Evaluation of Oxidative Stability of Full Fat Soybean Flour in Storage and Sensory Quality of Tuo Zaafi-Enriched with Soy Flour as Influenced by Traditional Processing Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment I–Ghana Field Study
2.1.1. Pretreatment Methods
2.1.2. Sensory Evaluation
2.1.3. Proximate Analysis of Soy-Enriched Tuo Zaafi
2.2. Experiment II—Oxidative Stability under Accelerated Conditions Study
2.3. Analyses of Physical Properties and Oxidative Stability Markers
2.3.1. Crude Soy Oil
2.3.2. Determination of Acid Value
2.3.3. Determination of Peroxide Value
2.3.4. Determination of p-Anisidine Value
2.3.5. Determination of Lipoxygenase Enzyme Activity
2.3.6. Determination of Color Characteristics
2.3.7. Determination of Moisture Content
2.3.8. Statistical Analysis
3. Results and Discussion
3.1. Experiment I—Ghana Field Study
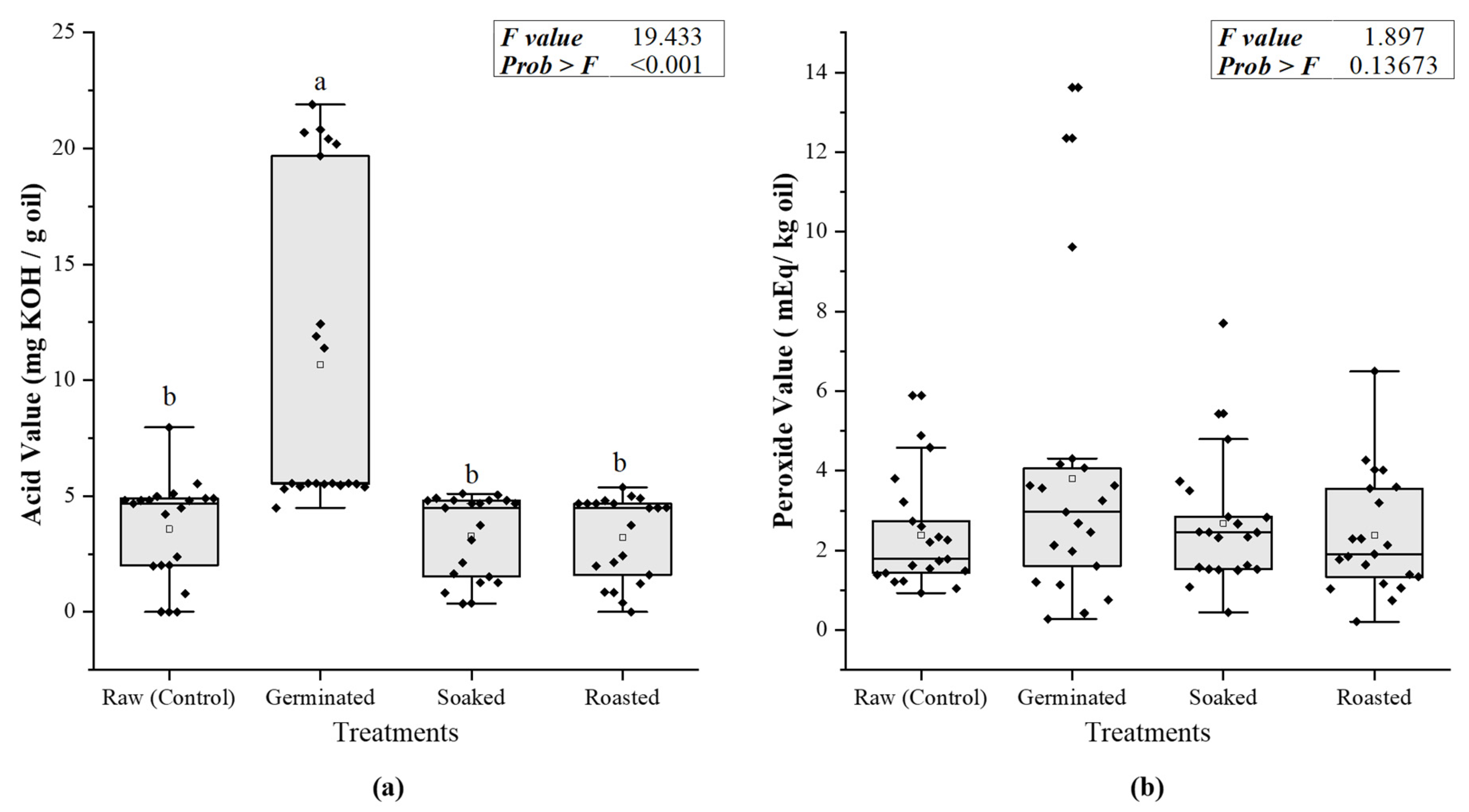
3.1.1. Oxidative Stability Markers
3.1.2. Sensory Study
3.1.3. Proximate Analysis of Soy-Enriched Tuo Zaafi
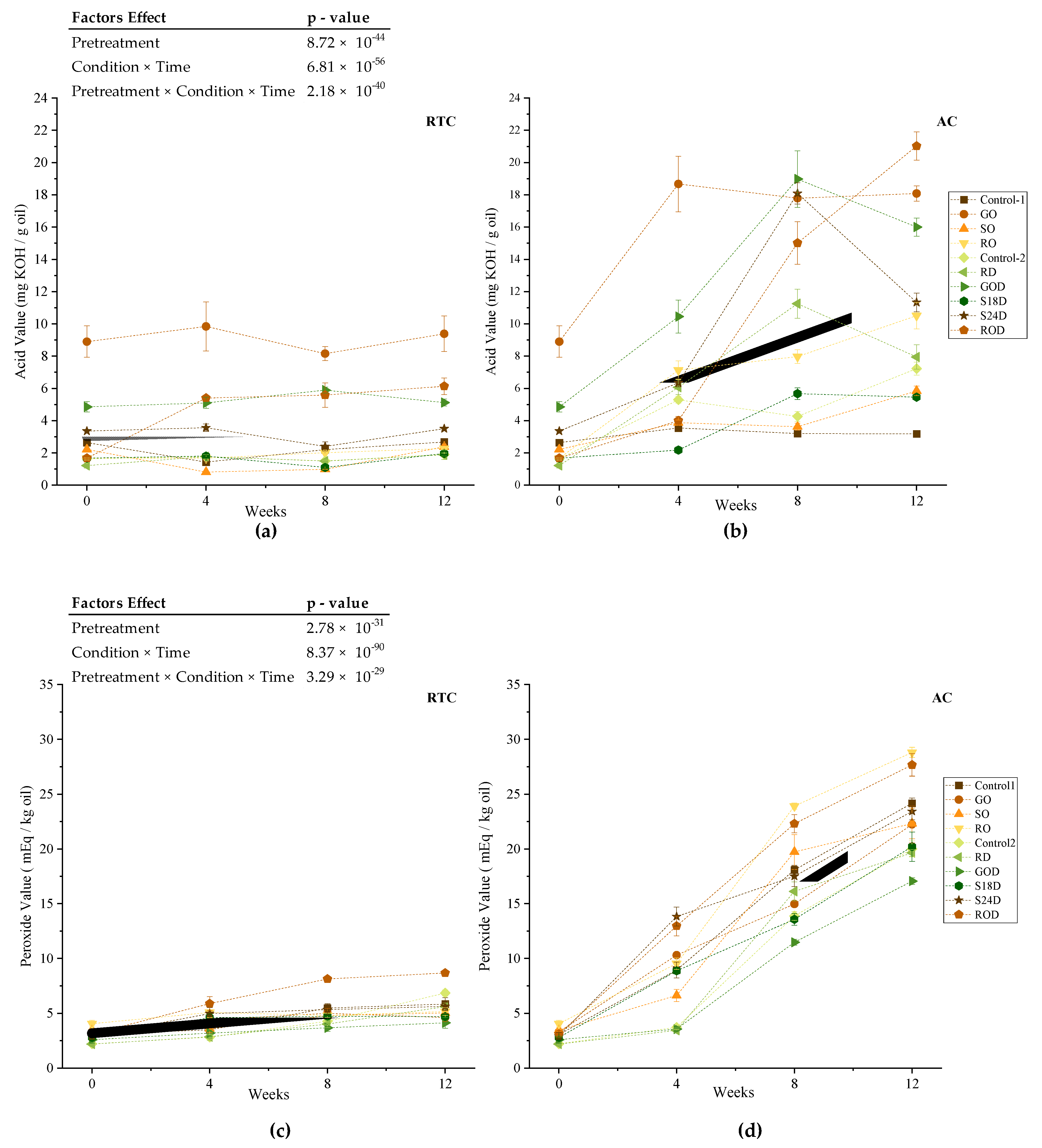
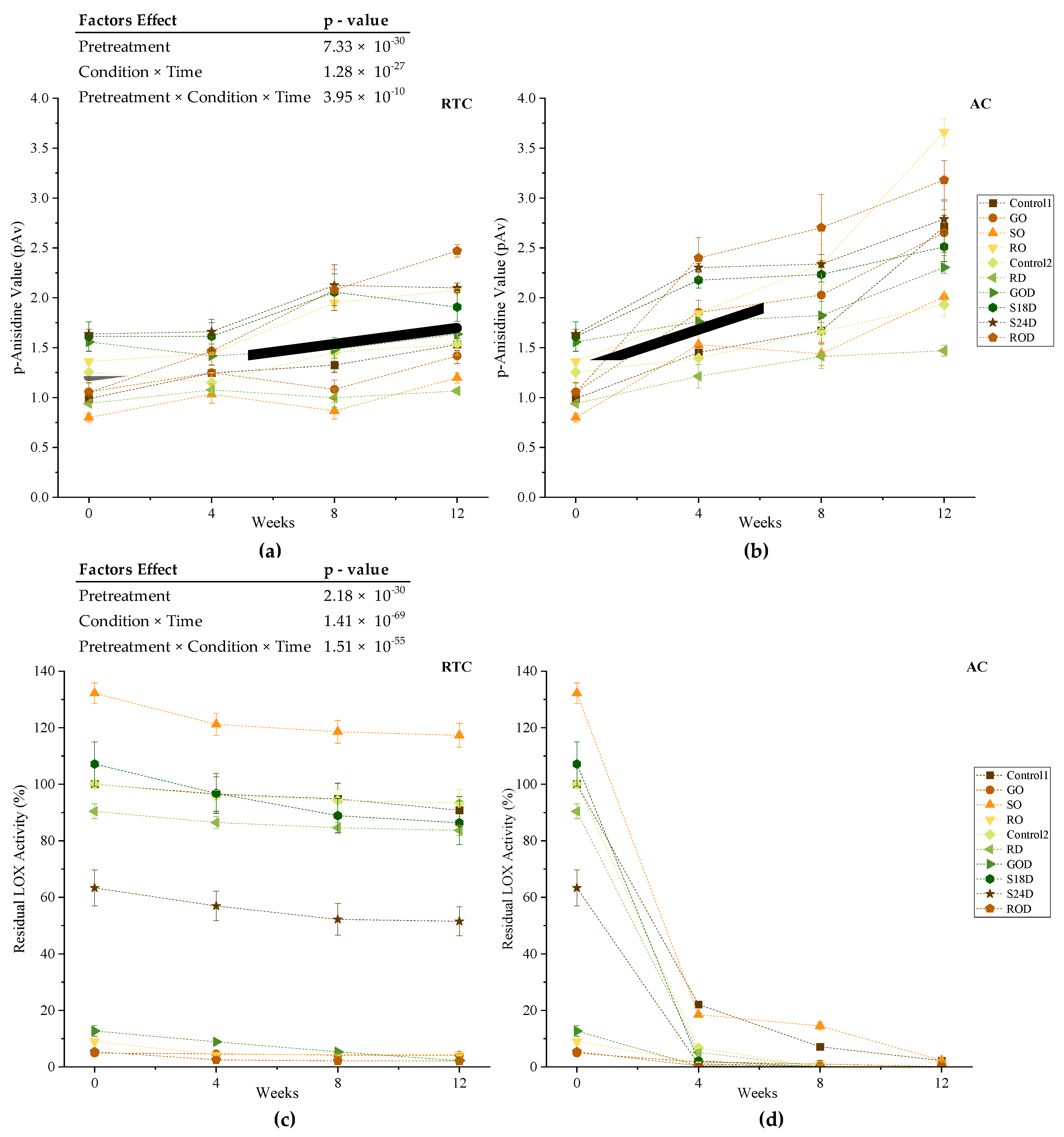
3.2. Experiment II—Oxidative Stability under Accelerated Conditions Study
3.2.1. Acid Value
3.2.2. Peroxide Value
3.2.3. p-Anisidine Value
3.2.4. Lipoxygenase Enzyme Activity
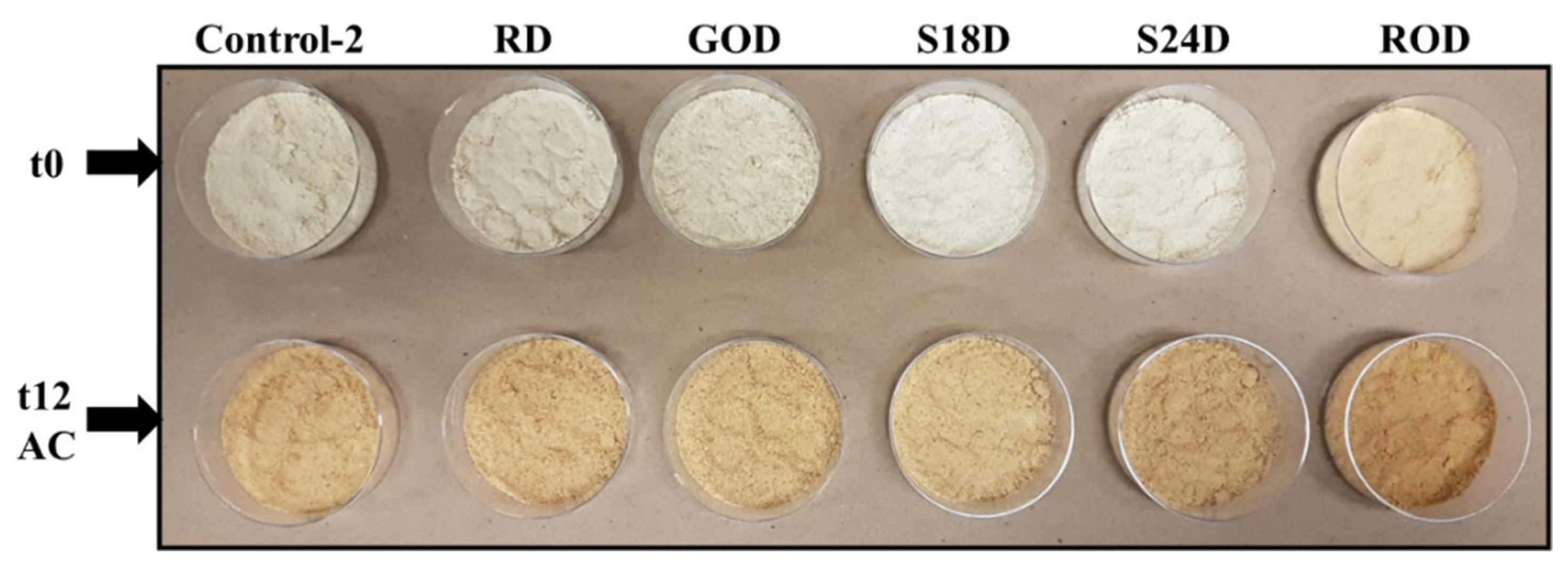
3.2.5. Color Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bain, L.E.; Awah, P.K.; Geraldine, N.; Kindong, N.P.; Sigal, Y.; Bernard, N.; Tanjeko, A.T. Malnutrition in Sub-Saharan Africa: Burden, causes and prospects. Pan Afr. Med. J. 2013, 15, 120. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO); International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Fanzo, J. The Nutrition Challenge in Sub-Saharan Africa; United Nations Development Programme, Regional Bureau for Africa: Addis Ababa, Ethiopia, 2012. [Google Scholar]
- Liu, K. Chemistry and nutritional value of soybean components. In Soybeans: Chemistry, Technology, and Utilization; Chapman & Hall: New York, NY, USA, 1997; pp. 25–95. [Google Scholar]
- Paul, K.N.; Hamdiyah, A.; Samuel, A.D. Food expenditure and household welfare in Ghana. Afr. J. Food Sci. 2014, 8, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Dlamini, T.S.; Tshabalala, P.; Mutengwa, T. Soybeans production in South Africa. OCL 2014, 21, D207. [Google Scholar] [CrossRef]
- Prabakaran, M.; Lee, K.J.; An, Y.; Kwon, C.; Kim, S.; Yang, Y.; Ahmad, A.; Kim, S.H.; Chung, I.M. Changes in Soybean (Glycine max, L.) flour fatty-acid content based on storage temperature and duration. Molecules 2018, 23, 2713. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Goyal, R.; Barwal, S. Domestic processing effects on physicochemical, nutritional and anti-nutritional attributes in soybean (Glycine max, L. merill). Int. Food Res. J. 2013, 20, 3203–3209. [Google Scholar]
- Prodanov, M.; Sierra, I.; Vidal-Valverde, C. Influence of soaking and cooking on the thiamin, riboflavin and niacin contents of legumes. Food Chem. 2004, 84, 271–277. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.; Varma, K. Effect of germination and dehulling on the nutritive value of soybean. Nutr. Food Sci. 2016, 46, 595–603. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Boge, E.L.; Boylston, T.D.; Wilson, L.A. Effect of cultivar and roasting method on composition of roasted soybeans. J. Sci. Food Agric. 2009, 89, 821–826. [Google Scholar] [CrossRef]
- Bolaji, O.T.; Apotiola, Z.O.; Ejagbomo, O.A. Impact of roasting conditions on the quality characteristics of soya bean. Ann. Food Sci. Technol. 2018, 19, 515–526. [Google Scholar]
- Madhujith, T.; Sivakanthan, S. Oxidative stability of edible plant oils. In Bioactive Molecules in Food; Springer International Publishing: Cham, Germany, 2018; pp. 529–551. [Google Scholar]
- Gotoh, N.; Wada, S. The importance of peroxide value in assessing food quality and food safety. J. Am. Oil Chem. Soc. 2006, 83, 473–474. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, U.N. Methods for measuring oxidative rancidity in fats and oils. In Food Lipids: Chemistry, Nutrition, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2002; pp. 387–403. [Google Scholar]
- O’Brien, R.D. Fats and Oils: Formulating and Processing for Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Warner, K.; Eskin, M. Methods to Access Quality and Stability of Oils and Fat-Containing Foods; Aocs Publishing: Champaign, IL, USA, 1995. [Google Scholar]
- Mandal, S.; Dahuja, A.; Santha, I.M. Lipoxygenase activity in soybean is modulated by enzyme-substrate ratio. J. Plant Biochem. Biotechnol. 2014, 23, 217–220. [Google Scholar] [CrossRef]
- Zhu, S.; Riaz, M.N.; Lusas, E.W. Effect of different extrusion temperatures and moisture content on lipoxygenase inactivation and protein solubility in soybeans. J. Agric. Food Chem. 1996, 44, 3315–3318. [Google Scholar] [CrossRef]
- Ham, J.R. Every day it’s tuo zaafi: Considering food preference in a food insecure region of Ghana. Agric. Hum. Values 2020, 37, 907–917. [Google Scholar] [CrossRef]
- Abas, R.; Cobbina, S.J.; Abakari, G.J.F. Microbial quality and antibiotic sensitivity of bacterial isolates in “Tuo-Zaafi” vended in the central business district of tamale. Food Sci. Nutr. 2019, 7, 3613–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Ikya, I.; Gernah, D.; Sengev, I. Proximate composition, nutritive and sensory properties of fermented maize, and full fat soy flour blends for agidi production. Afr. J. Food Sci. 2013, 7, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Xu, Z.; Prinyawiwatkul, W.; Losso, J.N.; King, J.M.; Godber, J.S. Comparison of soybean oils, gum, and defatted soy flour extract in stabilizing menhaden oil during heating. J. Food Sci. 2008, 73, C19–C23. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cd 3d-63 Acid Value Method; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- AOCS. AOCS Official Method Cd 8b-90 Peroxide Value Acetic Acid-Isooctane Method; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- AOCS. AOCS Official Method Cd 18-90 p-Anisidine Value Method; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- Ben-Aziz, A.; Grossman, S.; Ascarelli, I.; Budowski, P. Linoleate oxidation induced by lipoxygenase and heme proteins: A direct spectrophotometric assay. Anal. Biochem. 1970, 34, 88–100. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. Lipoxygenase from soybeans. In Methods in Enzymology; Lowenstein, J.M., Ed.; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioproc. Tech. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Mostafa, M.; Rahma, E.; Rady, A. Chemical and nutritional changes in soybean during germination. Food Chem. 1987, 23, 257–275. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United States (FAO); World Health Organization (WHO). Codex Standard for Named Vegetable Oils (CODEX-STAN 210–1999). 1999. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 30 September 2019).
- Park, S.-K.; Prabakaran, M.; An, Y.; Kwon, C.; Kim, S.; Yang, Y.; Kim, S.-H.; Chung, I.-M. Impact of storage stability on soybean (Glycine max, L.) flour stored in different conditions and package materials. Korean J. Crop. Sci. 2018, 63, 338–359. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Farhoosh, R.; Einafshar, S.; Sharayei, P. The effect of commercial refining steps on the rancidity measures of soybean and canola oils. Food Chem. 2009, 115, 933–938. [Google Scholar] [CrossRef]
- Ali, A.S.; Elozeiri, A.A. Metabolic processes during seed germination. In Advances in Seed Biology; InTech Open: London, UK, 2017. [Google Scholar]
- Temba, M.C.; Njobeh, P.B.; Adebo, O.A.; Olugbile, A.O.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554. [Google Scholar] [CrossRef]
- Kuttappan, V.A.; Lee, Y.S.; Erf, G.F.; Meullenet, J.-F.C.; McKee, S.R.; Owens, C.M. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 2012, 91, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Derera, J.; Siwela, M.; Veldman, F. Consumer acceptance of yellow, provitamin a-biofortified maize in KwaZulu-Natal. S. Afr. J. Clin. Nutr. 2011, 24, 186–191. [Google Scholar] [CrossRef]
- Guinard, J.-X.; Mazzucchelli, R. The sensory perception of texture and mouthfeel. Trends Food Sci. Technol. 1996, 7, 213–219. [Google Scholar] [CrossRef]
- Smith, P. Saturated fat reduction in sauces. In Reducing Saturated Fats in Foods; Elsevier: Amsterdam, The Netherlands, 2011; pp. 370–377. [Google Scholar]
- Bau, H.; Villaume, C.; Nicolas, J.; Méjean, L. Effect of germination on chemical composition, biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J. Sci. Food Agric. 1997, 73, 1–9. [Google Scholar] [CrossRef]
- Kilcast, D.; Lewis, D.F. Structure and texture—Their importance in food quality. Nutr. Bull. 1990, 15, 103–113. [Google Scholar] [CrossRef]
- Li, H.; Fitzgerald, M.A.; Prakash, S.; Nicholson, T.M.; Gilbert, R.G. The molecular structural features controlling stickiness in cooked rice, a major palatability determinant. Sci. Rep. 2017, 7, 43713. [Google Scholar] [CrossRef] [Green Version]
- Michelfelder, A.J. Soy: A complete source of protein. Am. Fam. Physician 2009, 79, 43–47. [Google Scholar] [PubMed]
- Etiosa, O.; Chika, N.; Benedicta, A. Mineral and proximate composition of soya bean. Asian J. Phys. Chem. Sci. 2018, 4, 2456–7779. [Google Scholar] [CrossRef]
- Balogun, M.; Kolawole, F.; Karim, O.; Fasakin, T. Proximate composition, microbial quality and consumer acceptability of gruel from fermented maize and soybean. Appl. Trop. Agric. 2016, 21, 53–57. [Google Scholar]
- Ezeokeke, C.T.; Onuoha, A.B. Nutrient composition of cereal (maize), legume (soybean) and fruit (banana) as a complementary food for older infants and their sensory assessment. J. Food Eng. 2016, 6, 139–148. [Google Scholar]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Kotoka, F.; Appiah, A.P.; Awuah, P.; Baiden, B.K.A. Effects of roasting and boiling on the yield, quality and oxidative stability of extracted soya bean oil. Therm. Sci. Eng. 2018, 1. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Hua, Y.; Zhang, C.; Kong, X.; Li, X.; Chen, Y. Effect of soaking conditions on the formation of lipid derived free radicals in soymilk. Food Chem. 2020, 315, 126237. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, Y.; Liu, Y. Volatile components of deep-fried soybean oil as indicator indices of lipid oxidation and quality degradation. Eur. Food Res. Technol. 2020, 246, 1183–1192. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Chauhan, G.S. Influence of germination temperature on oil content and fatty acid composition of soy sprouts. J. Food Sci. Technol. 2006, 43, 325–326. [Google Scholar]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; Van den Broeck, H.C.; Brouns, F.J.P.H.; De Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Kermasha, S.; Bisakowski, B.; Ramaswamy, H.; Van de Voort, F.R. Thermal and microwave inactivation of soybean lipoxygenase. LWT 1993, 26, 215–219. [Google Scholar] [CrossRef]
- Navicha, W.B.; Hua, Y.; Masamba, K.; Kong, X.; Zhang, C. Optimization of soybean roasting parameters in developing nutritious and lipoxygenase free soymilk. J. Food Meas. Charact. 2017, 11, 1899–1908. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Pandey, V.; Chauhan, G.S. Changes in lipoxygenase isozymes and trypsin inhibitor activity in soybean during germination at different temperatures. Food Chem. 2006, 99, 563–568. [Google Scholar] [CrossRef]
- Srivastav, P.P.; Das, H.; Prasad, S. Effect of roasting process variables on in-vitro protein digestibility of bengalgram, maize and soybean. Food Chem. 1990, 35, 31–37. [Google Scholar] [CrossRef]
- Bordingnon, J.R.; Ida, E.L.; Oliveira, M.C.; Mandarino, J.M. Effect of germination on the protein content and on the level of specific activity of lipoxygenase-1 in seedlings of three soybean cultivars. Arch. Latinoam. Nutr. 1995, 45, 222–226. [Google Scholar] [PubMed]
- Bau, H.M.; Villaume, C.; Méjean, L. Effects of soybean (Glycine max) germination on biologically active components, nutritional values of seeds, and biological characteristics in rats. Nahrung 2000, 44, 2–6. [Google Scholar] [CrossRef]
- Shin, D.-J.; Kim, W.; Kim, Y. Physicochemical and sensory properties of soy bread made with germinated, steamed, and roasted soy flour. Food Chem. 2013, 141, 517–523. [Google Scholar] [CrossRef]
- Maetens, E.; Hettiarachchy, N.; Dewettinck, K.; Horax, R.; Moens, K.; Moseley, D.O. Physicochemical and nutritional properties of a healthy snack chip developed from germinated soybeans. LWT 2017, 84, 505–510. [Google Scholar] [CrossRef]
- Wang, C.; Croft, K.P.; Jarlfors, U.; Hildebrand, D.F. Subcellular localization studies indicate that lipoxygenases 1 to 6 are not involved in lipid mobilization during soybean germination. Plant Physiol. 1999, 120, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedow, J.N. Plant lipoxygenase: Structure and function. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Tian, Q.; Hua, Y. Oxidation reactions in model systems simulating the processing of soybeans into soymilk: Role of lipase and lipoxygenase in volatile flavors formation. Int. J. Food Prop. 2021, 24, 192–202. [Google Scholar] [CrossRef]
- Mang, Y.D.; Njintang, Y.N.; Abdou, B.A.; Scher, J.; Bernard, C.; Mbofung, M.C. Dehulling reduces toxicity and improves in vivo biological value of proteins in vegetal milk derived from two mucuna (Mucuna pruriens, L.) seeds varieties. J. Food Sci. Technol. 2016, 53, 2548–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens Culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Y.; Ahmad, A. Impact of processing on nutritional and antinutritional factors of legumes: A review. Food Chem. 2018, 19, 199–215. [Google Scholar]
- Stephany, M.; Bader-Mittermaier, S.; Schweiggert-Weisz, U.; Carle, R. Lipoxygenase activity in different species of sweet lupin (Lupinus, L.) seeds and flakes. Food Chem. 2015, 174, 400–406. [Google Scholar] [CrossRef]
- Asbi, B.A.; Wei, L.S.; Steinberg, M.P. Effect of PH on the kinetics of soybean lipoxygenase-1. J. Food Sci. 1989, 54, 1594–1595. [Google Scholar] [CrossRef]
- Chinma, C.E.; Anuonye, J.C.; Simon, O.C.; Ohiare, R.O.; Danbaba, N. Effect of germination on the physicochemical and antioxidant characteristics of rice flour from three rice varieties from Nigeria. Food Chem. 2015, 185, 454–458. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Wu, H.; Jing, L.; Gong, B.; Gou, M.; Zhao, K.; Li, W. The compositional, physicochemical and functional properties of germinated mung bean flour and its addition on quality of wheat flour noodle. J. Food Sci. Technol. 2018, 55, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Raigar, R.K.; Dalbhagat, C.G.; Mishra, H.N. Effect of pilot scale roasting on color and textural attributes of soybean kernels. J. Food Process. Preserv. 2020, 44, e14883. [Google Scholar] [CrossRef]
- Yousif, A.M. Soybean grain storage adversely affects grain testa color, texture and cooking quality: Soybean storage effect on grain quality. J. Food Qual. 2014, 37, 18–28. [Google Scholar] [CrossRef]
- Kong, F.; Chang, S.K.C.; Liu, Z.; Wilson, L.A. Changes of soybean quality during storage as related to soymilk and tofu making. J. Food Sci. 2008, 73, S134–S144. [Google Scholar] [CrossRef] [PubMed]

| Batch ID | Pretreatment Methods | Sample Codes | Storage Conditions | Sample Collection | |
|---|---|---|---|---|---|
| 1 | Dehulled | Raw (untreated) | Control-1 | Room Temperature Conditions (RTC) Accelerated Conditions (AC) | Day zero Week 4 Week 8 Week 12 |
| Germinated | GO | ||||
| Soaked | SO | ||||
| Roasted | RO | ||||
| Raw (untreated) | Control-2 | ||||
| 2 | Nondehulled | Raw and dehulled | RD | ||
| Germinated and dehulled | GOD | ||||
| Soaked 18 h and dehulled | S18D | ||||
| Soaked 24 h and dehulled | S24D | ||||
| Roasted and dehulled | ROD | ||||
| Tuo Zaafi | Proximate (g/100 g) | Total Energy kcal/100 g | ||||
|---|---|---|---|---|---|---|
| Moisture | Crude Protein | Total Ash | Crude Fat | Total CHO | ||
| 10% Soy-enriched | 81.72 ± 0.04 | 12.75 ± 0.64 | 1.41 ± 0.09 | 1.40 ± 0.09 | 84.44 ± 0.52 | 387.54 ± 0.52 |
| 100% Maize | 82.80 ± 0.08 | 7.89 ± 0.36 | 0.41 ± 0.07 | 0.36 ± 0.06 | 91.33 ± 0.30 | 382.87 ± 1.55 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulkirpik, E.; Toc, M.; Atuna, R.A.; Amagloh, F.K.; Andrade Laborde, J.E. Evaluation of Oxidative Stability of Full Fat Soybean Flour in Storage and Sensory Quality of Tuo Zaafi-Enriched with Soy Flour as Influenced by Traditional Processing Methods. Foods 2021, 10, 2192. https://doi.org/10.3390/foods10092192
Gulkirpik E, Toc M, Atuna RA, Amagloh FK, Andrade Laborde JE. Evaluation of Oxidative Stability of Full Fat Soybean Flour in Storage and Sensory Quality of Tuo Zaafi-Enriched with Soy Flour as Influenced by Traditional Processing Methods. Foods. 2021; 10(9):2192. https://doi.org/10.3390/foods10092192
Chicago/Turabian StyleGulkirpik, Ece, Marco Toc, Richard A. Atuna, Francis K. Amagloh, and Juan E. Andrade Laborde. 2021. "Evaluation of Oxidative Stability of Full Fat Soybean Flour in Storage and Sensory Quality of Tuo Zaafi-Enriched with Soy Flour as Influenced by Traditional Processing Methods" Foods 10, no. 9: 2192. https://doi.org/10.3390/foods10092192







