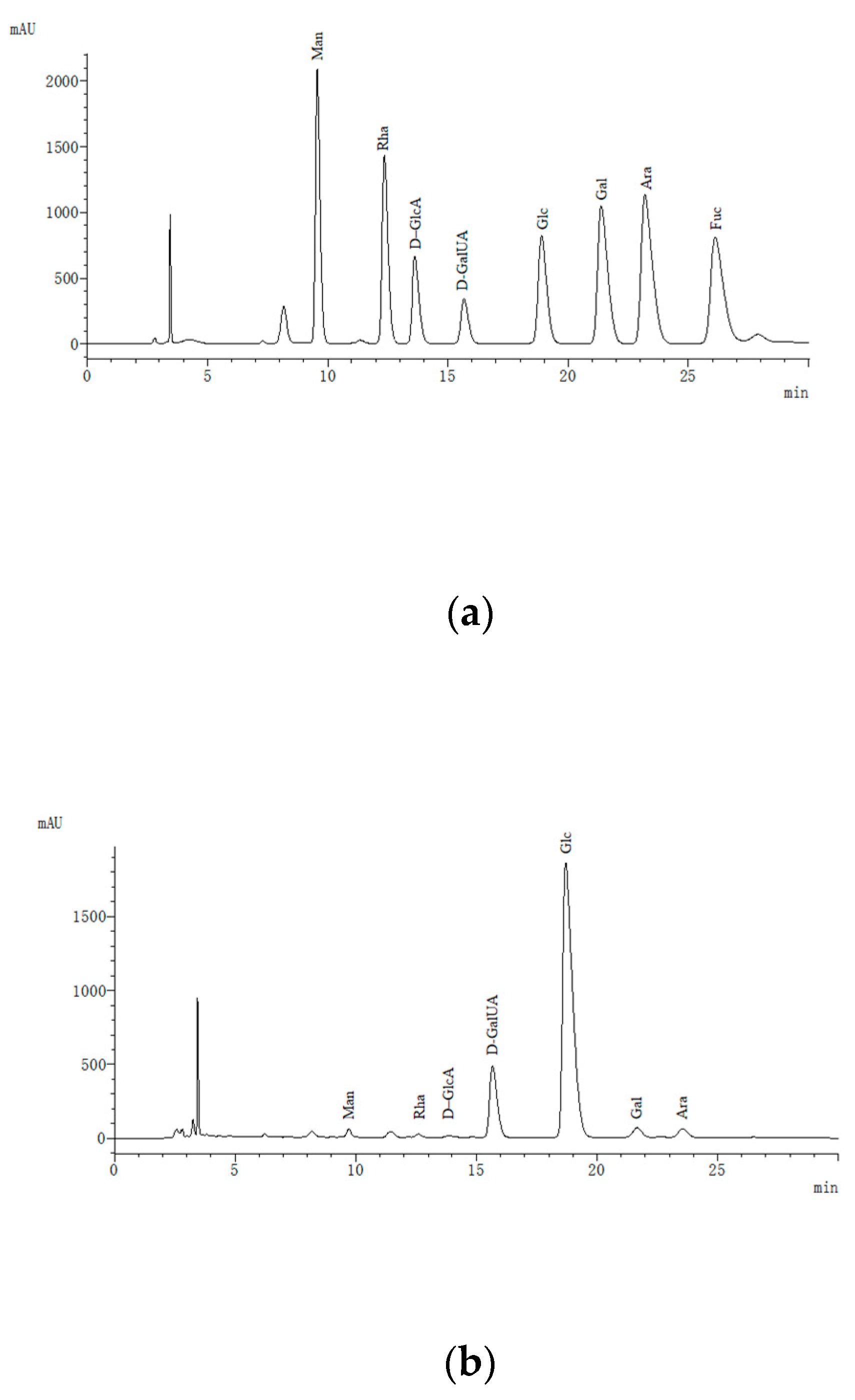
2.6.4. Analysis of Monosaccharide
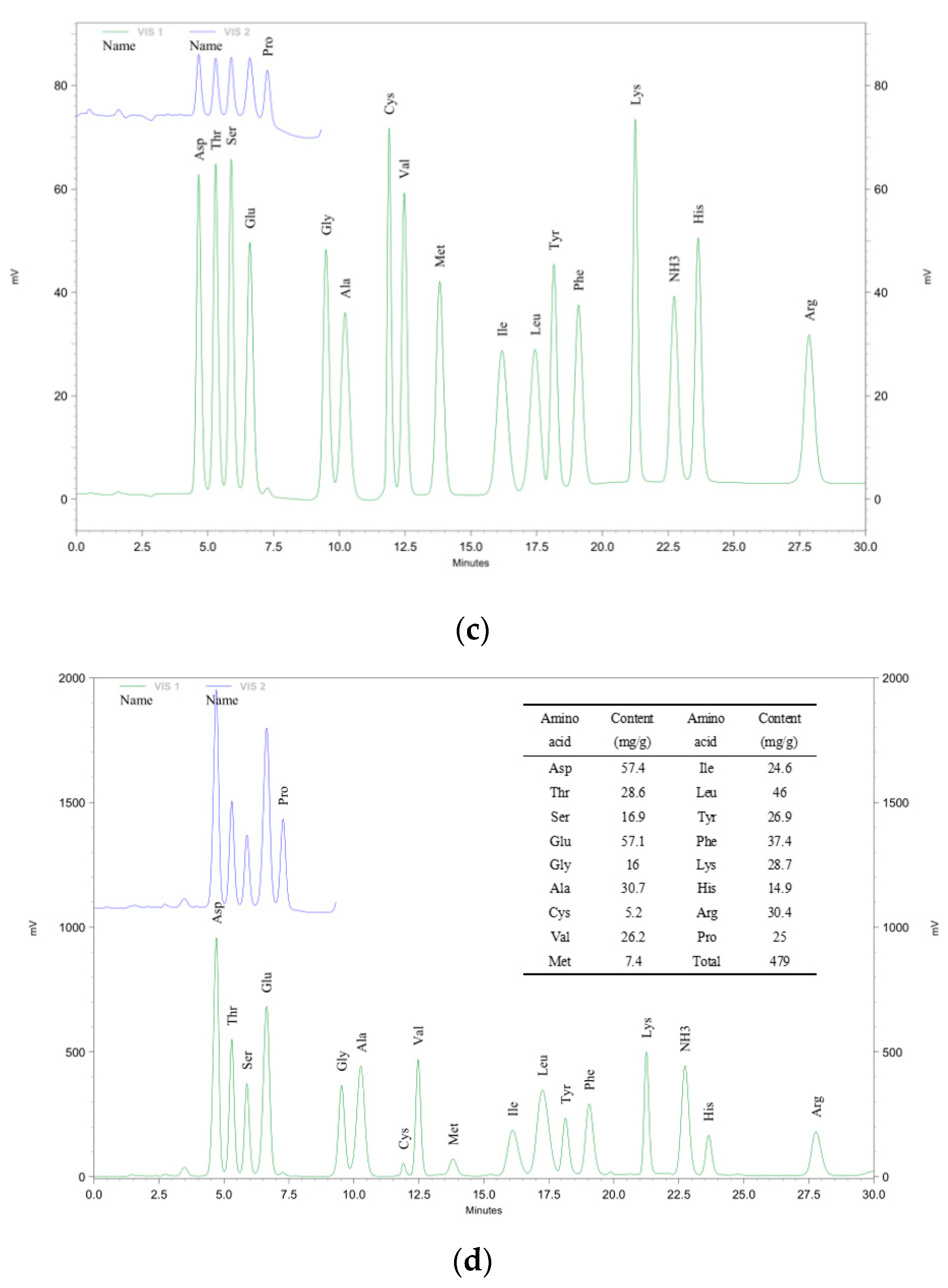
2.6.5. Analysis of Amino Acids
2.6.6. Mineral Analysis
2.7. SEM and EDS Analyses
2.8. FT-IR and XRD Analysis
2.9. Establishment of Chemical Composition Interaction Model
2.10. Data Analysis
3. Results and Discussion
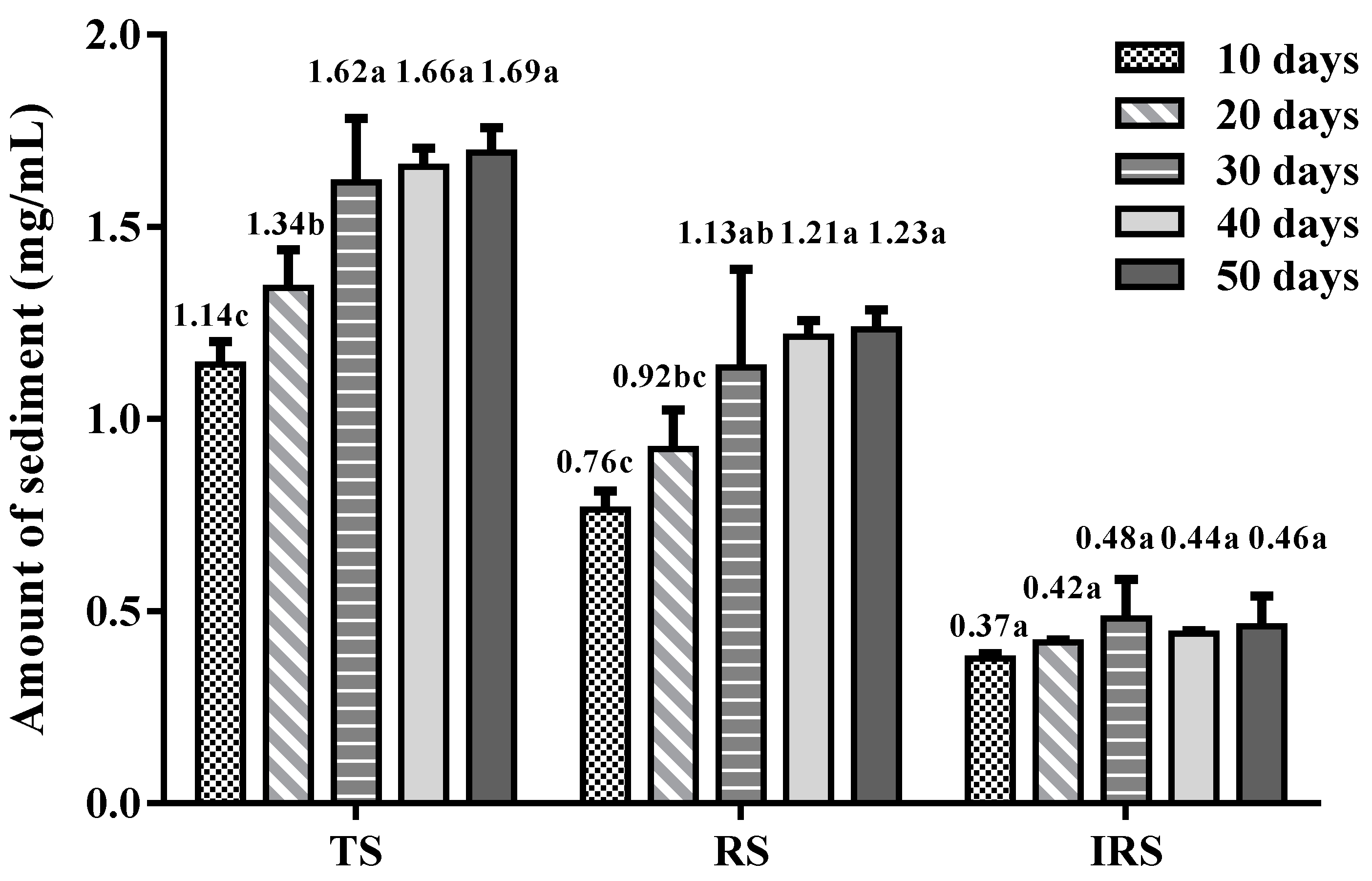
3.1. Observations of Sediment during Storage
3.2. Chemical Composition Analysis of the Extract and Sediments
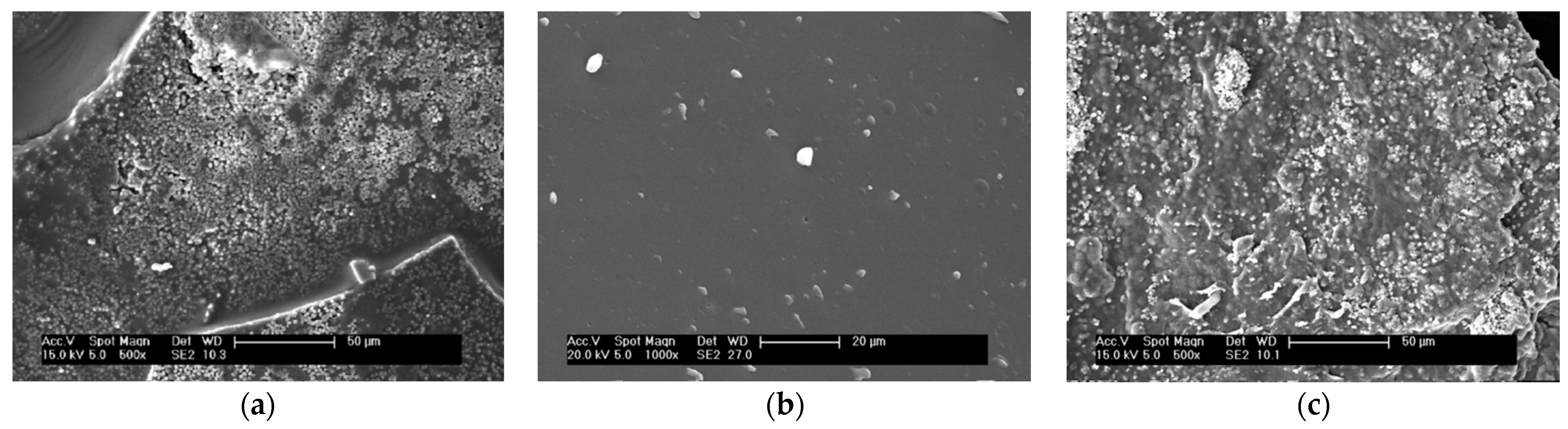
3.3. SEM and EDS Analysis
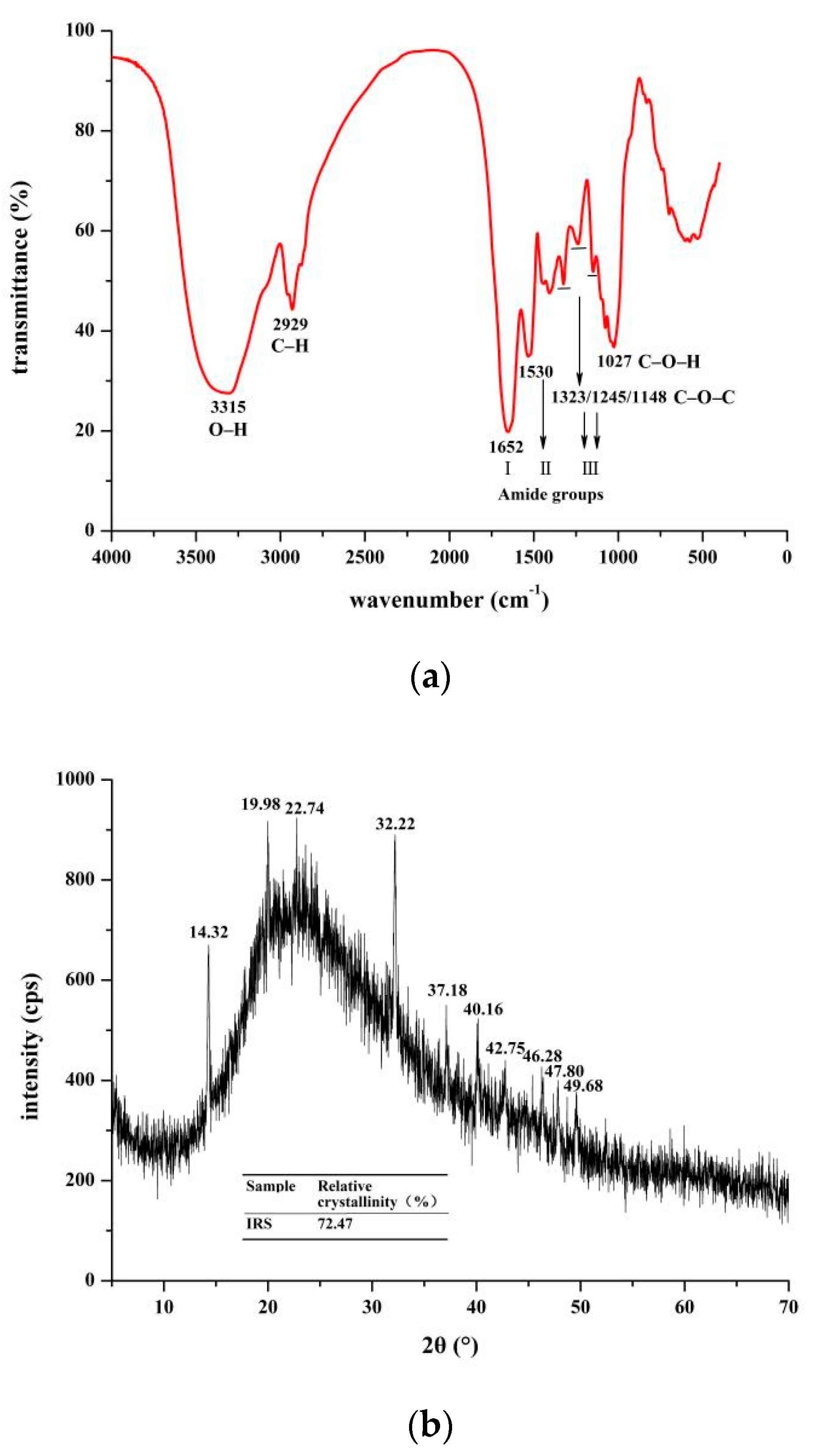
3.4. FT-IR and XRD Analyses
3.5. Monosaccharide and Amino Acid Composition Analysis of IRS
3.6. Verification of Chemical Composition Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jeong, H.; Park, D.H.; Seo, H.G.; Choi, M.-J.; Cho, Y. Effect of Roasting Time and Cryogenic Milling on the Physicochemical Characteristics of Dried Ginseng Powder. Foods 2020, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, Y.; Qiu, H.; Ju, Z.; Shi, Y.; Wang, Z.; Yang, L. Localization of constituents for determining the age and parts of ginseng through ultraperfomance liquid chromatography quadrupole/time of flight-mass spectrometry combined with desorption electrospray ionization mass spectrometry imaging. J. Pharm. Biomed. Anal. 2021, 193, 113722. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Qu, L.; Deng, X.; Duan, Z.; Fu, R.; Liang, L.; Fan, D. Ginsenoside Rk1 induces apoptosis and downregulates the expression of PD-L1 by targeting the NF-κB pathway in lung adenocarcinoma. Food Funct. 2020, 11, 456–471. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Z.; Wang, X.; Wang, C. Recent Advances in Panax ginseng C.A. Meyer as a Herb for Anti-Fatigue: An Effects and Mechanisms Review. Foods 2021, 10, 1030. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, J.; Wang, H.; Zhou, Y.; Liu, X.; Liu, Z. Structural characterization of ginseng oligopeptides and anti-aging potency evaluation in Caenorhabditis elegans. RSC Adv. 2020, 10, 39485–39494. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, C.; Li, W.; Hang, F.; Li, K.; Shi, C.; Doherty, W.O. Nutritional and antioxidant properties of non-centrifugal cane sugar derived from membrane clarified juice. LWT 2020, 131, 109717. [Google Scholar] [CrossRef]
- Shi, C.; Rackemann, D.W.; Moghaddam, L.; Wei, B.; Li, K.; Lu, H.; Xie, C.; Hang, F.; Doherty, W.O. Ceramic membrane filtration of factory sugarcane juice: Effect of pretreatment on permeate flux, juice quality and fouling. J. Food Eng. 2019, 243, 101–113. [Google Scholar] [CrossRef] [Green Version]
- LaClair, C.E.; Etzel, M.R. Ingredients and pH are Key to Clear Beverages that Contain Whey Protein. J. Food Sci. 2010, 75, C21–C27. [Google Scholar] [CrossRef]
- Malathy, R.; Prabakaran, M.; Kalaiselvi, K.; Chung, I.-M.; Kim, S.-H. Comparative Polyphenol Composition, Antioxidant and Anticorrosion Properties in Various Parts of Panax ginseng Extracted in Different Solvents. Appl. Sci. 2020, 11, 93. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, G.-S.; Wang, Q.-S.; Yuan, H.-B.; Feng, C.-H.; Yin, J.-F. Irreversible Sediment Formation in Green Tea Infusions. J. Food Sci. 2012, 77, C298–C302. [Google Scholar] [CrossRef]
- Siebert, K.J. Haze formation in beverages. LWT 2006, 39, 987–994. [Google Scholar] [CrossRef]
- Yang, W.; Deng, C.; Xu, L.; Jin, W.; Zeng, J.; Li, B.; Gao, Y. Protein-neutral polysaccharide nano- and micro-biopolymer complexes fabricated by lactoferrin and oat β-glucan: Structural characteristics and molecular interaction mechanisms. Food Res. Int. 2020, 132, 109111. [Google Scholar] [CrossRef]
- Wu, D.; Lin, Q.; Singh, H.; Ye, A. Complexation between whey protein and octenyl succinic anhydride (OSA)-modified starch: Formation and characteristics of soluble complexes. Food Res. Int. 2020, 136, 109350. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Desobry-Banon, S.; Hardy, J. Structure and Technofunctional Properties of Protein-Polysaccharide Complexes: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 689–753. [Google Scholar] [CrossRef]
- Akbulut, M.; Çalişir, S.; Marakoğlu, T.; Çoklar, H. Some physicomechanical and nutritional properties of barberry (Berberis vulgaris L.) fruits. J. Food Process. Eng. 2009, 32, 497–511. [Google Scholar] [CrossRef]
- Kherici, S.; Benouali, D.; Benyetou, M.; Ghidossi, R.; Lacampagne, S.; Mietton-Peuchot, M. Study of Potassium Hydrogen Tartrate Unseeded Batch Crystallization for Tracking Optimum Cooling Mode. Orient. J. Chem. 2015, 31, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.E.; Mousavi, M.; Kiani, H. Characterization and identification of sediment forming agents in barberry juice. Food Chem. 2020, 312, 126056. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zhong, X.-Y.; Yin, J.-F.; Yuan, H.-B.; Tang, P.; Du, Q.-Z. The impact of Ca2+ combination with organic acids on green tea infusions. Food Chem. 2013, 139, 944–948. [Google Scholar] [CrossRef]
- Rodriguez-Clemente, R.; Correa-Gorospe, I.; De Castro, J. A new method for the stabilization of wines with respect to the potassium bitartrate precipitation. J. Enol. 1990, 41, 16–20. [Google Scholar] [CrossRef]
- Zou, B.; Xu, Y.-J.; Wu, J.-J.; Yu, Y.-S.; Xiao, G.-S. Phenolic compounds participating in mulberry juice sediment formation during storage. J. Zhejiang Univ. Sci. B 2017, 18, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.-F.; Xu, Y.-Q.; Yuan, H.-B.; Luo, L.-X.; Qian, X.-J. Cream formation and main chemical components of green tea infusions processed from different parts of new shoots. Food Chem. 2009, 114, 665–670. [Google Scholar] [CrossRef]
- Nagalakshmi, S.; Ramaswamy, M.; Natarajan, C.; Seshadri, R. The rôle of added carbohydrates in tea ‘cream’ solubilisation. Food Chem. 1984, 13, 69–77. [Google Scholar] [CrossRef]
- Cuesta, G.; Suarez, N.; Bessio, M.I.; Ferreira, F.; Massaldi, H. Quantitative determination of pneumococcal capsular poly-saccharide serotype 14 using a modification of phenol-sulfuric acid method. J. Microbiol. Methods 2003, 52, 69–73. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, L.; Zhao, C.; Huang, W.; Chen, Q. Nondestructive Estimation of Total Free Amino Acid in Green Tea by Near Infrared Spectroscopy and Artificial Neural Networks. In IFIP Advances in Information and Communication Technology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 369, AICT (PART 2); pp. 43–53. [Google Scholar]
- Gao, K.; Chen, J.; Wang, Y.; Qi, Y.; Chen, L.; Li, S.; Sun, Y. Effects of 60Co-γ and Electron Beam Irradiation on Storage Quality of Panax ginseng. Food Bioprocess Technol. 2018, 11, 1627–1638. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Li, W.; Cai, X.; Qi, D.; Zhang, Y.; Han, C. Comparison of the structures and prebiotic-like effects in vitro of polysaccharides from Coprinus comatus fruit body and mycelium. Int. J. Biol. Macromol. 2020, 165, 2621–2629. [Google Scholar] [CrossRef]
- East, C.P.; Wallace, A.D.; Al-Hamzah, A.; Doherty, W.O.S.; Fellows, C.M. Effect of poly(acrylic acid) molecular mass and end-group functionality on calcium oxalate crystal morphology and growth. J. Appl. Polym. Sci. 2010, 115, 2127–2135. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A.; Mohebbi, M.; Malaekeh-Nikouei, B. New studies on basil (Ocimum bacilicum L.) seed gum: Part I—Fractionation, physicochemical and surface activity characterization. Food Hydrocoll. 2016, 52, 350–358. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Li, S.-C.; Yang, X.-M.; Ma, H.-L.; Yan, J.-K.; Guo, D.-Z. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohydr. Polym. 2015, 133, 24–30. [Google Scholar] [CrossRef]
- Guo, Q.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Liu, J.; Gao, Y. Production and characterization of pea protein isolate-pectin complexes for delivery of curcumin: Effect of esterified degree of pectin. Food Hydrocoll. 2020, 105, 105777. [Google Scholar] [CrossRef]
- Butto, N.; Vera, N.C.; Díaz-Soler, F.; Yazdani-Pedram, M.; Neira-Carrillo, A. Effect of Chitosan Electrospun Fiber Mesh as Template on the Crystallization of Calcium Oxalate. Crystals 2020, 10, 453. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.-Y.; Wang, F.-X.; Ouyang, J.-M. Regulation on Calcium Oxalate Crystallization and Protection on HK-2 Cells of Tea Polysaccharides with Different Molecular Weights. Oxidative Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein-polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Mou.écoucou, J.; Villaume, C.; Sanchez, C.; Méjean, L. β-Lactoglobulin/polysaccharide interactions during in vitro gastric and pancreatic hydrolysis assessed in dialysis bags of different molecular weight cut-offs. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2004, 1670, 105–112. [Google Scholar] [CrossRef]
- Mravec, J.; Kračun, S.K.; Rydahl, M.G.; Westereng, B.; Pontiggia, D.; De Lorenzo, G.; Domozych, D.S.; Willats, W.G.T. An oligogalacturonide-derived molecular probe demonstrates the dynamics of calcium-mediated pectin complexation in cell walls of tip-growing structures. Plant J. 2017, 91, 534–546. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Ji, X.; Lyu, F.; Liu, J.; Ding, Y. Heat stability and rheology of high-calorie whey protein emulsion: Effects of calcium ions. Food Hydrocoll. 2021, 114, 106583. [Google Scholar] [CrossRef]
- Van Dinh, Q.; Liu, J.; Dutta, P. Effect of Calcium ion on synaptotagmin-like protein during pre-fusion of vesicle for exocytosis in blood-brain barrier. Biochem. Biophys. Rep. 2020, 24, 100845. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium−Alginate Gels with X-ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Nakauma, M.; Funami, T.; Fang, Y.; Nishinari, K.; Draget, K.I.; Phillips, G.O. Calcium binding and calcium-induced gelation of normal low-methoxyl pectin modified by low molecular-weight polyuronate fraction. Food Hydrocoll. 2017, 69, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Bouton, S.; Leboeuf, E.; Mouille, G.; Leydecker, M.-T.; Talbotec, J.; Granier, F.; Lahaye, M.; Höfte, H.; Truong, H.-N. QUASIMODO1 Encodes a Putative Membrane-Bound Glycosyltransferase Required for Normal Pectin Synthesis and Cell Adhesion in Arabidopsis. Plant Cell 2002, 14, 2577–2590. [Google Scholar] [CrossRef] [Green Version]
- Domozych, D.S.; Sørensen, I.; Popper, Z.; Ochs, J.; Andreas, A.; Fangel, J.U.; Pielach, A.; Sacks, C.; Brechka, H.; Ruisi-Besares, P.; et al. Pectin Metabolism and Assembly in the Cell Wall of the Charophyte Green Alga Penium margaritaceum. Plant Physiol. 2014, 165, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, A. Complexation between milk proteins and polysaccharides via electrostatic interaction: Principles and applications—A review. Int. J. Food Sci. Technol. 2008, 43, 406–415. [Google Scholar] [CrossRef]
- Li, J.-E.; Wang, W.-J.; Zheng, G.-D.; Li, L.-Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Biol. Macromol. 2017, 95, 719–724. [Google Scholar] [CrossRef]
- Sperber, B.L.; Schols, H.A.; Stuart, M.A.C.; Norde, W.; Voragen, A.G. Influence of the overall charge and local charge density of pectin on the complex formation between pectin and β-lactoglobulin. Food Hydrocoll. 2009, 23, 765–772. [Google Scholar] [CrossRef]

| Storage (Days) | Observation | Amount of Sediments | Description | |
|---|---|---|---|---|
| Yellow Sediments | White Sediments | |||
| 0 |  | No | No | The extract was clarified without sediment. |
| 10 |  | Some | Little | Some quantity of yellow sediments and a small quantity of white sediment appeared. |
| 20 |  | Much | Little | Compared to the sediment stored for 10 days, the yellow sediment increased significantly, and the white sediment did not change significantly. |
| 30 |  | Much | Some | Compared to the sediment stored for 20 days, both the yellow sediment and the white sediment increased significantly. |
| 40 |  | Much | Some | Compared to the sediment stored for 30 days, both the yellow sediment and the white sediment increased but not significantly. |
| 50 |  | Much | Some | Compared to the sediment stored for 40 days, both the yellow sediment and the white sediment increased only slightly. |
| Chemical Components | Supernatant | RS | IRS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 10 Day | 20 Day | 30 Day | 40 Day | 50 Day | 10 Day | 20 Day | 30 Day | 40 Day | 50 Day | 10 Day | 20 Day | 30 Day | 40 Day | 50 Day | ||
| K | mg/kg | 1088 a | 1080 a | 1032 b | 1032 b | 1024 c | 1014 c | 31 c | 33 c | 39 b | 49 a | 51 a | 1551 b | 1706 b | 3672 a | 3236 a | 3614 a |
| Mg | 86 a | 85 a | 84 a | 82 a | 81 a | 80 a | 2.9 c | 3.6 c | 4.3 b | 4.9 ab | 5.1 a | 625 b | 630 b | 680 ab | 704 a | 735 a | |
| Na | 19 b | 18 b | 20 a b | 20 ab | 21 a b | 22 a | 11 a | 9.2 ab | 7.4 b | 6.5 b | 4.4 c | 357 c | 376 c | 412 bc | 536 ab | 601 a | |
| Ca | 47 a | 31 b | 29 b | 28 b | 26 b | 25 b | 9.1 a b | 11 a | 11 a | 10 a | 8.7 b | 6224 d | 14678 c | 16186 c | 20137 b | 25024 a | |
| Al | 2.8 a | 2.2 b | 2.0 b | 1.9 b | 1.4 b | 1.3 b | 2.3 a | 2.2 a | 1.6 b | 1.1 b c | 0.800 cd | 771 e | 964 d | 1210 c | 1738 b | 2048 a | |
| Fe | 2.6 a | 2.3 b | 2.2 b c | 2.0 cd | 2.0 c d | 1.9 e | 0.633 a | 0.621 a | 0.616 a | 0.446 b | 0.411 b | 213 c | 220 c | 224 c | 261 b | 312 a | |
| Mn | 20 a | 20 a | 19 a b | 19 ab | 19 a b | 19 a b | 0.234 a | 0.207 b | 0.200 b | 0.187 bc | 0.165 c | 50 d | 52 d | 58 c | 70 b | 74 a | |
| Ni | 0.150 a | 0.145 a b | 0.141 b | 0.139 b | 0.133 c | 0.133 c | 0.018 a | 0.013 b | 0.012 b | 0.012 b | 0.011 b | 1.1 e | 1.5 d | 1.8 c | 2.1 b | 2.4 a | |
| Sr | 0.248 a | 0.221 a b | 0.210 b | 0.219 b | 0.203 b | 0.198 b | 0.033 a | 0.031 a | 0.029 ab | 0.024 bc | 0.022 c | 33 d | 39 c | 40 c | 45 b | 53 a | |
| Ba | 0.054 a | 0.049 a b | 0.048 b | 0.045 b | 0.045 b | 0.044 b | 0.007 a | 0.007 a | 0.006 ab | 0.005 ab | 0.003 b | 15 c | 16 b | 16 b | 20 a | 20 a | |
| Free amino acid | mg/mL | 5.3 a | 5.6 a | 5.5 a | 5.8 a | 5.8 a | 5.8 a | 0.068 b | 0.069 b | 0.102 a | 0.106 a | 0.110 a | |||||
| Protein | 5.9 a | 5.5 a | 5.4 a | 5.1 a | 5.0 a | 5.0 a | 0.282 d | 0.333 cd | 0.374 bc | 0.436 ab | 0.506 a | ||||||
| Total sugar | 44 a | 44 a | 43 a | 42 a | 41 a | 40 a | 0.881 a | 1.0 a | 1.5 a | 1.5 a | 1.7 a | ||||||
| Total saponins | 4.1 a | 3.8 a b | 4.0 a | 3.8 ab | 3.7 ab | 3.7 ab | 0.141 b | 0.156 ab | 0.165 a b | 0.168 ab | 0.185 a | ||||||
| Rg1 | 0.409 a | 0.402 b | 0.397 c | 0.396 cd | 0.388 e | 0.392 d | 0.007 c | 0.012 b | 0.011 b | 0.015 a | 0.017 a | ||||||
| Re | 0.211 a | 0.306 a | 0.305 a | 0.307 a | 0.296 a | 0.291 a | 0.004 c | 0.008 b | 0.008 b | 0.012 a | 0.012 a | ||||||
| Rf | 0.217 c | 0.224 c | 0.150 d | 0.221 c | 0.422 a | 0.383 b | 0.003 c | 0.005 b | 0.005 b | 0.006 a | 0.005 b | ||||||
| Rb1 | 0.769 a | 0.743 b | 0.737 c | 0.737 c | 0.618 d | 0.454 e | 0.010 d | 0.009 d | 0.023 c | 0.024 b | 0.031 a | ||||||
| Rc | 0.285 a | 0.276 b | 0.266 c | 0.243 d | 0.231 e | 0.242 d | 0.004 d | 0.008 c | 0.010 b | 0.010 b | 0.012 a | ||||||
| Rb2 | 0.325 a | 0.295 c | 0.307 b | 0.280 d | 0.272 e | 0.188 f | 0.004 d | 0.009 c | 0.009 c | 0.011 b | 0.012 a | ||||||
| Rb3 | 0.038 a | 0.032 b | 0.028 c | 0.028 c | 0.022 d | 0.012 e | 0.001 a | 0.001 a | 0.001 a | 0.001 a | 0.001 a | ||||||
| Rd | 0.079 a | 0.075 b | 0.069 c | 0.052 d | 0.046 e | 0.041 f | 0.001 b | 0.001 b | 0.001 b | 0.002 a | 0.001 b | ||||||
| Total ginsenoside | 2.9 ab | 3.0 a | 2.8 bc | 2.8 bc | 2.7 c | 2.4 d | 0.034 e | 0.053 d | 0.072 c | 0.087 b | 0.093 a | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, D.; Hua, M.; Chen, J.-B.; Li, S.-S.; Wen, L.-K.; Sun, Y.-S. Formation and Characterization of Irreversible Sediment of Ginseng Extract. Foods 2021, 10, 2714. https://doi.org/10.3390/foods10112714
Qu D, Hua M, Chen J-B, Li S-S, Wen L-K, Sun Y-S. Formation and Characterization of Irreversible Sediment of Ginseng Extract. Foods. 2021; 10(11):2714. https://doi.org/10.3390/foods10112714
Chicago/Turabian StyleQu, Di, Mei Hua, Jian-Bo Chen, Shan-Shan Li, Lian-Kui Wen, and Yin-Shi Sun. 2021. "Formation and Characterization of Irreversible Sediment of Ginseng Extract" Foods 10, no. 11: 2714. https://doi.org/10.3390/foods10112714
APA StyleQu, D., Hua, M., Chen, J.-B., Li, S.-S., Wen, L.-K., & Sun, Y.-S. (2021). Formation and Characterization of Irreversible Sediment of Ginseng Extract. Foods, 10(11), 2714. https://doi.org/10.3390/foods10112714






