The Microbial Diversity and Biofilm-Forming Characteristic of Two Traditional Tibetan Kefir Grains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Activation of Tibetan Kefir Grains
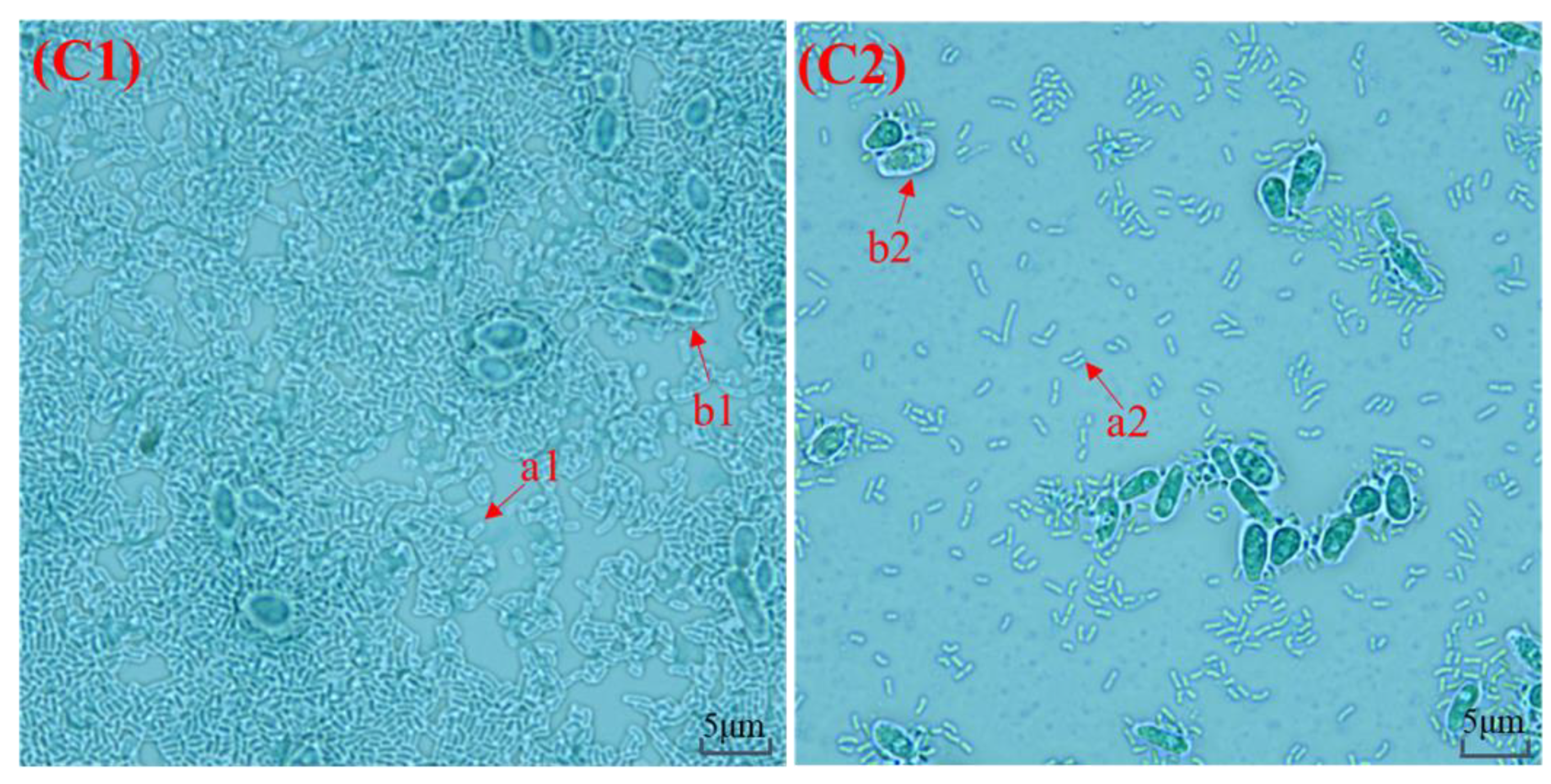
2.2. Macroscopic Observation and Microscopic Observation of Kefir Grains
2.3. High-Throughput Analysis of Microorganism Diversity on Kefir Grains and Kefir Milks
2.3.1. Bacterial and Fungal Total DNA Extraction and Polymerase Chain Reaction (PCR) Amplifycation
2.3.2. High-Throughput Sequencing and Bioinformatic Analysis
2.4. Isolation of Bacteria and Yeast from Kefir Grains
2.5. Determination of the Proliferation Rate of Kefir Grains
2.6. The Yield and Structural Investigation of Kefir EPS
2.6.1. Monosaccharide Composition Analysis of Kefir EPS
2.6.2. Fourier-Transform Infrared (FT-IR) Analysis of Kefir EPS
2.7. Determination of the Optimal Days for Biofilm Formation
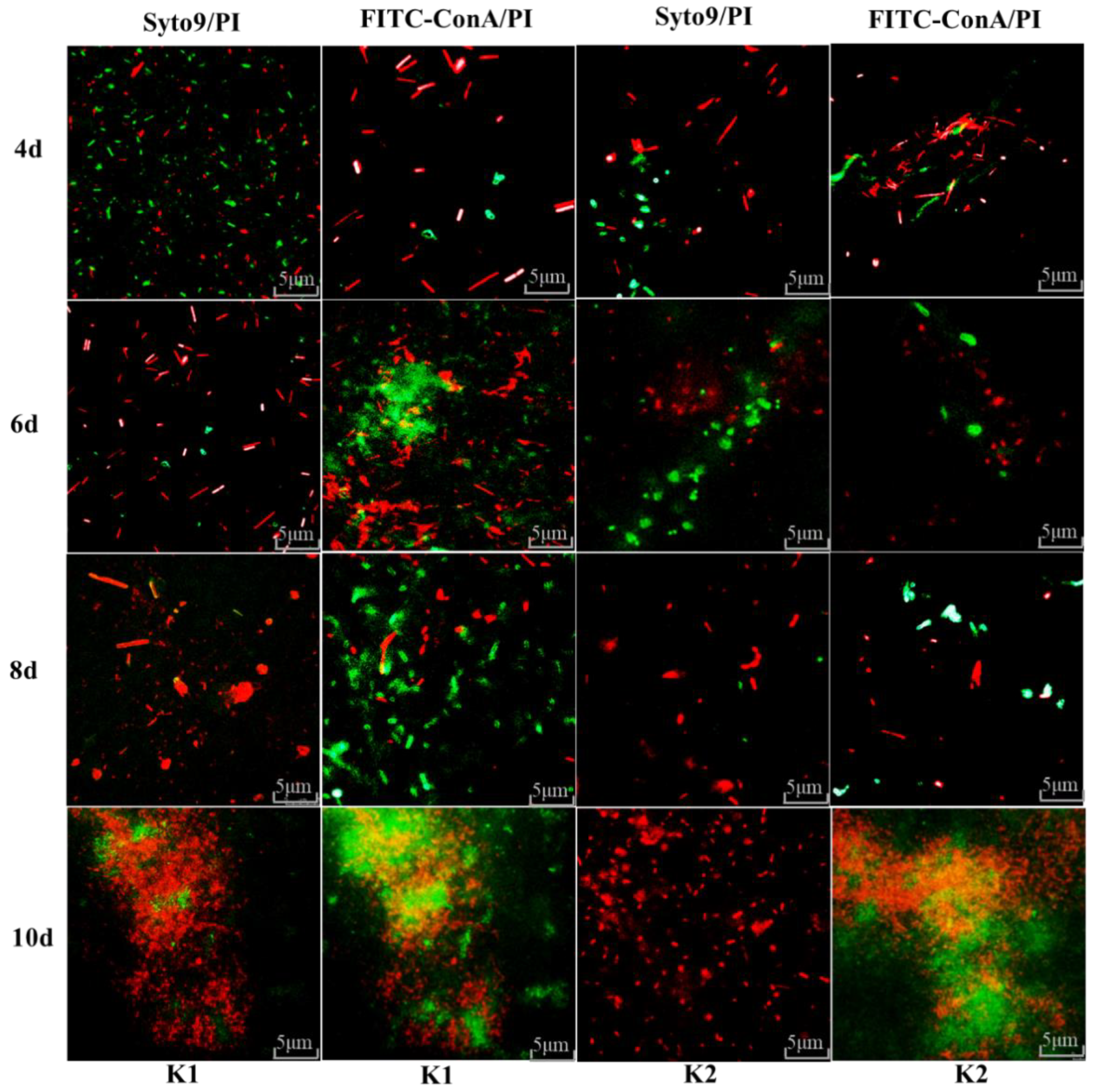
2.8. Fluorescence Microscope Observation in the Formation of Kefir Biofilm
2.9. Statistical Analysis
3. Results and Discussion
3.1. Macroscopic Observation and Microscopic Observation of Kefir Grains
3.2. Sequencing Results and Alpha Diversity Analysis
3.3. Microbial Community Composition Analysis
3.4. Isolation of Bacteria and Yeast from Kefir Grains
3.5. Determination of the Proliferation Rate of Kefir Grains
3.6. Determination of the Yield of Kefir EPS
3.7. Monosaccharide Composition Analysis of Kefir EPS
3.8. FT-IR Analysis of Kefir EPS
3.9. Determination of the Optimal Days for Biofilm Formation of K1 and K2
3.10. Fluorescence Microscope Observation for Biofilm Formation of K1 and K2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzel-Seydim, Z.B.; Gökırmakliı, Ç.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Alves, E.; Ntungwe, E.N.; Gregorio, J.; Rodrigues, L.M.; Pereira-Leite, C.; Caleja, C.; Pereira, E.; Barros, L.; Aguilar-Vilas, M.V.; Rosado, C.; et al. Characterization of Kefir Produced in Household Conditions: Physicochemical and Nutritional Profile, and Storage Stability. Foods 2021, 10, 1057. [Google Scholar] [CrossRef]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Kefir characteristics and antibacterial properties-potential applications in control of enteric bacterial infection. Int. J. Dairy Technol. 2021, 118, 105021. [Google Scholar] [CrossRef]
- Ilikkan, O.K.; Bagdat, E.S. Comparison of bacterial and fungal biodiversity of Turkish kefir grains with high-throughput metagenomic analysis. LWT-Food Sci. Technol. 2021, 152, 112375. [Google Scholar] [CrossRef]
- Han, X.; Yi, H.X.; Zhao, S.N.; Sun, J.L.; Wang, Y. Prospects of artificial kefir grains prepared by cheese and encapsulated vectors to mimic natural kefir grains. J. Food Qual. 2020, 2020, 8839135. [Google Scholar] [CrossRef]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001, 68, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.O.; Magalhaes-Guedes, K.T.; Braga, R.A.; Dias, D.R.; Schwan, R.F. Fermentation process for production of apple-based kefir vinegar: Microbiological, chemical and sensory analysis. Braz. J. Microbiol. 2017, 48, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.; O’Sullivan, O.; Cotter, P.D.; Ross, P.; Hill, C. High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol. Lett. 2011, 320, 56–62. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Genotypic diversity of bacteria and yeasts isolated from Tibetan kefir. Int. J. Food Sci. Technol. 2018, 53, 1535–1540. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germana, M.A.; Erten, H.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Teusink, B.; Molenaar, D. Systems biology of lactic acid bacteria: For food and thought. Curr. Opin. Syst. Biol. 2017, 6, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Z.; Liu, X.; Jiang, H.; Dong, M.S. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte, C.A. Food-derived biopolymer kefiran composites, nanocomposites and nanofibers: Emerging alternatives to food packaging and potentials in nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.Y.; Zhao, W.; Guo, T.; Cao, Y.Q.; Teng, J.W.; Hao, X.N.; Zhao, J.; Yang, Z.N. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Tang, W.; Geng, W.; Zheng, Y.; Wang, Y. In vitro and in vivo evaluation of the probiotic attributes of Lactobacillus kefiranofaciens XL10 isolated from Tibetan kefir grain. Appl. Microbiol. Biotechnol. 2017, 101, 2467–2477. [Google Scholar] [CrossRef]
- Bhawal, S.; Kumari, A.; Kapila, S.; Kapila, R. Physicochemical Characteristics of Novel Cell-Bound Exopolysaccharide from Probiotic Limosilactobacillus fermentum (MTCC 5898) and Its Relation to Antioxidative Activity. J. Agric. Food Chem. 2021, 69, 10338–10349. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xia, X.D.; Tang, W.Z.; Ji, J.; Rui, X.; Chen, X.H.; Jiang, M.; Zhou, J.Z.; Zhang, Q.Q.; Dong, M.S. Structural Characterization and Anticancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolin, C.; Croatti, V.; Laghi, L.; Giordani, B.; Tondi, M.R.; De Gregorio, P.R.; Foschi, C.; Vitali, B. Lactobacillus Biofilms Influence Anti-Candida Activity. Front. Microbiol. 2021, 12, 750368. [Google Scholar] [CrossRef]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; Garcia, A. Biofilm Forming Lactobacillus: New Challenges for the Development of Probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef]
- Dong, J.; Liu, B.; Jiang, T.M.; Liu, Y.P.; Chen, L.J. The biofilm hypothesis: The formation mechanism of Tibetan kefir grains. Int. J. Dairy Technol. 2018, 71, 44–50. [Google Scholar] [CrossRef]
- Tang, W.Z.; Dong, M.S.; Wang, W.W.; Han, S.; Rui, X.; Chen, X.H.; Jiang, M.; Zhang, Q.; Wu, J.; Li, W. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr. Polym. 2017, 173, 654–664. [Google Scholar] [CrossRef]
- You, X.; Li, Z.; Ma, K.; Zhang, C.; Chen, X.H.; Wang, G.; Yang, L.; Dong, M.S.; Rui, X.; Zhang, Q.; et al. Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr. Polym. 2020, 235, 115977. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.C.; Li, C.K.; Liu, Y.H.; Li, W.C.; Chen, Y.F.; Siqinbateer; Bao, Y.S.; Saqila, W.; Zhang, H.P.; Menghe, B.; et al. Koumiss consumption modulates gut microbiota, increases plasma high density cholesterol, decreases immunoglobulin G and albumin. J. Funct. Foods 2019, 52, 469–478. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.N.; Guo, M.R. Autogenic successions of bacteria and fungi in kefir grains from different origins when sub-cultured in goat milk. Food Res. Int. 2020, 138, 109784. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, K.N.; Lo, Y.M.; Chiang, M.L.; Chen, H.C.; Liu, J.R.; Chen, M.J. Investigation of microorganisms involved in biosynthesis of the kefir grain. Food Microbiol. 2012, 32, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Bicer, Y.; Telli, A.E.; Sonmez, G.; Turkal, G.; Telli, N.; Ucar, G. Comparison of commercial and traditional kefir microbiota using metagenomic analysis. Int. J. Dairy Technol. 2021, 74, 528–534. [Google Scholar] [CrossRef]
- Kumar, M.R.; Yeap, S.K.; Mohamad, N.E.; Abdullah, J.O.; Masarudin, M.J.; Khalid, M.; Leow, A.T.C.; Alitheen, N.B. Metagenomic and phytochemical analyses of kefir water and its subchronic toxicity study in BALB/c mice. BMC Complement. Med. Ther. 2021, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.A.; Chou, J.Y. Yeasts in fermented food and kefir: In vitro characterization of probiotic traits. J. Anim. Plant Sci. 2021, 31, 567–582. [Google Scholar] [CrossRef]
- Goktas, H.; Dikmen, H.; Demirbas, F.; Sagdic, O.; Dertli, E. Characterisation of probiotic properties of yeast strains isolated from kefir samples. Int. J. Dairy Technol. 2021, 74, 715–722. [Google Scholar] [CrossRef]
- Leonel, L.V.; Arruda, P.V.; Chandel, A.K.; Felipe, M.G.A.; Sene, L. Kluyveromyces marxianus: A potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Crit. Rev. Biotechnol. 2021, 41, 1131–1152. [Google Scholar] [CrossRef] [PubMed]
- Fadda, M.E.; Mossa, V.; Deplano, M.; Pisano, M.B.; Cosentino, S. In vitro screening of Kluyveromyces strains isolated from Fiore Sardo cheese for potential use as probiotics. LWT-Food Sci. Technol. 2017, 75, 100–106. [Google Scholar] [CrossRef]
- Moradi, Z.; Kalanpour, N. Kefiran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Khodaiyan, F.; Jahanbin, K.; Gharibzahedi, S.M.T.; Taheri, S. Structural investigation and response surface optimisation for improvement of kefiran production yield from a low-cost culture medium. Food Chem. 2012, 133, 383–389. [Google Scholar] [CrossRef]
- Prado, M.R.M.; Zibetti, R.G.M.; de Souza, D.; Pedroso, L.L.; Soccol, C.R. Anti-inflammatory and angiogenic activity of polysaccharide extract obtained from Tibetan kefir. Microvasc. Res. 2016, 108, 29–33. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.L.; Yang, Y.Z.; Wen, Y.Z.; Liu, S.X.; Li, C.F.; Hu, Z.Y.; Cheng, X.R.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.J.; Wang, X.M.; Zhang, X.L.; Zhang, C.P.; Chen, X.H.; Dong, M.S.; Rui, X.; Zhang, Q.Q.; Fang, Y.; Li, W. Isolation, structural characterization and neuroprotective activity of exopolysaccharide from Paecilomyces cicada TJJ1213. Int. J. Biol. Macromol. 2021, 183, 1034–1046. [Google Scholar] [CrossRef]
- Piermaria, J.A.; Pinotti, A.; Garcia, M.A.; Abraham, A.G. Films based on kefiran, an exopolysaccharide obtained from kefir grain: Development and characterization. Food Hydrocoll. 2009, 23, 684–690. [Google Scholar] [CrossRef]
- Koo, H.; Yamada, K.M. Dynamic cell-matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr. Opin. Cell Biol. 2016, 42, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, S.H.; Gomez, F.A.; Ramirez, R.; Nilo, L.; Henriquez, V. Biofilm generation by Piscirickettsia salmonis under growth stress conditions: A putative in vivo survival/persistence strategy in marine environments. Res. Microbiol. 2012, 163, 557–566. [Google Scholar] [CrossRef] [PubMed]

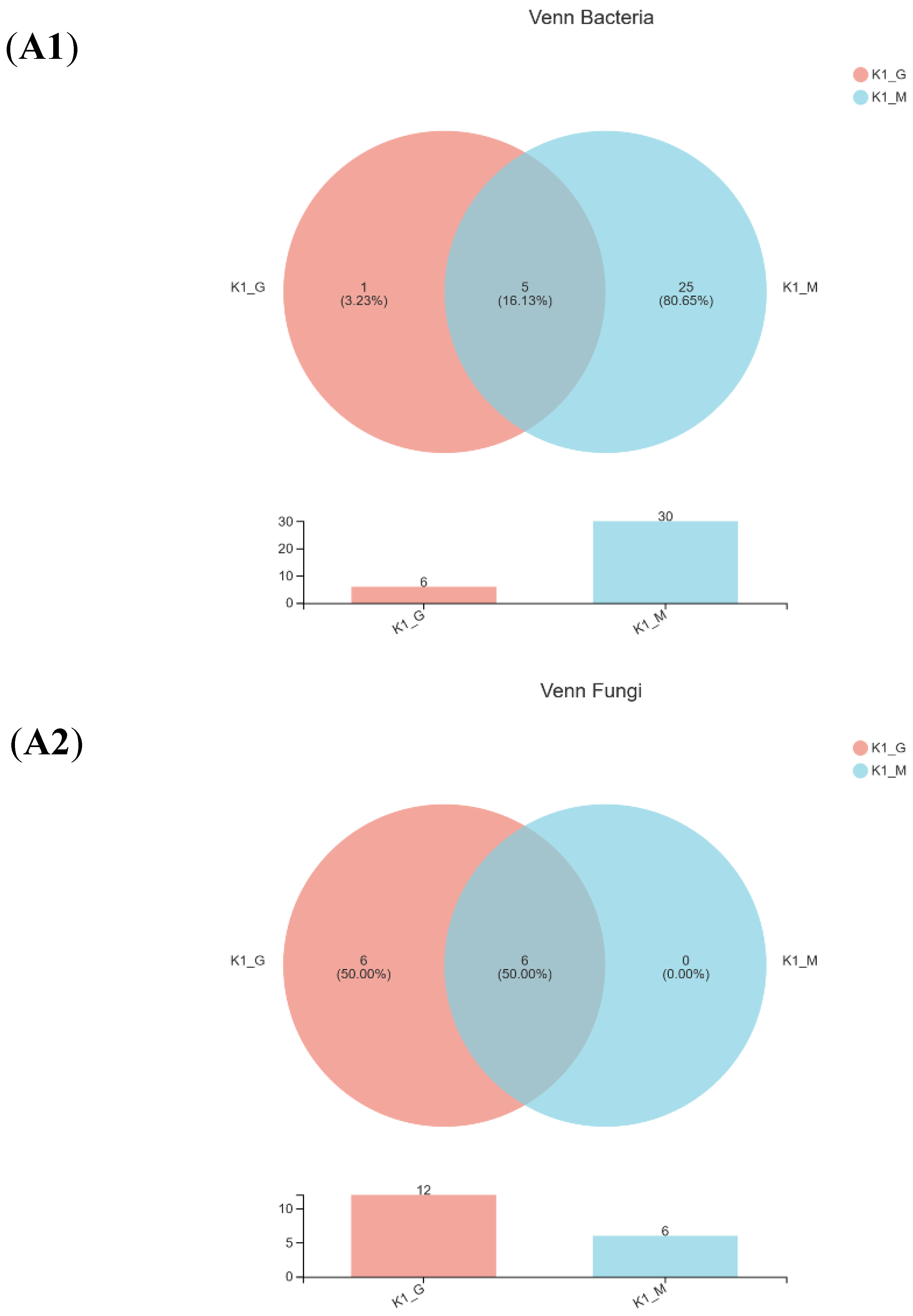





| Sample a | Observed OTUs | Number of Sequences | Shannon Index | Chao Index | Simpson Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| K1-G | 3 | 20 | 37,390 | 50,045 | 0.22609 | 0.1133 | 23 | 20 | 0.45443 | 0.96659 |
| K1-M | 23 | 6 | 40,148 | 74,209 | 0.31343 | 0.1891 | 5 | 6 | 0.33823 | 0.91517 |
| K2-G | 4 | 14 | 47,988 | 30,691 | 1.18108 | 0.3147 | 4 | 21 | 0.89401 | 0.84856 |
| K2-M | 4 | 23 | 43,221 | 32,009 | 0.90832 | 1.3493 | 4 | 27 | 0.83235 | 0.36902 |
| Strains (Identification %) | Colony Characteristics | Separation Sources | Medium |
| L. kefiri K1-M1 (99.93) | Round, dry, transparent, entire | K1-M | MRS |
| L. kefiri K1-M2 (99.97) | Round, moist, white, smooth | K1-M | MRS |
| L. kefiranofaciens K1 (99.96) | Round, moist, white, smooth | K1-M | MRS |
| L. kefiranofaciens G-M1 (99.98) | Round, moist, transparent, smooth | K1-G | MRS |
| L. kefiri G-M2 (99.94) | Round, dry, transparent, entire | K1-G | MRS |
| L. kefiranofaciens subsp kefiri granum G-M6 (99.93) | Round, moist, transparent, smooth | K1-G | MRS |
| K. marxianus Y1 (99.95) | Round, ropy, white, smooth | K1-M | PDA |
| K. marxianus G-Y3 (99.93) | Round, ropy, white, entire | K1-G | PDA |
| Strains (Identification %) | Colony Characteristics | Separation Sources | Medium |
| E. durans K2-M3 (99.97) | Round, dry, transparent, entire | K2-M | MRS |
| F. fructosus K2-Y6 (99.94) | Punctirorm, ropy, white, smooth | K2-M | MRS |
| L. kefiri K2-MY1 (99.94) | Round, moist, white, smooth | K2-M | MRS |
| L. paracasei K2-MX1 (99.93) | Round, moist, transparent, smooth | K2-M | MRS |
| K. marxianus K2-Y1 (99.98) | Round, ropy, white, entire | K2-M | PDA |
| A. fabarum K2-Y4 (99.96) | Punctirorm, ropy, transparent, smooth | K2-M | MRS |
| L. kefiri K2-GM5 (99.94) | Round, dry, transparent, entire | K2-G | MRS |
| E. durans K2-M2 (99.96) | Punctirorm, ropy, white, smooth | K2-G | MRS |
| K. marxianus K2-Y3 (99.97) | Round, ropy, white, entire | K2-G | PDA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, W.; Xu, M.; Tian, J.; Li, W. The Microbial Diversity and Biofilm-Forming Characteristic of Two Traditional Tibetan Kefir Grains. Foods 2022, 11, 12. https://doi.org/10.3390/foods11010012
Wang X, Li W, Xu M, Tian J, Li W. The Microbial Diversity and Biofilm-Forming Characteristic of Two Traditional Tibetan Kefir Grains. Foods. 2022; 11(1):12. https://doi.org/10.3390/foods11010012
Chicago/Turabian StyleWang, Xiaomeng, Wenpei Li, Mengjia Xu, Juanjuan Tian, and Wei Li. 2022. "The Microbial Diversity and Biofilm-Forming Characteristic of Two Traditional Tibetan Kefir Grains" Foods 11, no. 1: 12. https://doi.org/10.3390/foods11010012
APA StyleWang, X., Li, W., Xu, M., Tian, J., & Li, W. (2022). The Microbial Diversity and Biofilm-Forming Characteristic of Two Traditional Tibetan Kefir Grains. Foods, 11(1), 12. https://doi.org/10.3390/foods11010012







